Abstract
Type 2 diabetes mellitus is a major cause of morbidity and mortality worldwide, and the prevalence is set to increase dramatically over the coming decades. Understanding the metabolic pathways that lead to type 2 diabetes is therefore an important healthcare objective. Novel investigational techniques based on magnetic resonance spectroscopy (MRS) have allowed real-time insight into the molecular defects in patients with type 2 diabetes, revealing that insulin resistance is a product of decreased insulin-stimulated skeletal muscle glycogen synthesis, which can mostly be attributed to decreased insulin-stimulated glucose transport (Glut 4) activity. This defect appears to be a result of intracellular lipid-induced inhibition of insulin-stimulated insulin-receptor substrate (IRS)–1 tyrosine phosphorylation resulting in reduced IRS-1–associated phosphatidyl inositol 3 kinase activity. The hypothesis that insulin resistance is a result of accumulation of intracellular lipid metabolites (e.g., fatty acyl CoAs, diacylglycerol) in skeletal muscle and hepatocytes is supported by observations in patients and mouse models of lipodystrophy. Furthermore, the increase in hepatic insulin sensitivity observed in patients with type 2 diabetes following weight loss is also accompanied by a significant reduction in intrahepatic fat without any changes in circulating adipocytokines (interleukin-6, resistin, leptin). Finally, recent MRS studies in healthy, lean, elderly subjects and lean insulin-resistant offspring of parents with type 2 diabetes have demonstrated that reduced mitochondrial activity may also lead to increased intramyocellular lipid content and insulin resistance in skeletal muscle in these individuals. In summary, in vivo MRS has proved to be an important tool for elucidating the causal chain of events that causes insulin resistance. Understanding the cellular mechanism(s) of insulin resistance in turn offers the prospect of better targeted and more effective therapeutic interventions for treatment and prevention of type 2 diabetes.
Keywords: Fat, Insulin resistance, Insulin sensitivity, The metabolic syndrome, Obesity, Type 2 diabetes mellitus
The world faces a pandemic of type 2 diabetes mellitus, a fact that has attracted the attention not only of scientists and healthcare providers, but also of the popular media. In the United States, diabetes is already the leading cause of blindness among working-age adults,1 end-stage renal disease,2 and nontraumatic loss of limb3; it is also the fifth-leading cause of death.4 In the United States alone, the direct medical cost of diabetes amounts to US$92 billion annually, with indirect costs adding another US$40 billion.4 In Italy, the cost of type 2 diabetes was recently estimated at €5 billion, amounting to >6% of total private and public healthcare expenditure.5 Diabetes also causes a substantial economic burden in lower-income economies. For example, in Latin America and the Caribbean, the total annual cost associated with diabetes may be around US$65 billion.6
Once considered to be a disease of wealthy nations, type 2 diabetes now constitutes a truly global affliction. The International Diabetes Federation (IDF) anticipates that the worldwide incidence of diabetes among those aged 20 to 79 years will increase by around 70% in the next 20 years, from 194 million in 2003 to 333 million in 2025.7 The increase will affect all global regions, with projected increases ranging from 21% in Europe to 111% in Africa. Of particular concern is South East Asia, which will see an additional 40 million cases of diabetes by 2025.
Fortunately, research into the mechanisms that lead to type 2 diabetes is providing important new results. The connection between the epidemiologic risk factors (obesity, sedentary lifestyle) and insulin resistance has recently begun to yield to innovative investigative techniques. That is the subject of this review.
WHAT IS THE INITIAL DEFECT IN TYPE 2 DIABETES?
The presence of hyperglycemia implicates defects in several organs. In the pancreatic islets, impaired insulin secretion results from defects in the β-cells. In the liver, glucose production increases as a consequence of increased hepatic gluconeogenesis.8 However, before these events, and often anticipating them by decades, are pathologic alterations in the response of skeletal muscle to insulin. It is skeletal muscle, therefore, that has attracted the attention of our group to understand the initial stages of the disease.
In our laboratory, we have extensively used in vivo magnetic resonance spectroscopy (MRS) to investigate the cellular mechanisms of insulin resistance in humans.9 This technique is unique in that it can, by noninvasive means, measure the concentration of intracellular metabolites containing naturally occurring isotopes of 1H, 13C, and 31P in the human body. This is a major advance over the traditional muscle biopsy approach to assess the concentration of intracellular intermediates, which is subject to experimental artifact due to warm ischemia and the continuation of biochemical activity between the times of sample excision and freezing.10 Furthermore, because MRS is noninvasive, it involves no ionizing radiation, which makes it a very safe and powerful tool for human studies.
