Abstract
Supercompensated muscle glycogen can be achieved by using several carbohydrate (CHO)-loading protocols. This study compared the effectiveness of two “modified” CHO-loading protocols. Additionally, we determined the effect of light cycle training on muscle glycogen. Subjects completed a depletion (D, n = 15) or nondepletion (ND, n = 10) CHO-loading protocol. After a 2-day adaptation period in a metabolic ward, the D group performed a 120-min cycle exercise at 65% peak oxygen uptake (V̇O2 peak) followed by 1-min sprints at 120% V̇O2 peak to exhaustion. The ND group performed only 20-min cycle exercise at 65% V̇O2 peak. For the next 6 days, both groups ate the same high-CHO diets and performed 20-min daily cycle exercise at 65% V̇O2 peak followed by a CHO beverage (105 g of CHO). Muscle glycogen concentrations of the vastus lateralis were measured daily with 13C magnetic resonance spectroscopy. On the morning of day 5, muscle glycogen concentrations had increased 1.45 (D) and 1.24 (ND) times baseline (P < 0.001) but did not differ significantly between groups. However, on day 7, muscle glycogen of the D group was significantly greater (p < 0.01) than that of the ND group (130 ± 7 vs. 104 ± 5 mmol/l). Daily cycle exercise decreased muscle glycogen by 10 ± 2 (D) and 14 ± 5 mmol/l (ND), but muscle glycogen was equal to or greater than preexercise values 24 h later. In conclusion, a CHO-loading protocol that begins with a glycogen-depleting exercise results in significantly greater muscle glycogen that persists longer than a CHO-loading protocol using only an exercise taper. Daily exercise at 65% V̇O2 peak for 20 min can be performed throughout the CHO-loading protocol without negatively affecting muscle glycogen supercompensation.
Keywords: carbohydrate loading, detraining, 13C magnetic resonance spectroscopy
many endurance athletes practice muscle glycogen supercompensation or carbohydrate (CHO) loading, even though the factors affecting glycogen kinetics and the underlying mechanisms are not completely understood. A comprehensive CHO-loading protocol that achieves and maintains peak muscle glycogen until the event occurs without attenuating the metabolic adaptations acquired from endurance training is a challenge. Endurance athletes typically practice CHO loading to increase preexercise muscle glycogen concentrations, and this practice has been found to correlate positively with performance during submaximal exercise events lasting >90 min (2, 20). The early classical protocol included a depletion phase (i.e., an exhaustive exercise bout coupled with 3 days on a low-CHO diet) followed by a 3-day loading phase [i.e., no exercise and a high (>500 g/day)-CHO diet] (2, 20, 33).
Modified protocols (31, 34, 35) with various combinations of diet and exercise have been tested in an attempt to avoid the negative side effects (e.g., irritability, weakness) associated with the more severe classical protocol. Sherman et al. (35) and Roedde et al. (31) compared the classical protocol with modified protocols that included a taper in training volume, with conflicting results. Sherman et al. found no significant difference between the two protocols in achieving supercompensated muscle glycogen and time to perform a 20.9-km run. In contrast, Roedde et al. reported significantly greater muscle glycogen in subjects who followed the classical protocol. The relatively small number of subjects and different exercise modes (e.g., running vs. cycling) of these two studies may explain the conflicting findings.
Bergström and Hultman (3) used a one-leg exercise model to compare depletion with nondepletion and reported that exhaustive exercise activates a “local factor” in the exercised muscle that stimulates glucose uptake and glycogen supercompensation. Saltin and Hermansen (33) later reviewed the available research on glycogen storage and emphasized the importance of muscle glycogen depletion in achieving supercompensation.
The study of human muscle glycogen metabolism has typically relied on small sample sizes and use of the needle biopsy technique. Generally, the number of repetitive biopsies is limited to three to five per subject. Even this low number of biopsies, if taken from an area of muscle ≤7 cm2, can negatively affect glycogen synthesis (7). Other limitations of many CHO-loading studies include a lack of rigorous control of diet and/or exercise throughout the study and measurement of muscle glycogen for only 3–4 days of CHO loading, even when a plateau has not been reached.
Since the validation of 13C magnetic resonance spectroscopy (MRS) (36), it has become possible to make frequent, noninvasive measures of muscle glycogen without traumatizing the muscle. It is now possible to profile the time course of muscle glycogen kinetics with good precision (±5 mmol/l muscle) and determine the relative effects of important variables of CHO-loading protocols (e.g., diet and exercise).
When CHO loading, competitive athletes generally prefer moderate training to rest, out of a concern that detraining may occur and offset the potential benefits of supercompensated muscle glycogen. Their concerns are supported by studies reporting that even 3–6 days of rest can significantly reduce several metabolic adaptations acquired through endurance training (25, 26). On the other hand, CHO-loading protocols that include a high-intensity depletion exercise (90–180 min) and/or daily long (45- to 60-min) training sessions can prevent muscle glycogen super-compensation (10, 34).
The present study was designed to determine 1) the relative importance of an exhaustive depletion exercise bout and a high-CHO diet on the time course of muscle glycogen supercompensation and 2) the effect of daily light training sessions [20 min at 65% peak oxygen uptake (V̇O2 peak)] on muscle glycogen concentrations during and after CHO loading.
METHODS
Subjects
Twenty-five male volunteers from US Navy and Marine Corps Special Operations commands gave written informed consent to participate in this study. Subjects were engaged in 60–120 min of daily training that included aerobic training (running, swimming, cycling), calisthenics, and resistance exercises (weight lifting). This study was approved by the Human Investigation Committee of Yale University School of Medicine (New Haven, CT) and the Committee for the Protection of Human Subjects at the Naval Health Research Center (San Diego, CA). Partial data from this study were previously presented in a study examining the effects of caffeine on the neuroendocrine axis and muscle glycogen utilization during a 2-h cycle exercise bout (24).
