Summary
Background
Impaired glucose tolerance is common among obese adolescents, but the changes in insulin sensitivity and secretion that lead to this prediabetic state are unknown. We investigated whether altered partitioning of myocellular and abdominal fat relates to abnormalities in glucose homoeostasis in obese adolescents with prediabetes.
Methods
We studied 14 obese children with impaired glucose tolerance and 14 with normal glucose tolerance, of similar ages, sex distribution, and degree of obesity. Insulin sensitivity and secretion were assessed by the euglycaemichyperinsulinaemic clamp and the hyperglycaemic clamp. Intramyocellular lipid was assessed by proton nuclear magnetic resonance spectroscopy and abdominal fat distribution by magnetic resonance imaging.
Findings
Peripheral glucose disposal was significantly lower in individuals with impaired than in those with normal glucose tolerance (mean 35·4 [SE 4·0] vs 60·6 [7·2] μmoles per kg lean body mass per min; p=0·023) owing to a reduction in non-oxidative glucose disposal metabolism (storage). Individuals with impaired glucose tolerance had higher intramyocellular lipid content (3·04 [0·43] vs 1·99 [0·19]%, p=0·03), lower abdominal subcutaneous fat (460 [47] vs 626 [39] cm2, p=0·04), and slightly higher visceral fat than the controls (70 [11] vs 47 [6] cm2, p=0·065), resulting in a higher ratio of visceral to subcutaneous fat (0·15 [0·02] vs 0·07 [0·01], p=0·002). Intramyocellular and visceral lipid contents were inversely related to the glucose disposal and non-oxidative glucose metabolism and positively related to the 2 h plasma glucose concentration.
Interpretation
In obese children and adolescents with prediabetes, intramyocellular and intra-abdominal lipid accumulation is closely linked to the development of severe peripheral insulin resistance.
Introduction
The prevalence of type 2 diabetes has increased alarmingly among children worldwide during the past decade.1–3 Affected young people are typically grossly obese, have reached puberty, and have a family history of type 2 diabetes; in more developed countries, many belong to minority ethnic groups.1,2 The young age at presentation exposes these patients to a high risk of long-term complications.1
Progression from normal glucose tolerance to overt type 2 diabetes in adults involves an intermediate stage of impaired glucose tolerance, referred to as prediabetes.4 This state has been studied extensively in adults, but studies in children are rare. In a clinic population, we recently found a high frequency of impaired glucose tolerance among children and adolescents with obvious obesity.5 In that study, insulin resistance was the best predictor of the plasma glucose concentration at 2 h in an oral glucose tolerance test.5 The underlying mechanisms relevant to the putative changes in insulin sensitivity and secretion in the early stage of diabetes in youth have not been explored. Here, we used robust techniques, the euglycaemic-hyperinsulinaemic and hyperglycaemic clamps, to examine the roles of insulin resistance and β-cell function in obese young people with prediabetes.
In addition to an increase in visceral fat,6 the intramyocellular accumulation of lipid has lately emerged as a key modulator of insulin sensitivity in adults.7–10 Abnormalities in insulin signalling can arise as a result of overaccumulation of various lipids in skeletal muscle.11–14 In this study, we compared obese children and adolescents with normal or impaired glucose tolerance. To gain insights into the lipid composition of skeletal muscle, we used proton nuclear magnetic resonance (1H-NMR) spectroscopy to quantify non-invasively the intramyocellular and extramyocellular lipid content of the soleus muscle.15,16 Magnetic resonance imaging (MRI) was used to examine the contribution of the visceral and subcutaneous abdominal depots to changes in glucose metabolism.17 We hypothesised that altered myocellular and abdominal fat partitioning is present early in obese insulin-resistant children with prediabetes.
Methods
Participants
The study participants were 14 obese children and adolescents with impaired glucose tolerance and 14 with normal glucose tolerance, as assessed by a 2 h glucose tolerance test. They were recruited from a multiethnic cohort of obese children and adolescents drawn from the Pediatric Obesity Clinic at Yale-New Haven Hospital.5 To be eligible for this study, they had to be aged between 8 and 18 years, to be taking no medications that can alter glucose metabolism, and to be otherwise healthy. In all participants we did a complete physical examination and took a detailed medical history. All had a body-mass-index Z score larger than 2·00 (body-mass index >97·7th centile) for age and sex.18 Stage of development was assessed on the basis of breast development in girls and genital development in boys according to Tanner criteria. The study was approved by the Human Investigational Committee of the Yale School of Medicine. Written informed consent was obtained from the parents, and written assent was given by the participants.