We used MRS to measure the rate of insulin-stimulated muscle glycogen synthesis in healthy individuals and patients with type 2 diabetes.11 The equivalent of a postprandial state was simulated by performing a hyperglycemic–hyperinsulinemic clamp to establish levels of plasma insulin at approximately 400 pmol and glucose at approximately 10 mmol/L. The rate of insulin-stimulated muscle glycogen synthesis, assessed by the incorporation of [13C]glucose into gastrocnemius/soleus muscle glycogen, was decreased by >50% in patients with type 2 diabetes compared with healthy age- and weight-matched individuals. Extrapolation of these data to the whole body indicates that almost all of the insulin resistance observed in the patients with type 2 diabetes could be attributed to defects in insulin-stimulated muscle glycogen synthesis.
The glycogen synthesis pathway comprises several steps (Figure 1), and disruption of any one of these could potentially explain the defects observed in type 2 diabetes.12 Briefly, uptake of glucose into the cell occurs via glucose transporter 4 (GLUT 4), whereupon it is phosphorylated by hexokinase to glucose-6-phosphate (G6P). After isomerization to G1P and activation to uridine 5′-diphosphate (UDP)-glucose, the final step is polymerization into glycogen by glycogen synthase.
Figure 1.
The pathway of muscle glycogen synthesis. GLUT 4 = glucose transporter 4; UDP = uridine 5′-diphosphate.
By measuring the accumulation of precursors at each step of the pathway in response to stimulation by insulin, the rate-controlling step in this pathway can be determined. For example, 31P MRS can noninvasively assess the intramyocellular concentrations of phosphorus-containing compounds such as G6P. In healthy volunteers we found a decrease in phosphocreatine, an increase in inorganic phosphate, and, most importantly, an increase in G6P during a hyperglycemic-hyperinsulinemic clamp.12 However, no such increase in intramyocellular G6P was observed in patients with type 2 diabetes, suggesting that defects in insulin-stimulated glucose transport and phosphorylation activity are responsible for the reduced insulin-stimulated muscle glycogen synthesis activity in these individuals.
In order to assess whether defects in insulin-stimulated glucose transport activity or hexokinase activity were responsible for the reduced insulin-stimulated muscle glycogen synthesis, we developed a novel 13C MRS method to assess intracellular glucose concentrations in humans.13 Under conditions similar to those of the previous studies, we found that the intracellular concentration of glucose was approximately 50-fold lower than the extracellular concentration, with no significant differences between healthy subjects and patients with type 2 diabetes.13 Importantly, the intracellular concentration of glucose in patients with type 2 diabetes (0.24 mmol/L) was approximately 25 times lower than would be expected if hexokinase were the primary rate-controlling enzyme in insulin-stimulated muscle glycogen synthesis.
Taken together, these MRS studies led us to the conclusion that the reduced insulin-stimulated muscle glycogen synthesis, which underlies insulin resistance in patients with type 2 diabetes, is attributable mostly to reduced insulin-stimulated glucose transport into skeletal muscle cells. The identification of the primary metabolic defect that occurs early in the disease process is important for 2 reasons. First, it provides a clear target for pharmaceutical intervention. Second, it provides a focus for studies that seek to understand the process that leads to insulin resistance, which in turn connects the pathophysiology with environmental and genetic risk factors.
WHY IS GLUCOSE TRANSPORTER 4 DEFECTIVE IN PATIENTS WITH TYPE 2 DIABETES?
To investigate the earliest stages of the disease process that lead to type 2 diabetes, it is necessary to study a population of individuals who are known to be at high risk for developing the disease in later life. Such a population comprises young, healthy lean offspring of parents with type 2 diabetes. Their suitability as study subjects derives from the fact that, compared with patients with type 2 diabetes, they are younger, lean, healthy, and unlikely to have other confounding factors that might contribute to insulin resistance. The lifetime risk for the development of type 2 diabetes in such individuals is approximately 40%.14-16 This, in turn, underscores the importance of inherited factors in etiology of the disease.17 The strongest independent predictor of future risk in these individuals is insulin resistance, so it is this insulin-resistant subset that provides the ideal candidates for studies examining the earliest defects leading to insulin resistance.