Physical Characteristics
Anthropometric and V̇O2 peak measurements were made 1–2 wk before the study. Percent body fat was estimated from seven-site skinfold thickness (18). V̇O2 peak was determined by use of a multistage exercise test on a mechanically braked cycle ergometer (model 818-E, Monark). The test began with exercise at 60 W, which increased in a stepwise fashion by 60 W every 4 min until volitional fatigue or failure to maintain 60 rpm (19). Respiratory gases were measured over 15-s intervals with open-circuit spirometry (Horizon 4400, SensorMedics). Heart rate was monitored (Polar Vantage XL) during the final 15 s of each 4-min stage. Subjects were admitted to the Yale/New Haven Hospital General Clinical Research Center (GCRC), where they underwent medical screening that included a physical exam, medical history evaluation, and routine blood tests.
Experimental Design
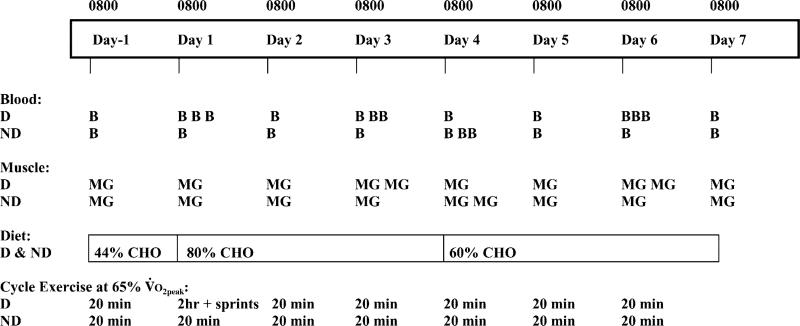
Subjects completed a depletion (n = 15) or nondepletion (n = 10) modified CHO-loading protocol (Fig. 1). Subjects were assigned to treatment groups matched for age, V̇O2 peak, body weight, and body fat. The study began with a 2-day adaptation period during which subjects stayed in the GCRC and consumed a normal mixed diet. On the morning after the adaptation period, groups performed one of two exercise protocols. For the remainder of the study, both groups performed 20 min of daily cycle exercise and consumed the same diets. Under fasting conditions at the same time every day (0800–1200), muscle glycogen concentrations in the vastus lateralis were measured using 13C MRS. Glycogen measurements also were made before and after daily 20-min cycle exercise bouts (twice for the depletion group and once for the nondepletion group) during the loading and maintenance phases.
Fig. 1.
Experimental design with time line for diet, exercise, muscle, and blood samples. D, depletion group; ND, nondepletion group; B, blood sampling; MG, muscle glycogen; CHO, carbohydrate; V̇O2peak peak oxygen uptake.
Urine output, food and water intake, and body weights were measured daily. Fasting venous blood samples were taken from all subjects at 8:00 AM on days 1–6 and on the morning of day 7 (end of study). Blood samples also were collected from the depletion group before exercise, at 120 min, and after exhaustion in the depletion exercise bout. Samples were analyzed for selected hormones, metabolites, and standard blood chemistry.
Exercise Protocols
On the first day of the loading protocol, the depletion group performed a depletion exercise by cycling at 70 rpm for 120 min at 65% V̇O2 peak, followed by repeated 1-min sprints at 90 rpm and 120% V̇O2 peak, separated by 1-min rest periods (13). Exercise ended with volitional fatigue or failure to maintain a pedal rate of ≥90 rpm. The nondepletion group performed 20 min of cycle exercise at 65% V̇O2 peak on the 1st day. On all other days, both groups performed 20 min of cycle exercise at 65% V̇O2 peak at 70 rpm.
Dietary Protocols
All meals were planned by a registered dietician and prepared in the metabolic kitchen of the GCRC. Diets for both treatment groups were designed to contain 195 kJ (46.65 kcal)·kg body wt−1·day−1; however, slight adjustments were made to maintain body weight over the course of the study. Subjects were encouraged to eat all of the food provided. Food and beverage consumption was carefully monitored, and any uneaten food was documented. During the 2-day adaptation period, subjects consumed a normal mixed diet (44% CHO, 38% fat, and 18% protein). During the 3-day loading phase (days 1–3), subjects consumed a high-CHO diet (~9 g CHO·kg−1·day−1, ~675–745 g CHO/day) composed of 80% CHO, 10% fat, and 10% protein. This diet included three bottles of a glucose-polymer beverage containing 105 g CHO/532 ml bottle (CarboForce, American Body-Building, Walterboro, SC). During the 3-day maintenance phase (days 4–6), subjects consumed a moderate-CHO diet (~6.5 g CHO·kg−1·day−1, or ~480–530 g CHO/day) composed of 56% CHO, 26% fat, and 18% protein, and including two bottles of CarboForce. Each day of the loading and maintenance phases, one bottle of CarboForce (~1.4 g CHO/kg body wt) was consumed within 30 min after exercise.
MRS
Natural abundance 13C MRS was performed daily to determine noninvasively the time course of muscle glycogen depletion and repletion during the CHO-loading and -post-loading periods. Baseline muscle glycogen concentration values were determined after the 2-day adaptation period in the GCRC. Muscle glycogen concentration was also measured before and after the exhausting (2-h) and moderate (20-min) cycle exercise bouts. Measurements were performed in a 2.1-T Bruker Biospec spectrometer with a 1-m-diameter magnet bore, as previously described (28). A mark was made on the subject's quadriceps muscle to facilitate repeated measures of muscle glycogen from the same site.
During the measurements, subjects remained supine, with the observation radio frequency (RF) probe resting above the quadriceps muscle. The probe consisted of a 9-cm-diameter inner coil for 13C acquisition and a 13-cm outer butterfly coil for 1H acquisition, image-guided positioning, and decoupling. Proton water line widths were shimmed to <50 Hz. The probe was positioned by an image-guided localization routine that used a T1-weighted gradient-echo image so that the observation volume was typically ~1 cm3 into the vastus lateralis muscle. The reference standard consisted of a microsphere containing [13C]formic acid (99% 13C enriched) fixed at the center of the double-tuned RF coil. Calibration of RF pulse widths was performed by determining the 180° flip angle at the center of the observation coil from the micro-sphere standard. Then the RF pulse width was set so that the 90° pulse was sent to the center of the muscle to obtain maximum suppression of the lipid signal from the subcutaneous fat layer and optimized signal from the muscle. 13C spectra were obtained with a 1H-decoupled pulse-acquired sequence in 10-min blocks consisting of 5,500 scans with a 90° pulse at coil center and a repetition time (TR) of 120 ms. Decoupling at the 1H frequency at a power of 15 W was applied at the C1 proton resonance frequency during the 25.6-ms acquisition period with a power deposition <4 W/kg (29).