Metabolic studies
All participants were instructed by a registered dietitian to follow a weight maintenance diet consisting of at least 250 g carbohydrate per day for 7 days before the study and to refrain from physical activity. The children arrived at the Yale Children's Clinical Research Center at 0730 h after an overnight fast of 10–12 h. Two intravenous catheters—one for blood sampling and one for infusion of glucose, insulin, and tracers—were inserted, one in the antecubital vein of each arm after local infiltration with lidocaine. The arm used for blood sampling was kept in a heated box for arterialisation of blood. The clamp studies were done in random order with an interval of at least 1 month.
Whole-body insulin sensitivity was measured by a two-step euglycaemic clamp19 by infusing insulin as a primed continuous infusion at 8 mU m-2 min-1 and 80 mU m–2 min–1. Each step lasted 2 h. A primed-continuous infusion of 6,6-deuterium-labelled glucose at a rate of 11·11 μmoles m–2 min–1 and a continuous infusion of 2H5-glycerol at a rate of 0·21 μmoles m–2 min–1, were used to quantify insulin's effects on glucose and glycerol turnover. To maintain the plasma enrichment of 2H-glucose constant at baseline value throughout the clamp, we used the Hot GINF method.20 Arterialised blood samples were collected every 5–10 min during the last 30 min of the baseline period and during each insulin infusion period for measurement of glucose and glycerol enrichments, hormones, and substrates. Indirect calorimetry was used at baseline and during the last 30 min of each step of the clamp to estimate net rates of carbohydrate and lipid oxidation.21 Non-oxidative glucose metabolism was calculated by subtracting the amount of glucose oxidised from the whole-body glucose uptake.
To quantify insulin secretion, the hyperglycaemic clamp was used. Blood glucose concentration was rapidly raised to 11·1 mmol/L by infusion of 20% dextrose at variable rates and kept at that value for 120 min.19
Localised 1H-NMR spectra of the soleus muscle were acquired on a 2·1 T Biospec system (Bruker Instruments, Inc, Billerica, MA, USA).17 One participant with impaired glucose tolerance had metal implants (bone fixation devices) so could not undergo 1H-NMR spectroscopy; all other participants did. The clinical status of the participants was concealed from the investigator who collected and analysed the data. All participants were instructed not to undertake any physical activity for 7 days before the test. Although we have not yet studied the reproducibility of intramyocellular and extramyo-cellular lipid measurements in our obese youngsters, we have found a coefficient of variation of 5% for intramyocellular lipid and 10% for extramyocellular lipid in young non-obese adults.
MRI was used to quantify visceral and abdominal subcutaneous fat depots.22 This procedure was done in 13 of the 14 participants with normal glucose tolerance (eight girls—four white, three African-American, one hispanic; and five boys—one white, two African-American, two hispanic) and in ten of the 14 participants with impaired glucose tolerance (five girls—three white, two African-American; and five boys—two white, one African-American, two hispanic). Total body composition was measured by dual-energy X-ray absorptiometry with a Hologic scanner (Boston, MA, USA). The clinical status of the participants was concealed from the investigator who collected and analysed these data.
Analytical procedures and calculations
Plasma and urine glucose concentrations were measured by the glucose oxidase method with a glucose analyser (Beckman Instruments, Brea, CA, USA). Plasma insulin, C-peptide, leptin, and adiponectin concentrations were measured by double-antibody radioimmunoassays. Plasma fatty acids were assayed by a colorimetric method. Analysis of enrichments of 2H-glucose and 2H5-glycerol in plasma and infusates by gas chromatography and mass spectrometry was done as described elsewhere.23,24
The glucose infusion rates were calculated during the last 30 min of the low and high insulin clamp and expressed as moles glucose per kg of lean body mass per min. Endogenous hepatic glucose production and glycerol turnover at baseline and during the two steps of the insulin clamp, along with the clamped glucose disposal rates, were calculated as previously reported.23,24
In the basal state, we calculated an index of hepatic insulin resistance as the product of endogenous glucose production and the fasting insulin concentrations.25 The rate of glucose metabolism and the insulin sensitivity during the hyperglycaemic clamp procedure were calculated as previously reported.19
During the hyperglycaemic clamp, first-phase (2–10 min) and late-phase (10–120 min) insulin responses were calculated as the mean hormone concentration during the respective times. Insulin secretion was estimated by application of a minimum model of glucose-induced insulin secretion to the glucose and C-peptide curves of each individual.26,27 The disposition index was calculated as the product of insulin sensitivity and the first-phase insulin secretion.27
Statistical analysis
To test group differences (impaired vs normal glucose tolerance) in metabolic and fat deposition we used t tests for independent samples. Variables that were not normally distributed (fasting insulin, phase-1 and phase-2 insulin secretion, disposition index, fasting C-peptide, intramyocellular lipid content, and visceral-tosubcutaneous fat ratio) were log-transformed for analysis, or non-parametric statistics were calculated (adiponectin and leptin). However, for clarity of interpretation, results are expressed as untransformed values. For the main hypotheses (insulin sensitivity, intramyocellular, visceral, and subcutaneous fat), both unadjusted and Holm-adjusted p values are presented to correct for multiple testing. Plasma glucose and insulin concentrations during the euglycaemic clamp were compared by two-factor (group and time) repeated-measures ANOVA. Spearman rank correlations were used to assess associations of muscle lipid deposition and visceral fat with insulin resistance and 2 h plasma glucose concentrations. All statistical analyses were done with SAS (version 8.2).
