Abstract
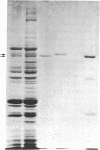
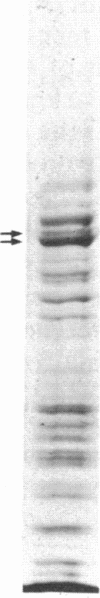
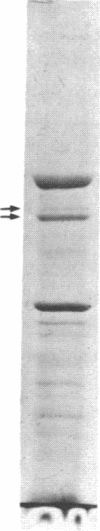
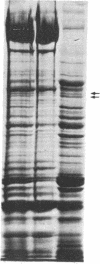
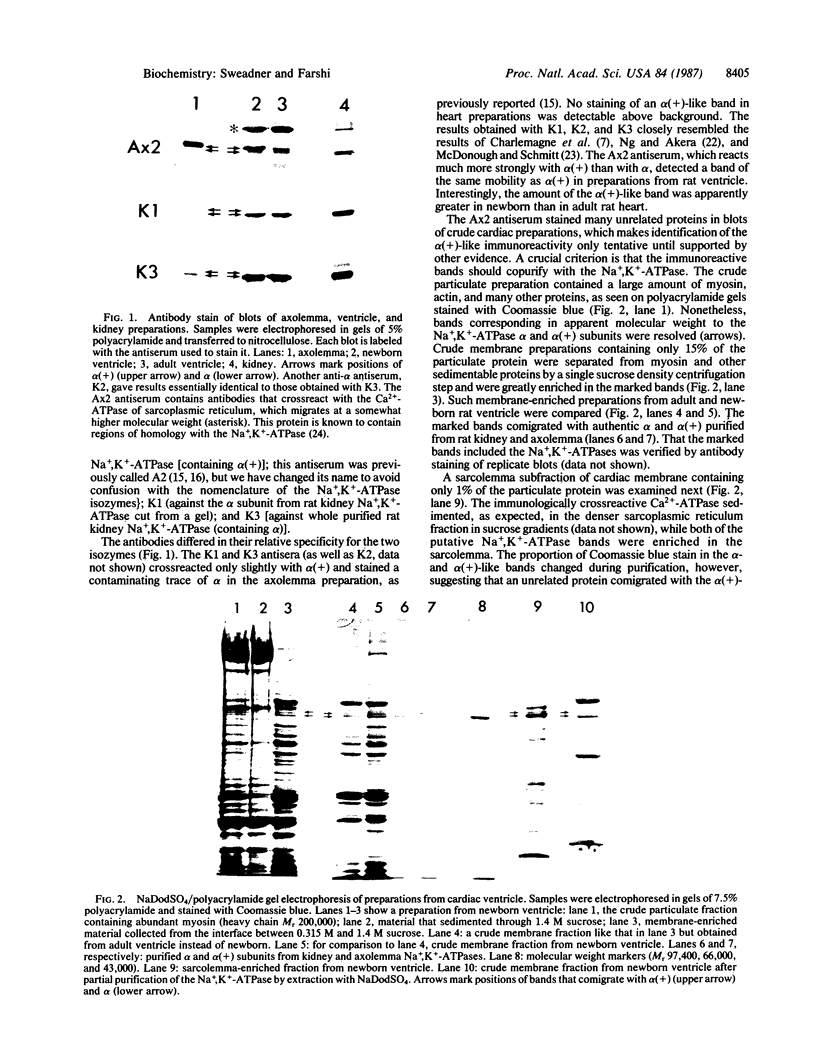
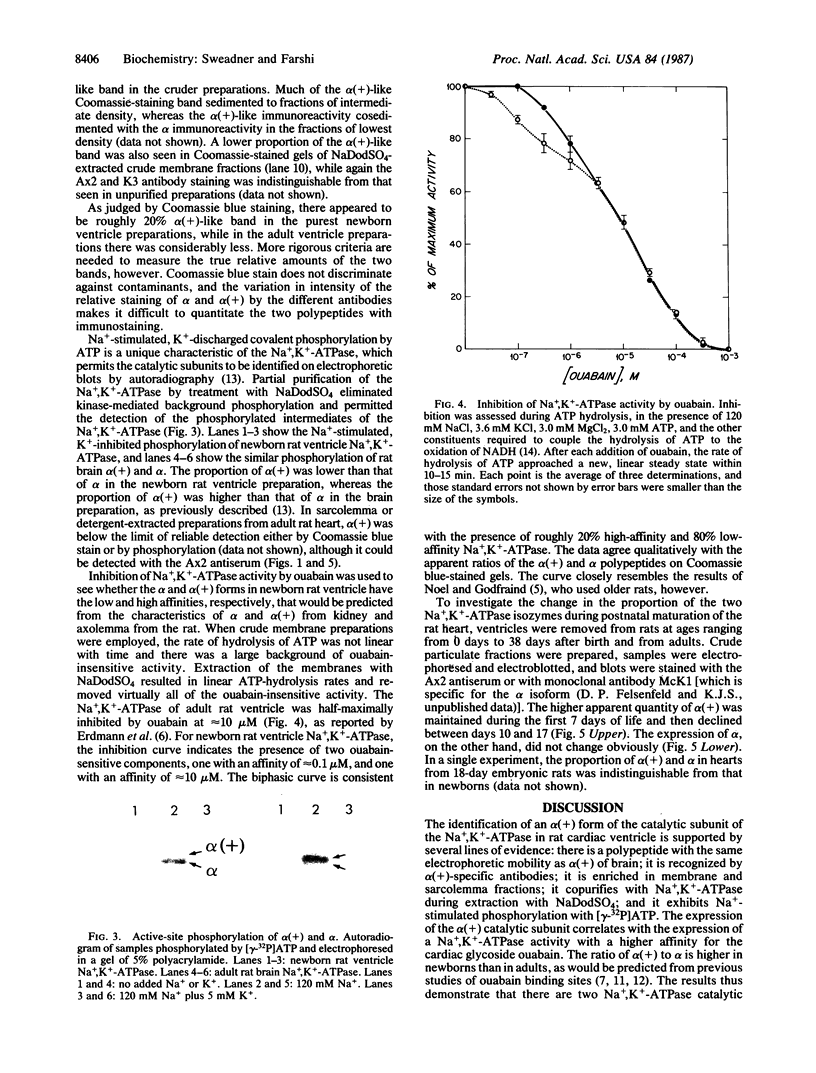
The mechanism of the inotropic effect of cardiac glycosides on the heart has long been controversial. Inotropic effects at low concentrations of cardiac glycosides indicate more than one class of receptor or more than one cellular mechanism. In the brain of the rat, high- and low-affinity cardiac glycoside receptors have been shown to be associated with two structurally different isoforms of the catalytic subunit of the Na+,K+-ATPase, termed alpha and alpha(+). Evidence is presented here that the high- and low-affinity sites in rat cardiac ventricle are associated with Na+,K+-ATPase catalytic subunit forms similar to the alpha(+) and alpha forms in the brain. Membranes from the rat ventricle contained polypeptides with the electrophoretic mobilities of alpha and alpha(+), which could be stained by isoform-specific anti-Na+,K+-ATPase antibodies on electrophoretic blots. Both polypeptides also displayed Na+-stimulated phosphorylation with [gamma-32P]ATP. Inhibition of Na+,K+-ATPase activity by ouabain demonstrated the presence of both high- and low-affinity ATPases proportional to the presence of the alpha(+) and alpha polypeptides. The ratios of the two isoforms changed with postnatal maturation, paralleling known changes in cardiac physiology and cardiac glycoside sensitivity. Cardiac glycoside sensitivity can evidently be regulated at the level of gene expression by developmental signals.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. J., Schwartz A., Grupp G., Grupp I., Lee S. W., Wallick E. T., Powell T., Twist V. W., Gathiram P. High-affinity ouabain binding site and low-dose positive inotropic effect in rat myocardium. Nature. 1982 Mar 11;296(5853):167–169. doi: 10.1038/296167a0. [DOI] [PubMed] [Google Scholar]
- Charlemagne D., Maixent J. M., Preteseille M., Lelievre L. G. Ouabain binding sites and (Na+,K+)-ATPase activity in rat cardiac hypertrophy. Expression of the neonatal forms. J Biol Chem. 1986 Jan 5;261(1):185–189. [PubMed] [Google Scholar]