Intramuscular glycogen concentrations were determined by comparison with signal from an external standard solution (150 mmol/l glycogen in 50 mmol/l KCl). The KCl in the glycogen standard allows for the phantom to have properties similar to a human leg. 13C spectra were processed by methods that have previously been described (28). Briefly, Gaussian broadened spectra (30 Hz) were baseline corrected by ±300 Hz on either side of the [1-13C]glycogen resonance of both subject spectra and standard spectra. Peak areas were then assessed at ±150 Hz of the resonance. Greater precision in measuring the exercise-induced change in muscle glycogen concentration was gained by using differential spectral analysis. The 13C MRS glycogen signal was corrected for the sensitive volume of the 13C coil. An image of the glycogen phantom solution was acquired using a dedicated proton coil of the same size as the 13C coil and a fully relaxed gradient-echo sequence with a 90° excitation pulse. The sensitive volume of that image was manually drawn and compared with the set of images previously recorded from each individual by means of the butterfly coil. The corresponding filling coefficient was calculated according to the ratio of the regions of interest defined from the leg muscles and the in vitro data set (1.18 in average). In addition, the 13C MRS signal was corrected for the load of the 13C coil. A fully relaxed spectrum (4 pulses, TR = 15 s, pulse length 100 μs) of the formic acid sphere was recorded both in vivo and in the presence of the phantom solution of glycogen. The ratio of the formate peak area obtained in both conditions was used as the loading correction factor (0.91 in average). The detection threshold for minimum change is ~5 mmol/l muscle, which is 7% of the baseline concentration. This method has a reported coefficient of variation of 4.3% in resting subjects and is lower than the one typically reported for the biopsy technique (36). The 13C MRS technique for assessing intramuscular glycogen concentrations has been validated in situ in frozen rabbit muscle (12) and by comparison with human gastrocnemius muscle biopsies (36).
Blood Analyses
Plasma glucose was measured by the glucose oxidase method (Beckman glucose analyzer, Fullerton, CA). Plasma immunoreactive insulin, glucagon, cortisol, and human growth hormone (hGH) concentrations were measured using commercially available double-antibody radioimmunoassay kits (insulin: Diagnostic System Laboratories, Webster, TX; glucagon: Linco Research, St. Charles, MO; cortisol: Diagnostic Products, Los Angeles, CA; hGH: Sanofi Diagnostics Pasteur, Chaska, MN). Plasma triglycerides were determined fluorometrically at 340 nm with a quantitative enzymatic assay kit (Sigma Diagnostics, St. Louis, MO). Plasma lactate concentrations were measured by the lactate dehydrogenase method (15). Plasma fatty acids were measured using a microfluorimetric assay (27). Plasma catecholamine (epinephrine and norepinephrine) concentrations were determined using a three-step procedure involving adsorption onto alumina (pH = 8.6), elution with a dilute acid, and analysis by high-pressure liquid chromatography.
Statistical Analyses
Blood metabolites, hormones, and muscle glycogen concentrations are presented as means ± SE in the text, Figs. 1–3, and Tables 1–4, unless noted otherwise. Statistical comparisons were made using a two-way repeated-measures analysis of variance (ANOVA). For within-subject comparisons, paired t-tests were used. When significant interactions were observed, unpaired t-tests were performed for time-point comparisons. When both multiple paired and unpaired t-tests were used, the alpha level was adjusted using the Bonferroni procedure. Significant differences between values and groups were accepted at P < 0.05.
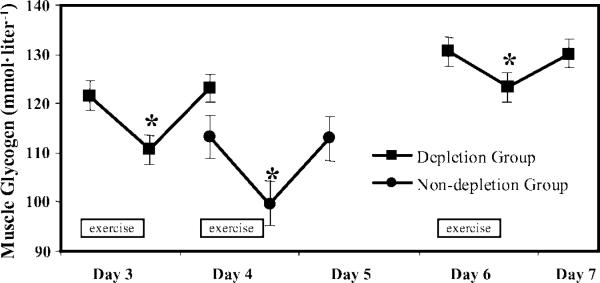
Fig. 3.
Muscle glycogen concentrations in response to 20 min of cycle exercise at 65% V̇O2peak during carbohydrate loading for depletion group (n = 15) and nondepletion group (n = 10). *P < 0.05 vs. preexercise levels.
Table 1.
Plasma metabolite concentrations in response to glycogen depletion exercise bout
| Glycogen, mmol/l | Glucose, mg/dl | Lactate, mM | FFA, μM | Triglycerides, mg/dl | |
|---|---|---|---|---|---|
| Baseline | 89 ± 4 | 89 ± 9 | 0.8 ± 0.2 | 237 ± 21 | 104 ± 9 |
| After 2 h of exercise | ND | 81 ± 3* | 1.8 ± 0.2† | 1,465 ± 157† | 125 ± 10* |
| After 5–21 sprints | 38 ± 6† | 91 ± 7 | 6.5 ± 0.6‡ | 1,295 ± 157† | 121 ± 9* |
Values are means ± SE of 15 subjects in the depletion carbohydrate-loading protocol. FFA, free fatty acids.
P < 0.05 vs. baseline
P < 0.01 vs. baseline
P < 0.01 vs. after sprints.
Table 4.