In our previously published study of a large cohort of children and adolescents with normal or impaired glucose tolerance,5 we observed a large standardised difference (d=1·25) in insulin resistance as measured by the crude HOMA index. To observe similar differences in insulin sensitivity by the euglycaemic clamp, group sizes of 14 provide 80% power at α=0·013. We expected greater power owing to the robust measurement of insulin sensitivity provided by the euglycaemic clamp technique. Similarly, this sample size provides 80% power to detect absolute group differences of 1·0 in intramyocellular lipid content, an effect size two-thirds of that we reported between lean and obese adolescents.17 Finally, with group sizes of 13 and 10, this study had 80% power to detect only large standardised effects (d=1·50) that translate into group differences of 150 cm2 and 30 cm2 in subcutaneous and visceral fat, respectively, based on variability reported from our laboratory.17
Role of the funding source
The funding sources had no involvement in the study design, data collection, data analysis, data interpretation, writing of the paper, or the decision to submit the paper for publication.
Results
In each group, there were four obese prepubertal children and ten obese adolescents. Each group had the same number of girls and boys, and the ethnic composition was similar (table 1). Age, pubertal stage of development, body-mass-index Z score, and percentage body fat were similar in the two groups. Within each group, there were no differences between girls and boys in percentage body fat, body fat distribution, or intramyocellular lipid content; therefore, all data are presented with boys and girls grouped together.
Table 1.
Demographic and anthropometric characteristics of participants
| |
Normal glucose tolerance (n=14) |
Impaired glucose tolerance (n=14) |
|---|---|---|
| Demographic | ||
| Age (years) | 13·5 (2·1) | 13·1 (3·4) |
| Male/female* |
6/8 |
6/8 |
| Ethnic origin* | ||
| White | 4 | 5 |
| African-American | 5 | 3 |
| Hispanic |
5 |
6 |
| Anthropometric | ||
| Height (cm) | 163·1 (8·1) | 160·1 (13·3) |
| Weight (kg) | 104·6 (16·9) | 96·6 (25·4) |
| Body-mass index (kg/m2) | 39·3 (5·5) | 37·0 (5·8) |
| Body-mass-index Z score | 2·54 (0·24) | 2·48 (0·29) |
| Body surface area (m2) | 2·04 (0·19) | 1·97 (0·33) |
| % body fat | 43·3 (4·4) | 41·8 (6·3) |
| Lean body mass (kg) | 55·9 (9·4) | 53·2 (15·2) |
Data mean (SD) or *number of participants.
Fasting glucose concentrations and haemoglobin A1C concentrations were similar in the two groups, but fasting insulin and C-peptide concentrations were significantly higher in the group with impaired glucose tolerance than in the group with normal glucose tolerance (table 2). Fasting leptin concentrations were similar in the two groups, reflecting their equivalent amounts of adiposity. By contrast, plasma adiponectin concentrations were significantly lower in the group with impaired glucose tolerance (p=0·045).
Table 2.
Mean (SE) biochemical variables of participants
| Variable |
Normal glucose tolerance |
Impaired glucose tolerance |
Difference (95% CI) |
p |
|---|---|---|---|---|
| Fasting glucose (mmol/L) | 5·05 (0·08) | 5·07 (0·17) | –0·02 (–0·52 to 0·29) | 0·57 |
| 2 h glucose (mmol/L) | 6·14 (0·25) | 8·98 (0·25) | –2·84 (–3·52 to –2·10) | <0·0001 |
| Fasting insulin (mU/L) | 32·0 (3·0) | 49·2 (6·0) | –17·2 (–31 to –3) | 0·02 |
| Fasting C-peptide (pmol/L) | 982 (90) | 1179 (90) | –197 (–506 to 57) | 0·11 |
| Leptin (μg/L) | 32·3 (4·0) | 24·6 (2·1) | 7·7 (–1·8 to 17·2) | 0·10 |
| Adiponectin (mg/L) | 8·51 (0·99) | 5·41 (0·78) | 3·10 (0·41 to 5·18) | 0·045 |
| Haemoglobin A1c (%) | 5·10 (0·08) | 5·25 (0·10) | 0·15 (–0·39 to 0·25) | 0·65 |
2 h values are from the oral glucose tolerance test; other variables were measured under fasting conditions.