- Erdmann E., Philipp G., Scholz H. Cardiac glycoside receptor, (Na+ + K+)-ATPase activity and force of contraction in rat heart. Biochem Pharmacol. 1980 Dec;29(24):3219–3229. doi: 10.1016/0006-2952(80)90295-6. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cells from adult human, dog, cat, rabbit, rat, and frog hearts and from fetal and new-born rat ventricles. Ann N Y Acad Sci. 1978 Apr 28;307:491–522. doi: 10.1111/j.1749-6632.1978.tb41979.x. [DOI] [PubMed] [Google Scholar]
- Hansen O. Interaction of cardiac glycosides with (Na+ + K+)-activated ATPase. A biochemical link to digitalis-induced inotropy. Pharmacol Rev. 1984 Sep;36(3):143–163. [PubMed] [Google Scholar]
- Herzig S., Mohr K. Action of ouabain on rat heart: comparison with its effect on guinea-pig heart. Br J Pharmacol. 1984 May;82(1):135–142. doi: 10.1111/j.1476-5381.1984.tb16450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inturrisi C. E., Papaconstantinou M. C. Ouabain sensitivity of the Na+, K+-ATPase from rat neonatal and human fetal and adult heart. Ann N Y Acad Sci. 1974;242(0):710–716. doi: 10.1111/j.1749-6632.1974.tb19132.x. [DOI] [PubMed] [Google Scholar]
- Kent R. B., Fallows D. A., Geissler E., Glaser T., Emanuel J. R., Lalley P. A., Levenson R., Housman D. E. Genes encoding alpha and beta subunits of Na,K-ATPase are located on three different chromosomes in the mouse. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5369–5373. doi: 10.1073/pnas.84.15.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer G. A., Brady A. J., Tan S. T., Serena D. Correlation of the glycoside response, the force staircase, and the action potential configuration in the neonatal rat heart. Circ Res. 1975 Jun;36(6):744–752. doi: 10.1161/01.res.36.6.744. [DOI] [PubMed] [Google Scholar]
- Lytton J. The catalytic subunits of the (Na+,K+)-ATPase alpha and alpha(+) isozymes are the products of different genes. Biochem Biophys Res Commun. 1985 Oct 30;132(2):764–769. doi: 10.1016/0006-291x(85)91198-2. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Brandl C. J., Korczak B., Green N. M. Amino-acid sequence of a Ca2+ + Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature. 1985 Aug 22;316(6030):696–700. doi: 10.1038/316696a0. [DOI] [PubMed] [Google Scholar]
- Maixent J. M., Charlemagne D., de la Chapelle B., Lelievre L. G. Two Na,K-ATPase isoenzymes in canine cardiac myocytes. Molecular basis of inotropic and toxic effects of digitalis. J Biol Chem. 1987 May 15;262(14):6842–6848. [PubMed] [Google Scholar]
- Matsuda T., Iwata H., Cooper J. R. Specific inactivation of alpha (+) molecular form of (Na+ + K+)-ATPase by pyrithiamin. J Biol Chem. 1984 Mar 25;259(6):3858–3863. [PubMed] [Google Scholar]