Plasma hormone concentrations in response to 20 min of cycle exercise at 65% V̇O2peak during CHO loading
| Insulin, μU/ml | Glucagon, pg/ml | Epinephrine, pg/ml | Norepinephrine, pg/ml | Cortisol, μg/dl | |
|---|---|---|---|---|---|
| D | |||||
| Fasted baseline | 10.2 ± 1.2 | 54.7 ± 4.6 | 27 ± 5.4 | 314 ± 48 | 19.9 ± 1.8 |
| Preexercise | 58.2 ± 12.1† | 59.0 ± 5.4 | 19 ± 2.4 | 316 ± 55 | 16.5 ± 1.0 |
| Postexercise | 14.3 ± 2.1‡ | 79.1 ± 7.2 | 60 ± 9.6‡ | 838 ± 140† | 16.1 ± 0.6* |
| ND | |||||
| Fasted baseline | 8.5 ± 0.6 | 49.4 ± 4.7 | 46 ± 3.5 | 287 ± 56 | 17.6 ± 2.5 |
| Preexercise | 70.6 ± 18.3† | 52.6 ± 4.6 | 43 ± 5.9 | 283 ± 42 | 15.5 ± 2.1 |
| Postexercise | 14.4 ± 2.7‡ | 78.2 ± 8.5 | 49 ± 6.5 | 687 ± 234‡ | 17.7 ± 2.3 |
Values are means ± SE of 15 D group and 10 ND group subjects.
P < 0.05 vs. baseline
P < 0.01 vs. baseline
P < 0.01 vs. preexercise.
RESULTS
Subject Characteristics
There were no significant differences between the depletion and nondepletion groups for any anthropomorphic or physiological characteristic. The depletion and nondepletion groups had mean ages (25.7 ± 1.1 and 27.1 ± 1.8 yr), heights (177.6 ± 1.6 and 175.3 ± 2.6 cm), weights (80.7 ± 2.0 and 75.1 ± 3.4 kg), body fat values (9.3 ± 0.7 and 9.2 ± 0.9%), and V̇O2 peak values (49.1 ± 1.4 and 47.4 ± 1.1 ml·kg−1·min−1), respectively.
Dietary Intake
There were no significant differences in the diets consumed by the two treatment groups in total kilocalories and absolute or percent intake of macronutrients (i.e., CHO, fat, and protein) during any phase of the study. Daily CHO intake for depletion and nondepletion groups averaged 9.2 and 9.0 g/kg total body mass (675–745 g CHO/day) during the loading phase and 6.6 and 6.4 g/kg (480–530 g CHO/day) during the maintenance phase, respectively. Daily caloric intake for both groups averaged 185 kJ/kg during the adaptation phase, 193 and 189 kJ/kg during the loading phase, and 197 and 191 kJ/kg during the maintenance phase for depletion and nondepletion groups, respectively. These daily intake values approximated the target value of 195 kJ/kg.
Muscle Glycogen Utilization
Effects of depletion exercise (2 h at 65% V̇O2 peak) and sprints
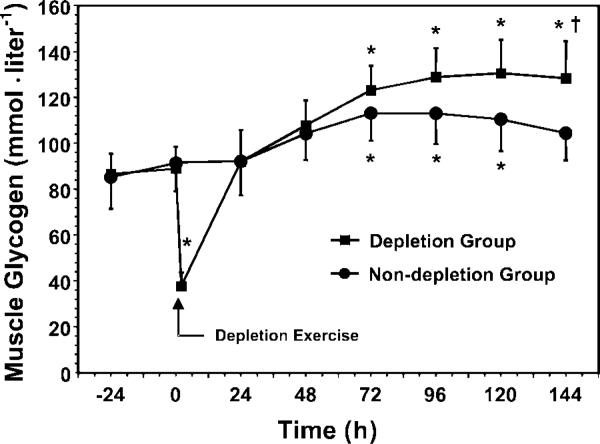
Baseline (preexercise) muscle glycogen concentrations for the depletion (89 ± 4 mmol/l) and the nondepletion (91 ± 5 mmol/) groups did not differ significantly (Fig. 2). After the depletion exercise, the muscle glycogen of the depletion group decreased to 38 ± 6 mmol/l (i.e., 40 ± 19% of the preexercise value).
Fig. 2.
Profile of muscle glycogen during depletion and nondepletion exercise regimens. Measurements were made daily between 8:00 AM and 12:00 noon for depletion group (n = 15) and nondepletion group (n = 10). *P < 0.01 vs. baseline; †P < 0.01 vs. nondepletion group.
Effects of moderate exercise (20 min at 65% V̇O2 peak)
The muscle glycogen used by the depletion group during the 20 min of cycle exercise on day 3 of CHO loading and on day 5 (2nd day of maintenance diet) averaged 11 ± 3 and 9 ± 3 mmol/l, respectively (Fig. 3). The muscle glycogen of the nondepletion group during the 20-min exercise bout on day 4 (1st day of maintenance diet) decreased by 14 ± 5 mmol/l. The mean decrease in muscle glycogen during the 20-min cycle exercise bout under CHO-loaded conditions did not differ significantly between the two groups and had a pooled average of 11 ± 2 mmol/l for the 20-min bout.
Muscle Glycogen Repletion during CHO Loading
High-CHO diet/loading phase (days 1–3)
During the first 24 h after the depletion exercise, muscle glycogen of the depletion group increased significantly from 40 to 92 mmol/l muscle (Fig. 2). In contrast, after 24 h on the same diet, muscle glycogen of the nondepletion group remained approximately the same (85–92 mmol/l muscle). After 3 days of loading, the muscle glycogen of the depletion and nondepletion groups had increased to levels that were significantly (P < 0.01) greater (138 and 124%, respectively) than those at baseline. We found no significant correlation between the relative or absolute level of muscle glycogen depletion of individuals and their initial rate of repletion or peak muscle glycogen value.
Moderate CHO diet/maintenance phase (days 4–6)
During this phase, muscle glycogen concentrations of the depletion group continued to increase (nonsignificantly) on days 4 and 5 and peaked on day 6 at 147% of baseline (Fig. 2). This level of supercompensation persisted until the final measurement on the morning of day 7. In contrast, muscle glycogen levels of the non-depletion group peaked on day 4 (at 124% of baseline), and then decreased back to baseline concentration on day 7. On day 7, the muscle glycogen of the nondepletion group (104 ± 5 mmol/l) was ~25% lower than that of the depletion group (130 ± 7 mmol/l; Fig. 2). This approximates the average amount of muscle glycogen (i.e., 26 mmol/l) used per hour during the 2-h depletion exercise performed by the depletion group.