Baseline plasma concentrations of fatty acids and glycerol, glycerol turnover (table 3), and hepatic glucose production rates (figure 1) did not differ between the groups. The index of hepatic insulin resistance, however, was significantly higher in the group with impaired glucose tolerance than in the group with normal glucose tolerance (142 [SE 20] vs 92 [9]; difference 50 [95% CI 7·5–92·5]; p=0·002), suggesting some degree of basal hepatic insulin resistance in the prediabetic state.
Table 3.
Baseline plasma fatty acids, glycerol, glycerol turnover, and lipid oxidation rates during low-dose and high-dose insulin clamp
| |
Normal glucose tolerance |
Impaired glucose tolerance |
Difference (95% CI) |
|---|---|---|---|
| Plasma fatty acids (μmol/L) | |||
| Baseline | 584 (29) | 654 (27) | –70 (–157 to 17) |
| Low-dose insulin | 138 (19) | 158 (12) | –20 (–74 to 33) |
| High-dose insulin |
33 (4) |
58 (7) |
–25 (–42 to –8·6)* |
| Plasma glycerol (μmol/L) | |||
| Baseline | 120 (8) | 110 (9) | 10 (–16 to 35) |
| Low-dose insulin | 79 (8) | 77 (7) | 2 (–23 to 27) |
| High-dose insulin |
64 (10) |
53 (5) |
11 (–16 to 38) |
| Glycerol turnover (μmoles per kg lean body mass per min) | |||
| Baseline | 8·7 (0·8) | 10·2 (1·2) | –1·5 (–4·4 to 1·4) |
| Low-dose insulin | 5·3 (0·4) | 6·6 (0·9) | –1·3 (–3·0 to 0·4) |
| High-dose insulin |
4·4 (0·4) |
4·9 (0·6) |
–0·5 (–2·0 to 1·0) |
| Lipid oxidation rate (μmoles per kg lean body mass per min) | |||
| Baseline | 2·29 (0·2) | 2·73 (0·3) | –0·44 (–1·20 to 0·35) |
| Low-dose insulin | 2·12 (0·1) | 2·5 (0·3) | –0·38 (–1·00 to 0·27) |
| High-dose insulin | 1·24 (0·2) | 1·63 (0·4) | –0·39 (–1·15 to 0·35) |
Data are mean (SE).
p=0·009.
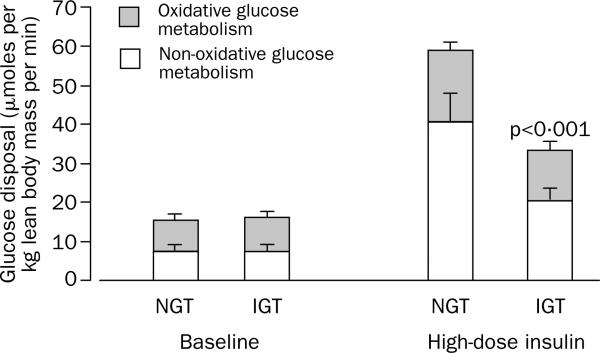
Figure 1.
Total body glucose disposal during the basal state and euglycaemic insulin clamp (80 mU m-2 min-1)
NGT=normal glucose tolerance; IGT=impaired glucose tolerance.
During the two steps of the clamp, plasma glucose concentrations were maintained at baseline values, and similar steady-state plasma concentrations of insulin were achieved in the groups with impaired or normal glucose tolerance during the last 60 min of each step (first step 58 [5] vs 54 [9] mU/L; second step 250 [20] vs 230 [18] mU/L).
Hepatic glucose production was totally suppressed during both steps of the clamp in all participants. Peripheral glucose disposal changed little during the low-dose insulin infusion in both groups. By contrast, the high-dose insulin infusion stimulated peripheral glucose disposal in both groups. However, the magnitude of insulin-stimulated glucose metabolism was lower in participants with impaired glucose tolerance than in those whose glucose tolerance was normal (35·4 [4] vs 60·6 [7·2] μmoles per kg lean body mass per min; difference 25·2 [5·2–45·5]; p<0·001, adjusted p=0·023; figure 1). This defect was mainly accounted for by a reduction in non-oxidative glucose disposal (storage), since rates of glucose oxidation did not differ significantly between the groups (figure 1). The ability of insulin to suppress systemic lipolysis during both clamp steps was of similar magnitude in the two groups (table 3). Lipid oxidation rates were slightly though not significantly higher in the group with impaired glucose tolerance; the limited sample size of this study may have prevented detection of a significant difference.
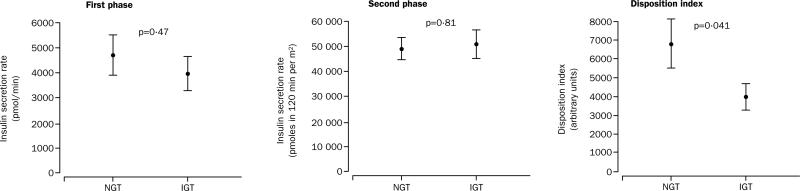
Plasma insulin concentrations and insulin secretion rates during both the phases of the hyperglycaemic clamp were similar in the two groups. However, the disposition index was significantly lower in impaired than in normal glucose tolerance (figure 2). Moreover, the insulin sensitivity index was significantly lower in the group with impaired glucose tolerance (table 4).