- McDonough A., Schmitt C. Comparison of subunits of cardiac, brain, and kidney Na+-K+-ATPase. Am J Physiol. 1985 Mar;248(3 Pt 1):C247–C251. doi: 10.1152/ajpcell.1985.248.3.C247. [DOI] [PubMed] [Google Scholar]
- Ng Y. C., Akera T. Two classes of ouabain binding sites in ferret heart and two forms of Na+-K+-ATPase. Am J Physiol. 1987 May;252(5 Pt 2):H1016–H1022. doi: 10.1152/ajpheart.1987.252.5.H1016. [DOI] [PubMed] [Google Scholar]
- Noel F., Godfraind T. Heterogeneity of ouabain specific binding sites and (Na+ + K+)-ATPase inhibition in microsomes from rat heart. Biochem Pharmacol. 1984 Jan 1;33(1):47–53. doi: 10.1016/0006-2952(84)90369-1. [DOI] [PubMed] [Google Scholar]
- Schwalb H., Dickstein Y., Heller M. Interactions of cardiac glycosides with cardiac cells. III. Alterations in the sensitivity of (Na+ +K+)-ATPase to inhibition by ouabain in rat hearts. Biochim Biophys Acta. 1982 Jul 28;689(2):241–248. doi: 10.1016/0005-2736(82)90256-5. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Greeb J., Lingrel J. B. Molecular cloning of three distinct forms of the Na+,K+-ATPase alpha-subunit from rat brain. Biochemistry. 1986 Dec 16;25(25):8125–8132. doi: 10.1021/bi00373a001. [DOI] [PubMed] [Google Scholar]
- Sweadner K. J. Enzymatic properties of separated isozymes of the Na,K-ATPase. Substrate affinities, kinetic cooperativity, and ion transport stoichiometry. J Biol Chem. 1985 Sep 25;260(21):11508–11513. [PubMed] [Google Scholar]
- Sweadner K. J., Gilkeson R. C. Two isozymes of the Na,K-ATPase have distinct antigenic determinants. J Biol Chem. 1985 Jul 25;260(15):9016–9022. [PubMed] [Google Scholar]
- Sweadner K. J. Two molecular forms of (Na+ + K+)-stimulated ATPase in brain. Separation, and difference in affinity for strophanthidin. J Biol Chem. 1979 Jul 10;254(13):6060–6067. [PubMed] [Google Scholar]
- Werdan K., Wagenknecht B., Zwissler B., Brown L., Krawietz W., Erdmann E. Cardiac glycoside receptors in cultured heart cells--II. Characterization of a high affinity and a low affinity binding site in heart muscle cells from neonatal rats. Biochem Pharmacol. 1984 Jun 15;33(12):1873–1886. doi: 10.1016/0006-2952(84)90542-2. [DOI] [PubMed] [Google Scholar]
- Young R. M., Lingrel J. B. Tissue distribution of mRNAs encoding the alpha isoforms and beta subunit of rat Na+,K+-ATPase. Biochem Biophys Res Commun. 1987 May 29;145(1):52–58. doi: 10.1016/0006-291x(87)91286-1. [DOI] [PubMed] [Google Scholar]