Metabolite and Hormonal Data
Fasted and rested conditions
Throughout the study, resting plasma triglyceride, fatty acid, glucose, and lactate concentrations were within normal limits and did not differ between groups, with one minor exception (Table 1). On the morning after the depletion exercise, fatty acid values were within normal limits but significantly greater in the depletion (346 ± 41 μM) than in the nondepletion group (226 ± 40 μM). Throughout the study period, there were no significant differences between the depletion and nondepletion groups in fasting plasma insulin, glucagon, epinephrine, norepinephrine, and growth hormone concentrations (Table 2). Fasting plasma cortisol concentrations remained within normal limits at all times but were significantly greater in the depletion group than in the nondepletion group (24 ± 1 vs. 16 ± 3 μg/dl, P < 0.05) on the morning of day 7.
Table 2.
Plasma hormone concentrations in response to glycogen depletion exercise bout
| Time | Insulin, μU/ml | Glucagon, pg/ml | Epinephrine, pg/ml | Norepinephrine, pg/ml | Cortisol, μg/dl | hGH, μg/ml |
|---|---|---|---|---|---|---|
| Baseline | 9.2 ± 0.8 | 60 ± 5 | 21 ± 5 | 208 ± 29 | 22.0 ± 1.6 | 0.9 ± 0.1 |
| After 2 h of exercise | 5.3 ± 0.8** | 126 ± 17** | 217 ± 34** | 857 ± 120* | 32.0 ± 2.1* | 8.8 ± 1.0* |
| After 5–21 sprints | 4.5 ± 0.7** | 119 ± 16** | 336 ± 83** | 1,372 ± 159† | 39.0 ± 1.9* | 12.7 ± 1.4* |
Values are means ± SE of 15 subjects. hGH, human growth hormone.
P < 0.05 vs. baseline
P < 0.01 vs. baseline
‡P < 0.01 vs. after sprints.
Postexercise condition
depletion bout (2 h at 65% V̇O2 peak) AND SPRINTS. After the 2-h depletion exercise, plasma lactate, free fatty acids, epinephrine, norepinephrine, glucagon, cortisol, and growth hormone had increased significantly, whereas insulin was significantly reduced (Tables 1 and 2). Blood glucose concentrations also decreased from 89 ± 2 to 81 ± 3 mg/dl after 2 h of cycle exercise but returned to preexercise values (91 ± 7 mg/dl) immediately after the last sprint. After2hof cycle exercise, lactate levels increased from 0.9 ± 0.2 to 1.8 ± 0.2 mM, but after the last sprint (volitional exhaustion), they had increased (P < 0.01) to 6.5 ± 0.6 mM. Plasma norepinephrine and cortisol also increased significantly (P < 0.05) after the last sprint. Plasma glucose, free fatty acids, triglycerides, insulin, glucagon, growth hormone, and epinephrine were unchanged after the last sprint (Tables 1 and 2).
moderate exercise (20 min at 65% V̇O2 peak). During the adaptation period (normal mixed diet), 20 min of cycle exercise had no effect on plasma glucose and triglycerides except for plasma lactate concentrations, which increased in both groups to ~2.0 mM (Table 3). Additionally, the plasma fatty acids decreased significantly in the depletion and nondepletion groups from 272 ± 19 to 194 ± 19 μM and from 224 ± 25 to 155 ± 14 μM, respectively. The hormonal response to the cycle exercise during this period did not differ between groups: insulin decreased significantly; glucagon, epinephrine, and norepinephrine increased significantly; and plasma growth hormone and cortisol were unchanged (Table 4).
Table 3.
Plasma metabolite concentrations in response to 20 min of cycle exercise at 65% V̇O2peak during CHO loading
| Glucose, mg/dl | Lactate, mM | FFA, μM | Triglycerides, mg/dl | |
|---|---|---|---|---|
| D | ||||
| Fasted baseline | 84.4 ± 2.4 | 1.2 ± 0.1 | 261 ± 25 | 111 ± 15 |
| Preexercise | 86.8 ± 4.7 | 2.0 ± 0.2 | 152 ± 14* | 130 ± 14 |
| Postexercise | 71.4 ± 7.1 | 4.2 ± 0.6† | 168 ± 11* | 168 ± 18 |
| ND | ||||
| Fasted baseline | 89.4 ± 2.3 | 1.1 ± 0.1 | 235 ± 19 | 145 ± 24 |
| Preexercise | 101.4 ± 9.5 | 2.2 ± 0.2 | 174 ± 18* | 156 ± 25 |
| Postexercise | 81.6 ± 2.6 | 4.0 ± 0.5† | 152 ± 23* | 179 ± 26 |
Values are means ± SE of 15 subjects in depletion (D) protocol and 10 subjects in nondepletion (ND) protocol. V̇O2 peak, peak oxygen uptake; CHO, carbohydrate.
P < 0.05
P < 0.01 vs. preexercise.
The same exercise performed on day 3 of the high-CHO diet produced no change in the plasma glucose, triglycerides, or free fatty acids, but it increased plasma lactate significantly to ~4.0 ± 0.5 mM in both groups. Plasma lactate returned to preexercise values (0.7–2.4 mM) at 60 min postexercise for both groups under both dietary conditions. As expected, this relatively short exercise again produced no significant increase in plasma cortisol or growth hormone concentrations for either group. Plasma glucagon showed a nonsignificant increase in both groups. However, plasma epinephrine increased significantly only in the depletion group, and norepinephrine increased significantly in both groups (Table 4).