Figure 2.
Insulin secretion rates and disposition index during hyperglycaemic clamp
Table 4.
Insulin concentrations, glucose infusion rates, and insulin sensitivity during the hyperglycaemic clamp
| Variable |
Normal glucose tolerance |
Impaired glucose tolerance |
Difference (95% CI) |
p |
|---|---|---|---|---|
| First-phase insulin (mU/L) | 140 (18) | 137 (18) | 3 (–48 to 56) | 0·87 |
| Second-phase insulin (mU/L) | 242 (38) | 231 (33) | 11 (–91 to 115) | 0·82 |
| Glucose infusion rate (mmoles per kg lean body mass per min) | 0·07 (0·006) | 0·05 (0·004) | 0·02 (–0·002 to 0·033) | 0·08 |
| Insulin sensitivity | 0·059 (0·007) | 0·042 (0·006) | 0·017 (0·005 to 0·040) | 0·04 |
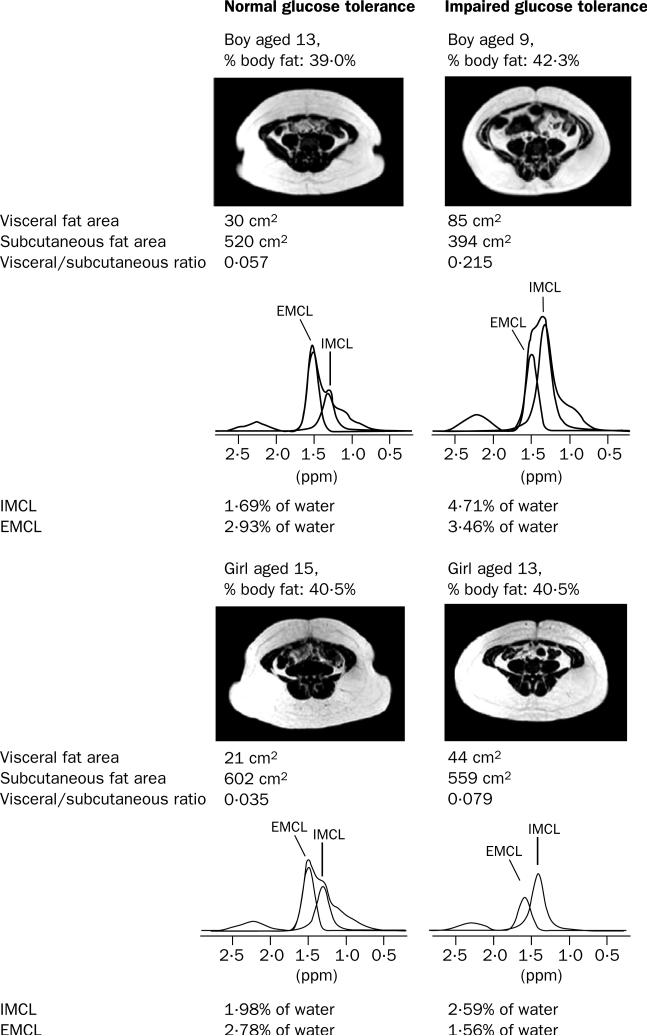
The intramyocellular lipid content of the soleus muscle was higher in the participants with impaired glucose tolerance than in those with normal glucose tolerance (3·04 [0·43] vs 1·99 [0·19]%; difference 1·05 [0·11–1·98]; p=0·018, adjusted p=0·03). No significant differences were observed between the groups in extramyocellular muscle lipid content. Representative spectra from one boy and girl with normal glucose tolerance and one boy and girl with impaired glucose tolerance are shown in figure 3.
Figure 3.
Transverse abdominal MRI scans and 1H-NMR soleus muscle spectra
L4 vertebral level 1, fat appears white with T1 weighting.
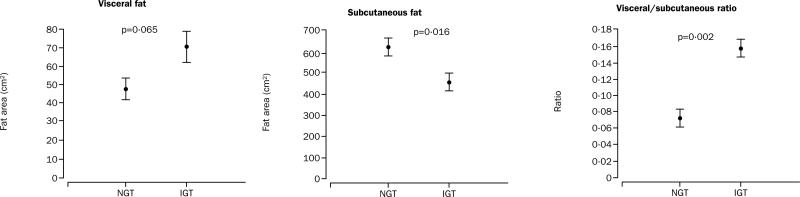
Participants with impaired glucose tolerance had significantly lower abdominal subcutaneous fat areas than those with normal glucose tolerance (460 [47] vs 626 [39] cm2; difference 166 [35–297] cm2; p=0·016, adjusted p=0·04; figure 4). Their visceral fat areas were slightly higher than those in the normal glucose tolerance group (70 [11] vs 47 [6] cm2; difference 23 [–1·57 to 47] cm2; adjusted p=0·065). The ratio of visceral to subcutaneous fat was higher in the participants with impaired glucose tolerance (0·15 [0·02] vs 0·07 [0·01]; difference 0·08 [0·03–0·12]; p=0·002). Representative MRIs are shown in figure 3.