One metabolic parameter that is commonly abnormal in the offspring of parents with type 2 diabetes is the concentration of plasma fatty acids (FAs), which is frequently increased compared with controls.18 Insulin resistance in these individuals, determined by the euglycemic–hyperinsulinemic clamp, has been shown to be significantly correlated with plasma FAs, but not with other indicators of metabolic status.18
The correlation becomes even stronger when intramyocellular lipid is considered. A negative correlation between glycogen synthase activation by insulin and muscle triglyceride content has been demonstrated in muscle tissue obtained with punch biopsies.19 Subsequently, 1H MRS has been used to assess intramyocellular and extramyocellular lipid content.20 In normal-weight, nondiabetic adults, a negative correlation (r2) of 0.34 was found between insulin sensitivity and the intramyocellular lipid content of the soleus muscle.21 Lipid content was not, however, correlated with body mass index (BMI), age, or fasting plasma concentrations of triglycerides, glucose, or insulin. These findings have been confirmed in lean offspring of parents with type 2 diabetes, in whom a higher correlation with insulin resistance was found with intramyocellular triglyceride content (r2 = 0.29) than with plasma FAs (r2 = 0.21).22
Although the presence of high plasma FAs in patients with insulin resistance has been recognized for some decades,23 early attempts to place the connection on a mechanistic footing have more recently been shown likely to be incorrect. For example, it was proposed that FAs cause insulin resistance in muscle through inhibition of pyruvate dehydrogenase activity24 and glycogen synthase activity.25
However, recent 13C and 31P MRS studies, which have measured intracellular glucose and G6P concentrations in healthy volunteers who have had their plasma FA concentrations increased by an intralipid-heparin infusion, have demonstrated that fatty acids induce insulin resistance in skeletal muscle by directly inhibiting insulin activation of glucose transport activity.26,27
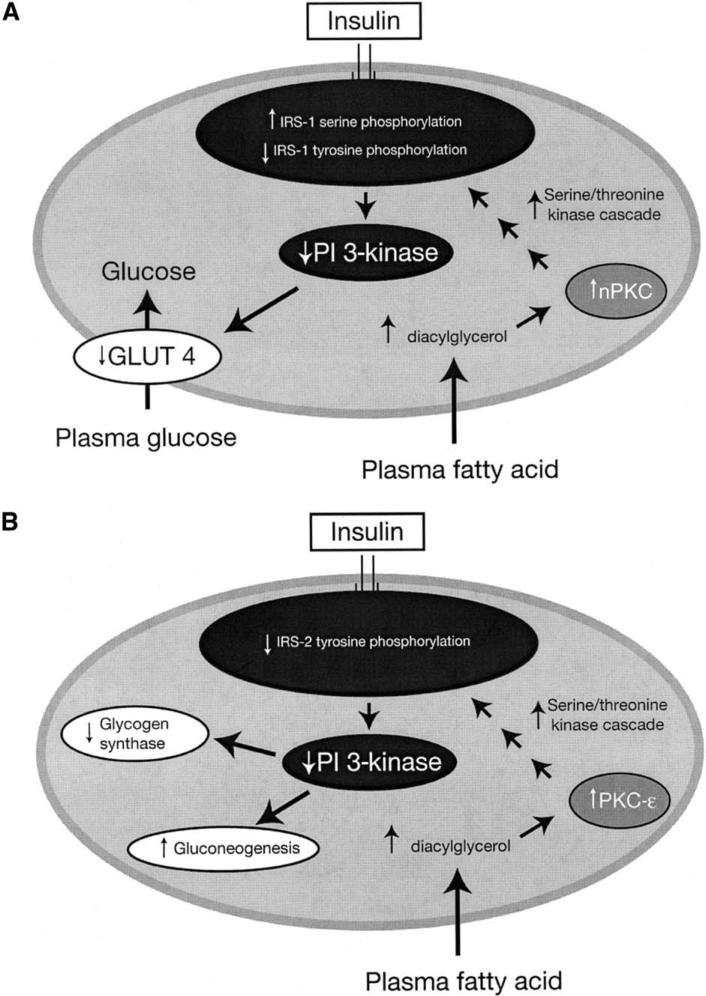
The mechanism of this effect has now been largely unraveled (Figure 2).28 A key player in insulin-stimulated glucose transport activity is phosphatidylinositol (PI) 3-kinase, which plays an essential role in insulin-induced glucose transport activity in skeletal muscle.29 In healthy individuals, insulin stimulation of GLUT 4 activity in skeletal muscle acts via increased insulin-stimulated phosphorylation of insulin receptor substrate (IRS)–1 that allows it to bind and activate PI 3-kinase activity. This, in turn, results in activation of GLUT 4 activity.27 Our group has shown that raising plasma FA levels in humans abolishes insulin activation of IRS-1–associated PI 3-kinase activity.27 Studies in rats have further shown that infusion of lipid metabolites activates protein kinase C (PKC)–θ, which, via a serine-threonine kinase cascade, blunts insulin-stimulated IRS-1 tyrosine phosphorylation. This, in turn, results in 50% reduction in insulin stimulation of PI 3-kinase activity.30 Furthermore, PKC-θ knockout mice are protected from lipid-induced insulin resistance in skeletal muscle.31
Figure 2.