DISCUSSION
The classical CHO-loading protocol (3, 20, 35) used by endurance athletes in the 1960s and 1970s has been largely replaced by less demanding modified protocols (24, 31, 35). Modified protocols include a training taper and may or may not begin with a depletion bout of exercise. Using 13C MRS to follow the time course of muscle glycogen supercompensation, we demonstrated that moderately trained males achieve greater muscle glycogen concentration and maintain it longer when they follow a modified protocol that begins with a depletion exercise bout. We also found that 20 min of moderate cycle exercise can be performed daily while CHO loading without negatively affecting muscle glycogen supercompensation. Specifically, we found that, when exhaustive cycle exercise was performed and followed by a high-CHO diet (~9 g·kg−1·day−1, ~675–745 g CHO/day), muscle glycogen concentration returned to 103% of baseline within 24 h. Consuming this diet for two more days increased muscle glycogen to 138% of baseline. Muscle glycogen repletion slowed over the next 2 days, peaking at 147% of baseline and persisting until the end of study (day 7). In contrast, the group that performed only 20 min of cycle exercise (decreasing muscle glycogen to 90% of baseline) and ate the same high-CHO diet showed no change in muscle glycogen at 24 h. Muscle glycogen concentration of the nondepletion group peaked at 72 h (124% of baseline) and was not different from baseline on day 7. These results agree with earlier findings (2, 31, 38) that muscle glycogen depletion (i.e., exhaustive exercise) affects the initial rate of muscle glycogen synthesis and the level of repletion (4, 7).
Several studies have suggested that when muscle glycogen is severely depleted, glycogen resynthesis is markedly activated (6, 16, 29). Muscle biopsy studies (6, 16) have found that when glycogen concentration in the vastus lateralis is decreased to 66–70 mmol/kg wet wt, glycogen synthase activity increases rapidly (6, 16). More recent studies using MRS reported an increase in the resynthesis rate when muscle glycogen was decreased to 30–40 mmol/l, or 25% of baseline (29). These findings agree with the rapid glycogen synthesis observed in our depletion group, whose mean postexercise muscle glycogen was 38 ± 6 mmol/l.
Glycogen synthase activity is increased by conversion of its D form to the active I form (8, 22). Glycogen repletion after exercise is biphasic (21) and is controlled by the rates of glucose transport and disposal. During the early rapid phase (0–6 h postexercise), glucose transport across the muscle membrane is maximally stimulated and insulin independent (29). Originally attributed to a “local factor” present in the muscle after exercise (3), it is now known to result from the translocation of an intracellular pool of the GLUT4 isoform of glucose transporter proteins (9), possibly secondary to activation of AMP-activated protein kinase (1, 37). Ren et al. (30) reported a rapid increase in the number of GLUT4 glucose transport receptors in rats in response to prolonged exercise. Kua et al. (23) reported that this increase in GLUT4 protein is controlled by both pretranslational and posttranslational mechanisms. This may explain why the depletion group achieved and maintained significantly greater muscle glycogen than the nondepletion group. When provided sufficient glucose, muscle glycogen synthesis continues during the slow phase (6–72 h) so that pre-exercise levels of glycogen can be reached by 24 h. Muscle glycogen can exceed normal levels by 72 h if a high-CHO diet is consumed and exercise is limited. It is well established that, during the slow phase, muscle glycogen can reach 1.5–2.0 times resting levels (16, 33); however, only a few studies have previously monitored muscle glycogen content longer than 72 h of CHO loading (11, 24).
Our second finding has practical applications for competitive endurance athletes who may prefer exercise to rest while CHO loading. Previous research suggests there may be trade-offs associated with continuing training while attempting to achieve and maintain glycogen supercompensation. For example, because the rate of muscle glycogenolysis is most rapid during the early minutes of exercise, even 20 min of moderate-intensity (60–75% V̇O2 peak) exercise can significantly decrease muscle glycogen by 30–58% (1, 14). Rapid rates of glycogenolysis also occur with high exercise intensity and high initial muscle glycogen concentrations. In a study comparing two modified CHO-loading protocols (5), the muscle glycogen of well-trained endurance runners did not differ when they performed 40 min of “easy” daily running instead of resting while CHO loading. Because these researchers did not report the intensity of the exercise or the amount or timing of the postexercise CHO intake, it is difficult to directly compare our findings.
In our study, subjects used only 10–15% of their muscle glycogen during 20 min of daily cycle exercise at 65% V̇O2 peak. The rate of glycogen resynthesis is maximal during the first 1–2 h postexercise (29), and maximum resynthesis occurs when 1.5 g glucose/kg body wt is consumed during this period (17). By consuming a CHO supplement (1.4 g glucose/kg body wt) immediately after exercise, within 24 h our subjects had replaced all of the muscle glycogen used during daily exercise. This demonstrates that an athlete can exercise daily during CHO loading without negatively affecting muscle glycogen supercompensation.
Metabolic and hormonal responses of our subjects were typical for the exercise stress and dietary conditions. The 2-h depletion exercise significantly increased plasma levels of fatty acids and triglycerides consistent with increased lipolysis. Elevated plasma fatty acid levels at 2 h typically occur with endurance exercise combined with glycogen sparing and the absence of lactate accumulation (32). Plasma lactate levels <2 mM at 2 h indicate that subjects exercising at 65% V̇O2 peak were below their “lactate threshold.” However, the postsprint plasma lactate concentrations indicate a significant involvement of anaerobic glycolysis. During the first 2 h of cycle exercise, insulin decreased and glucagon increased significantly to maintain glucose availability and was unchanged after the sprints. The hormonal responses of our subjects (e.g., increased plasma epinephrine, norepinephrine, cortisol, and growth hormone) after cycling 2 h at 65% V̇O2 peak and after the series of intense sprints are consistent with changes typically seen after prolonged exhaustive exercise (32). There were no significant differences between treatment groups in insulin sensitivity as estimated by homeostasis model assessment-estimated insulin resistance at any point in the study.
CHO-loading studies generally do not measure muscle glycogen in every subject each day, nor do they study the maintenance of supercompensated muscle glycogen. We previously reported that supercompen-sated muscle glycogen can persist for ≥3 days after completion of a classical CHO-loading protocol, if subjects abstain from exercise (11). The current study is an extension of that work and profiled muscle glycogen during and after subjects completed two modified CHO-loading protocols, which included 20 min of daily cycle exercise.