Figure 4.
Differences in visceral and subcutaneous abdominal fat and the visceral-to-subcutaneous ratio
Scales differ for visceral and subcutaneous fat.
Intramyocellular and visceral fat content were inversely correlated with the glucose disposal (r=–0·51, p=0·01 and r=–0·63, p=0·0048, respectively) and with non-oxidative glucose metabolism in the two groups together (r=–0·45, p=0·035 and r=–0·48, p=0·043, respectively). Intramyocellular lipid and visceral-to-subcutaneous fat ratio were positively related to the 2 h plasma glucose during the oral glucose tolerance test in the two groups together (r=0·38, p=0·05 and r=0·57, p=0·007 respectively).
Discussion
Our study offers a novel insight into the pathogenesis of prediabetes in obese children and adolescents—namely, that changes in glucose homoeostasis are closely linked with altered partitioning of fat in both skeletal muscle and adipose tissues. We found that obese children and adolescents with impaired glucose tolerance had: profound peripheral insulin resistance with major defects in the non-oxidative pathway of glucose metabolism; no compensatory increases in insulin secretion; low adiponectin concentrations; and similar magnitude of suppression of total body lipid oxidation, plasma fatty acids, and glycerol turnover. Most importantly, early in the development of type 2 diabetes in obese young people, increased intramyocellular lipid accumulation, along with an increased visceral fat mass, are related to insulin resistance. These differences are unlikely to be due to differences in percentage body fat, age, sex, or pubertal stage of development, because the two groups had similar distributions of these variables and we adjusted for them in the analysis.
Obese children and adolescents with prediabetes are a useful group for studying the initial pathophysiological changes relevant to the alterations in glucose metabolism, because they are free from the confounding effects of ageing on insulin sensitivity and secretion and reflect the earliest stage of prediabetes. Our study clearly showed that obese young people with impaired glucose tolerance show pronounced defects in the nonoxidative pathway of glucose metabolism. This metabolic defect is similar to that observed in adults with overt type 2 diabetes.28
Our study showed also that intramyocellular lipid accumulation is associated with insulin resistance in children with prediabetes, thus further supporting the view that increased lipid content in myocytes is a marker of impaired insulin action.10 Indeed, abnormalities in insulin signalling have been found to arise as a result of overaccumulation of various lipid moieties in myocytes, such as long-chain fatty acyl-CoA, which interferes directly with insulin signalling and glucose transport.11–14 Consistent with these findings is the inverse relation between intramyocellular lipid content and non-oxidative glucose disposal we found. Further evidence of cause and effect between intramyocellular lipid and insulin resistance came from a study by Greco and colleagues,29 which showed that selective depletion of intramyocellular fat stores restored normal insulin sensitivity in obese adults, despite a persistent excess of total body fat mass. In our study, sex and ethnicity did not significantly affect the intramyocellular lipid content. No differences in extramyocellular lipid between our groups were found, perhaps because the 1H-NMR technique is limited in its ability to quantify extramyocellular fat.30
As a possible mediator of triglyceride accumulation, the adipocyte-derived hormone adiponectin has emerged as an important player in the genesis of insulin resistance.31 The low adiponectin concentrations found in the obese children with impaired glucose tolerance imply a role of adiponectin in the genesis of insulin resistance in such individuals.
In this study, baseline plasma fatty acid concentrations did not differ significantly between the groups. This finding should, however, be interpreted in light of the hyperinsulinaemia in the group with impaired glucose tolerance, which suggests that baseline lipolysis may be resistant to the suppressive effects of insulin. However, both fatty acid concentrations and glycerol turnover during the low-dose and high-dose insulin infusions were similar in the two groups, which argues against a reduced antilipolytic effect of insulin in prediabetes. Similarly, the suppression of lipid oxidation rates was of similar magnitude in both groups. These findings suggest that insulin resistance is mainly confined to muscle tissue and that defective suppression of lipolysis may not contribute to the increased intramyocellular lipid content in these young people. The baseline hepatic glucose production rates were similar in the two groups, suggesting that hepatic insulin resistance did not have a major role in early prediabetes in our participants.
Visceral fat accumulation is known to be associated with features of the insulin resistance syndrome in adults and obese children,6 although the nature of this association and the relative importance of visceral and subcutaneous abdominal fat remains a matter of debate. An intriguing suggestion in this study was that altered distribution of fat between the abdominal subcutaneous and visceral compartments is associated with the development of impaired glucose tolerance. The participants with impaired glucose tolerance had more visceral fat and less abdominal subcutaneous fat than those whose glucose tolerance was normal. Therefore, the visceral-to-subcutaneous ratio was significantly greater in those with impaired glucose tolerance. Both the enlarged visceral depot and the visceral-tosubcutaneous ratio were inversely related to the insulin-stimulated glucose metabolism after adjustment for overall adiposity. Although the two groups participating in the MRI study had similar percentages of body fat, there were some differences—albeit small—in the sex and ethnic distribution. Therefore, these differences in abdominal fat partitioning between the two groups require further investigation. Owing to the small sample size, we were unable to detect any significant sex or ethnic differences in visceral adipose tissue and intramyocellular lipid content within or between groups. Adequately powered studies are warranted to address these important issues.
First-phase and second-phase insulin secretion rates were similar in both groups. These data should be viewed cautiously, because they are absolute measurements. When we estimated insulin secretion in the context of the “resistant milieu” of the participants with impaired glucose tolerance, the secretion of insulin was not able to compensate for the increased resistance, resulting in a pronounced decrease in insulin-stimulated glucose metabolism. This feature can be viewed as a “relative” β-cell failure due to the inability of these participants to overcome the extraordinary insulin resistance.
The results of this study shed new light on the findings of Weyer and colleagues,32 who studied progression to diabetes in adult Pima Indians. As in their findings, a reduction in insulin-stimulated glucose disposal, mainly in the nonoxidative pathway, characterised the progressors to diabetes. In that study, progressors gained more weight, but the tissue localisation of lipid deposition was not directly assessed. Our results emphasise that lipid deposition in intramyocellular and visceral compartments—and not necessarily increased weight per se—are related to the reduction in insulin sensitivity. In contrast to Weyer and colleagues’ findings, we did not detect an absolute reduction in the acute insulin response in patients with impaired glucose tolerance, although the cross-sectional design of our study limits the comparison. This discrepancy may imply that β-cell failure is a late development in adolescents. The differences in the populations studied may explain other differences in their metabolic profiles.
In summary, early in the natural history of type 2 diabetes in obese young people, altered partitioning of fat in both skeletal muscle and abdominal adipose tissues is closely linked to insulin resistance. Increased intramyocellular and intra-abdominal fat accumulation is strongly related to post-glucose hyperglycaemia in obese prediabetic young people.
Acknowledgments
We thank all participants in the study, Aida Groszmann and Andrea Belous for technical assistance in measuring all hormones, and Yanna Kossover for measuring all stable isotopes. This study was supported by grants R01 HD 40787 and R01 HD 28016 to SC, R01 DK-49230 to GIS, M01 RR-00125 and MO1 RR-06022 from the National Institutes of Health, MURST 60% Grant from the University of Verona to RCB, and the Stephen I Morse Pediatric Diabetes Research Fund to RW. SC is a recipient of a K24 HD 01464 award for patient-oriented research.
GLOSSARY
- ADIPONECTIN
The protein is expressed exclusively in adipocytes, reduced concentrations of which are found in obese human beings.
- DISPOSITION INDEX
The product of insulin sensitivity and acute insulin response, reflecting the β-cell function in the context of whole-body insulin sensitivity.
- IMPAIRED GLUCOSE TOLERANCE
A transition phase between normal glucose tolerance and diabetes, also referred to as prediabetes.
- INTRAMYOCELLULAR LIPID
Lipid deposited within the cytoplasm of the myocyte.
- PERIPHERAL INSULIN RESISTANCE
Failure of target tissues to increase whole-body glucose disposal in response to insulin.
Footnotes
Conflict of interest statement
None declared.