The potential military application of CHO loading involves possible conditions that would not occur in sports. The most obvious is that missions can be delayed for a variety of reasons and the CHO-loaded personnel may be required to wait several days before deploying. Athletic endurance events, however, are postponed for only minutes, not days. Thus, unlike the sports athlete, military special operations personnel may need to maintain glycogen supercompensation and avoid physical detraining during the period of postponement. The findings of this study may, however, have application to the recreational athlete who could benefit by knowing the time profile of peak muscle glycogen and the effect of daily exercises on super-compensated muscle glycogen.
In conclusion, we have demonstrated that modified CHO-loading protocols that begin with an exhaustive depletion exercise achieve greater muscle glycogen concentrations that will persist longer than nondepletion protocols (e.g., training taper). These findings agree with earlier studies. However, equally important, we demonstrated that 20 min of moderate cycle exercise can be performed daily during all phases of CHO loading. These exercise bouts do not alter the profile muscle glycogen concentrations if a CHO supplement (~1.4 g CHO/kg) is consumed within 30 min after exercise. This suggests that endurance athletes can continue moderate daily exercise while CHO loading and still achieve and maintain muscle glycogen supercompensation.
We thank Carole Franklin, Donna Caseria, and the staff of the Yale/New Haven Hospital General Clinical Research Center for their assistance with this study.
DISCLOSURES
This work was supported by National Institutes of Health Grants R01 DK-49230, K-23 02734, R01 DK-063192, P30 DK-45735, and M01 RR-00125. Dr. G. I. Shulman is an investigator of the Howard Hughes Medical Institute. This work was also supported by the Office of Naval Research, Arlington, VA, under work unit no. 06062233N-M3P30.002–6807. The views and opinions expressed in this report are those of the authors and do not reflect the official policy or position of the Department of the Navy, the Department of Defense, or the US Government. This study has been approved for public release; distribution is unlimited.
REFERENCES
- 1.Bergeron R, Russell RR, Young LH, Ren JM, Marcucci M, Shulman GI. Effect of AMPK activation on muscle glucose metabolism in conscious rats. Am J Physiol Endocrinol Metab. 1999;276:E938–E944. doi: 10.1152/ajpendo.1999.276.5.E938. [DOI] [PubMed] [Google Scholar]
- 2.Bergström J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol Scand. 1967;71:140–150. doi: 10.1111/j.1748-1716.1967.tb03720.x. [DOI] [PubMed] [Google Scholar]
- 3.Bergström J, Hultman E. Muscle glycogen synthesis after exercise: an enhancing factor localized in the muscle cells of man. Nature. 1966;210:309–310. doi: 10.1038/210309a0. [DOI] [PubMed] [Google Scholar]
- 4.Bergström J, Hultman E, Roch-Norlund AE. Muscle glycogen synthase in normal subjects: basal values, effect of glycogen depletion by exercise and of a carbohydrate-rich diet following exercise. Scand J Clin Lab Invest. 1972;29:231–236. doi: 10.3109/00365517209081080. [DOI] [PubMed] [Google Scholar]
- 5.Blom PCS, Costill DL, Vollestad NK. Exhaustive running: inappropriate as a stimulus of glycogen supercompensation. Med Sci Sports Exerc. 1987;19:398–403. [PubMed] [Google Scholar]
- 6.Bogardus C, Thuillez P, Ravussin E, Vasquez B, Narimiga M, Azhar S. Effect of muscle glycogen depletion on in vivo insulin action in man. J Clin Invest. 1983;72:1605–1610. doi: 10.1172/JCI111119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costill DL, Pearson DR, Fink WL. Impaired muscle glycogen storage after muscle biopsy. J Appl Physiol. 1988;64:2245–2248. doi: 10.1152/jappl.1988.64.5.2245. [DOI] [PubMed] [Google Scholar]
- 8.Danforth WH. Glycogen synthetase activity in skeletal muscle. J Biol Chem. 1965;240:588–593. [PubMed] [Google Scholar]
- 9.Douen AG, Ramlal T, Rastogi S, Bulan P, Catree GD, Vranic M, Holloszy JO, Klip A. Exercise induces recruitment of the “insulin-responsive glucose transporter.”. J Biol Chem. 1990;265:13427–13430. [PubMed] [Google Scholar]
- 10.Fogelholm GM, Tikkanen HO, Naveri HK, Naveri LS, Harkonen MHA. Carbohydrate loading in practice: high muscle glycogen concentration is not certain. Br J Sports Med. 1991;25:41–44. doi: 10.1136/bjsm.25.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goforth HW, Jr, Arnall DA, Bennett BL, Law PG. Persistence of supercompensated muscle glycogen in trained subjects after carbohydrate loading. J Appl Physiol. 1997;82:342–347. doi: 10.1152/jappl.1997.82.1.342. [DOI] [PubMed] [Google Scholar]
- 12.Gruetter R, Prolla TA, Shulman RG. 13C NMR visibility of rabbit muscle glycogen in vivo. Magn Reson Med. 1991;20:327–332. doi: 10.1002/mrm.1910200216. [DOI] [PubMed] [Google Scholar]
- 13.Heigenhauser GJF, Sutton JR, Jones NL. Effect of glycogen depletion on the ventilatory response to exercise. J Appl Physiol. 1983;54:470–474. doi: 10.1152/jappl.1983.54.2.470. [DOI] [PubMed] [Google Scholar]
- 14.Hermansen L, Hultman E, Saltin B. Muscle glycogen during prolonged severe exercise. Acta Physiol Scand. 1967;71:129–139. doi: 10.1111/j.1748-1716.1967.tb03719.x. [DOI] [PubMed] [Google Scholar]