References
- 1.Rosenbloom AL, Joe JR, Young RS, Winter WE. Emerging epidemic of type 2 diabetes in youth. Diabetes Care. 1999;22:345–54. doi: 10.2337/diacare.22.2.345. [DOI] [PubMed] [Google Scholar]
- 2.Dabelea D, Pettitt DJ, Jones KL, Arslanian SA. Type 2 diabetes mellitus in minority children and adolescents: an emerging problem. Endocrinol Metab Clin North Am. 1999;28:709–29. doi: 10.1016/s0889-8529(05)70098-0. [DOI] [PubMed] [Google Scholar]
- 3.Kitagawa T, Owada M, Urakami T, Yamauchi K. Increased incidence of non-insulin dependent diabetes mellitus among Japanese schoolchildren correlates with an increased intake of animal protein and fat. Clin Pediatr. 1998;37:111–15. doi: 10.1177/000992289803700208. [DOI] [PubMed] [Google Scholar]
- 4.Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes. 1997;46:701–10. doi: 10.2337/diab.46.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinha R, Fisch G, Teague B, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med. 2002;346:802–10. doi: 10.1056/NEJMoa012578. [DOI] [PubMed] [Google Scholar]
- 6.Montague CT, O'Rahilly S. The perils of portliness: causes and consequences of visceral adiposity. Diabetes. 2000;49:883–88. doi: 10.2337/diabetes.49.6.883. [DOI] [PubMed] [Google Scholar]
- 7.McGarry DJ. Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- 8.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–83. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 9.Perseghin G, Ghosh S, Gerow K, Shulman GI. Metabolic defects in lean nondiabetic offspring of NIDDM parents: a cross-sectional study. Diabetes. 1997;46:1001–09. doi: 10.2337/diab.46.6.1001. [DOI] [PubMed] [Google Scholar]
- 10.Krssak M, Falk Petersen K, Dresner A, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H-NMR spectroscopy study. Diabetologia. 1999;42:113–16. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 11.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–11. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 12.Dresner A, Laurent D, Marcucci M, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253–59. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffin ME, Marcucci MJ, Cline GW, et al. Free fatty acid induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–74. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 14.Yu C, Chen Y, Cline GW, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–36. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 15.Schick F, Eismann B, Jung WI, Bongers H, Bunse M, Lutz O. Comparison of localized proton NMR signals of skeletal muscle and fat tissue in vivo: two lipid compartments in muscle tissue. Magn Reson Med. 1993;29:158–67. doi: 10.1002/mrm.1910290203. [DOI] [PubMed] [Google Scholar]
- 16.Szczepaniak LS, Babcock EE, Schick F, et al. Measurement of intracellular triglyceride stores by 1H spectroscopy: validation in vivo. Am J Physiol. 1999;276:E977–89. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 17.Sinha R, Dufour S, Petersen KF, et al. Assessment of skeletal muscle triglyceride content by 1H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes. 2002;51:1022–27. doi: 10.2337/diabetes.51.4.1022. [DOI] [PubMed] [Google Scholar]
- 18.Kuczmarski RJ, Ogden CL, Guo SS, et al. CDC growth charts for the United States: methods and development. Vital Health Stat. 2000;2002;246:1–190. [PubMed] [Google Scholar]
- 19.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 20.Finegood DT, Bergman RN, Vranic M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps: comparison of unlabeled and labeled exogenous glucose infusates. Diabetes. 1987;36:914–24. doi: 10.2337/diab.36.8.914. [DOI] [PubMed] [Google Scholar]
- 21.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–34. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 22.Caprio S, Hyman LD, Limb C, et al. Central adiposity and its metabolic correlates in obese adolescent girls. Am J Physiol. 1995;269:E118–26. doi: 10.1152/ajpendo.1995.269.1.E118. [DOI] [PubMed] [Google Scholar]
- 23.Mayerson AB, Hundal RS, Dufour S, et al. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 2002;51:797–802. doi: 10.2337/diabetes.51.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson C, Tamborlane WV, Maggs DG, et al. Effect of insulin on glycerol production in obese adolescents. Am J Physiol. 1998;274:E737–43. doi: 10.1152/ajpendo.1998.274.4.E737. [DOI] [PubMed] [Google Scholar]
- 25.DeFronzo RA, Ferrannini E, Simonson DC. Fasting hyperglycemia in non-insulin-dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism. 1989;38:387–95. doi: 10.1016/0026-0495(89)90129-7. [DOI] [PubMed] [Google Scholar]
- 26.Toffolo G, DeGrandi F, Cobelli C. Estimation of beta-cell sensitivity from intravenous glucose tolerance test C-peptide data: knowledge of the kinetics avoids errors in modeling the secretion. Diabetes. 1995;44:845–54. doi: 10.2337/diab.44.7.845. [DOI] [PubMed] [Google Scholar]
- 27.Bonadonna RC, Stumvoll M, Fritsche A, et al. Altered homeostatic adaptation of first- and second-phase beta-cell secretion in the offspring of patients with type 2 diabetes: studies with a minimal model to assess beta-cell function. Diabetes. 2003;52:470–80. doi: 10.2337/diabetes.52.2.470. [DOI] [PubMed] [Google Scholar]
- 28.Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990;322:223–28. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- 29.Greco A, Mingione G, Giancaterini A, et al. Insulin resistance in morbid obesity: reversal with intramyocellular fat depletion. Diabetes. 2002;51:144–51. doi: 10.2337/diabetes.51.1.144. [DOI] [PubMed] [Google Scholar]
- 30.Boesch C, Kreis R. Observation of intramyocellular lipids by 1H-magnetic resonance spectroscopy. Ann NY Acad Sci. 2000;904:25–31. [PubMed] [Google Scholar]
- 31.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 32.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–94. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]