- 15.Hohorst HJ. l-(+)-Lactate determination with lactic dehydrogenase and DPN. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Verlag Chemie; Basel, Switzerland: 1965. pp. 266–270. [Google Scholar]
- 16.Hultman E, Bergström J, Roch-Norlund AE. Glycogen storage in human skeletal muscle. Adv Exp Med Biol. 1971;11:273–288. [Google Scholar]
- 17.Ivy JL, Lee MC, Brozinick JT, Jr, Reed MJ. Muscle glycogen storage after different amounts of carbohydrate ingestion. J Appl Physiol. 1988;65:2018–2023. doi: 10.1152/jappl.1988.65.5.2018. [DOI] [PubMed] [Google Scholar]
- 18.Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40:497–504. doi: 10.1079/bjn19780152. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs I, Kaiser P. Lactate in blood, mixed skeletal muscle, and FT or ST fibers during exercise in man. Acta Physiol Scand. 1982;14:461–466. doi: 10.1111/j.1748-1716.1982.tb07010.x. [DOI] [PubMed] [Google Scholar]
- 20.Karlsson JL, Saltin B. Diet, muscle glycogen, and endurance performance. J Appl Physiol. 1971;31:203–206. doi: 10.1152/jappl.1971.31.2.203. [DOI] [PubMed] [Google Scholar]
- 21.Kochan RG, Lamb DR, Lutz SA, Perrill CV, Reimann EM, Schlender KK. Glycogen synthetase activation in human skeletal muscle: effects of diet and exercise. Am J Physiol Endocrinol Metab Gastrointest Physiol. 1979;236:E660–E666. doi: 10.1152/ajpendo.1979.236.6.E660. [DOI] [PubMed] [Google Scholar]
- 22.Kochan RG, Lamb DR, Reimann EM, Schlender KK. Modified assays to detect activation of glycogen synthase following exercise. Am J Physiol Endocrinol Metab. 1981;240:E197–E202. doi: 10.1152/ajpendo.1981.240.2.E197. [DOI] [PubMed] [Google Scholar]
- 23.Kua CH, Browning KS, Ivy JL. Regulation of GLUT4 protein expression and glycogen storage after prolonged exercise. Acta Physiol Scand. 1999;165:193–201. doi: 10.1046/j.1365-201x.1999.00489.x. [DOI] [PubMed] [Google Scholar]
- 24.Laurent D, Schneider KE, Prusaczyk WK, Franklin C, Vogel SM, Krssak M, Petersen KF, Goforth HW, Shulman GI. Effects of caffeine on muscle glycogen utilization and the neuroendocrine axis during exercise. J Clin Endocrinol Metab. 2000;85:2170–2175. doi: 10.1210/jcem.85.6.6655. [DOI] [PubMed] [Google Scholar]
- 25.Martin WH, Coyle EF, Joyner M, Santeusanio D, Ehsani AA, Holloszy JO. Effects of stopping exercise training on epinephrine-induced lipolysis in humans. J Appl Physiol. 1984;56:845–848. doi: 10.1152/jappl.1984.56.4.845. [DOI] [PubMed] [Google Scholar]
- 26.Mikines KJ, Sonne B, Tronier B, Galbo H. Effects of acute exercise and detraining on insulin action in trained men. J Appl Physiol. 1989;66:704–711. doi: 10.1152/jappl.1989.66.2.704. [DOI] [PubMed] [Google Scholar]
- 27.Miles J, Glasscock R, Aikens J, Gerich J, Haymond M. A microfluorometric method for the determination of free fatty acids in plasma. J Lipid Res. 1983;24:96–99. [PubMed] [Google Scholar]
- 28.Perseghin G, Price TB, Petersen KF, Roden M, Cline G, Gerow K, Rothman DL, Shulman GI. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. New Eng J Med. 1996;335:1357–1362. doi: 10.1056/NEJM199610313351804. [DOI] [PubMed] [Google Scholar]
- 29.Price TB, Rothman DL, Taylor R, Avison MJ, Shulman GI, Shulman RG. Human muscle glycogen resynthesis after exercise: insulin-dependent and -independent phases. J Appl Physiol. 1994;76:104–111. doi: 10.1152/jappl.1994.76.1.104. [DOI] [PubMed] [Google Scholar]
- 30.Ren JM, Semenkovich CF, Gulve EA, Jao J, Holloszy JO. Exercise induces rapid increases in GLUT4 expression glucose transport capacity and insulin stimulated glycogen storage in muscle. J Biol Chem. 1994;269:14369–14401. [PubMed] [Google Scholar]
- 31.Roedde S, MacDougall JD, Sutton JR, Green HJ. Supercompensation of muscle glycogen in trained and untrained subjects. Can J Appl Spl Sci. 1986;11:42–46. [PubMed] [Google Scholar]
- 32.Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate in relation to exercise intensity and duration. Am J Physiol Endocrinol Metab. 1993;265:E380–E391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- 33.Saltin B, Hermansen L. Glycogen stores and prolonged severe exercise. In: Blix G, editor. Nutrition and Physical Exercise. Vol. 5. Almqvist and Wiksell; Uppsala, Sweden: 1967. pp. 32–46. [Google Scholar]
- 34.Sherman WM, Costill DL, Fink WJ, Hagerman FC, Armstrong LE, Murray TF. Effect of a 4.2.2-km footrace and subsequent rest or exercise on muscle glycogen and enzymes. J Appl Physiol. 1983;55:1219–1224. doi: 10.1152/jappl.1983.55.4.1219. [DOI] [PubMed] [Google Scholar]
- 35.Sherman WM, Costill DL, Fink WJ, Miller JM. Effect of exercise-diet manipulation on muscle glycogen and its subsequent utilization during performance. Int J Sports Med. 1981;2:114–118. doi: 10.1055/s-2008-1034594. [DOI] [PubMed] [Google Scholar]
- 36.Taylor R, Price TB, Rothman DL, Shulman RG, Shulman GI. Validation of 13C NMR measurement of human skeletal muscle glycogen by direct biochemical assay of needle biopsy samples. Magn Reson Med. 1992;27:13–20. doi: 10.1002/mrm.1910270103. [DOI] [PubMed] [Google Scholar]
- 37.Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol Endocrinol Metab. 1999;277:E1–E10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- 38.Zachwieja JJ, Costill DL, Pascoe DD, Robergs RA, Fink WJ. Influence of muscle glycogen depletion on the rate of resynthesis. Med Sci Sports Exerc. 1991;23:44–48. [PubMed] [Google Scholar]