The mechanism of fatty acid-induced insulin resistance in muscle (A) and liver (B). GLUT 4 = glucose transporter 4; IRS = insulin-receptor substrate; PI 3-kinase = phosphatidylinositol 3-kinase; nPKC = novel protein kinase C. (Adapted from J Clin Invest.28)
The finding that increased intracellular content of lipid metabolites can directly inhibit insulin-stimulated glucose transport activity has important ramifications for disease management, especially because a similar mechanism for fat-induced insulin resistance likely occurs in the liver, where accumulation of intracellular lipid metabolites activate a serine kinase cascade involving PKC-ε, leading to decreased tyrosine phosphorylation of IRS-2—a key mediator of insulin action in the liver.32,33 Recent studies in mitochondrial glycerol phosphate acyl-CoA transferase (GPAT) knockout mice strongly suggest that diacylglycerol, a known activator of PKC-ε, is the trigger in this process.34
One of the most important implications of these findings is that it is not obesity per se that is the driver of insulin resistance. Rather, it is the accumulation of intracellular lipid metabolites (e.g., diacylglycerol) that triggers the insulin resistance. Evidence for this hypothesis is provided by examining patients with congenital generalized lipodystrophy.
LIPODYSTROPHY AND INSULIN RESISTANCE
Congenital generalized lipodystrophy is a very rare, devastating disease, affecting approximately 1 in 10 million people. Patients have a paucity of fat, severe insulin resistance, hypertriglyceridemia, fatty infiltration of the liver and other tissues, and a deficiency of adipocyte hormones (e.g., leptin).35 A transgenic, fatless mouse model of severe lipodystrophy has been developed by constitutive expression of the A-ZIP/F-1 protein, which inhibits the DNA binding and function of specific B-ZIP transcription factors.36 The result is a mouse almost totally devoid of white adipose tissue and with dramatically reduced amounts of brown adipose tissue. These mice also have severe liver and muscle insulin resistance, and develop hyperglycemia. The intracellular fatty acyl-CoA content in both muscle and liver is approximately 2-fold higher than in wild-type mice, and they also have defects in the insulin activation of IRS-1 and IRS-2 associated PI 3-kinase activity in muscle and liver, respectively.37
Remarkably, the insulin resistance and other metabolic abnormalities could be reversed by transplanting adipose tissue from wild-type mice subcutaneously into the lipodystrophic mice.37 Most notably, fat transplantation significantly reduced liver and muscle lipid content, which probably explains why muscle glucose uptake was increased and the ability of insulin to suppress hepatic glucose production was restored.
Furthermore, insulin resistance seen in another mouse model of lipodystrophy, along with many other metabolic abnormalities, was reversed by chronic low-dose leptin treatment.38 To determine if the effects of leptin in lipodystrophic mice studies would translate to patients with lipodystrophy, we examined the effects of leptin-replacement therapy in patients with severe lipodystrophy.39 Recombinant human leptin was administered subcutaneously to the lipodystrophic patients at 12-hour intervals for 3 to 5 months, which resulted in normalization of their plasma leptin levels.39 The patients in this study all had severe, generalized lipodystrophy, hyperglycemia, increased glycosylated hemoglobin reflecting poor glycemic control despite treatment with multiple oral hypoglycemic therapies, severe fasting hyperinsulinemia (approximately 26 μU/mL), hepatic steatosis, and very low baseline concentrations of leptin (around 0.6 ng/mL). Severe muscle and liver insulin resistance was demonstrated by the euglycemic–hyperglycemic clamp. Leptin therapy resulted in a near normalization of fasting plasma glucose concentrations, along with significant improvements in both muscle and liver insulin sensitivity. These effects were accompanied by a reduction in intramyocellular triglyceride and a near normalization of liver fat, both assessed using 1H MRS.
THIAZOLIDINEDIONE MECHANISM OF ACTION
Thiazolidinediones (TZDs) improve insulin sensitivity in patients with type 2 diabetes. They act primarily to enhance insulin-stimulated glucose uptake in muscle by increasing insulin-stimulated GLUT 4 activity and muscle glycogen synthesis, which results in an increased insulin-stimulated glucose disposal rate of ≤45%.40,41 TZDs operate on the peroxisome proliferator-activated receptor–γ (PPAR-γ) to induce the expression of several tissue-specific target genes.
It is therefore somewhat of a paradox as to how the TZDs accomplish this insulin-sensitizing effect in skeletal muscle,41 because the receptor for TZDs (PPAR-γ) is located mostly in adipocytes.42 We hypothesize that the insulin-sensitizing effect of TZDs occurs through their ability to increase insulin sensitivity in the adipocyte, resulting in more efficient storage of fat in the adipocyte. This results in a redistribution of fat away from the hepatocyte and myocyte and into the adipocyte.
Recent studies in patients with type 2 diabetes support this hypothesis. Using microdialysis to assess glycerol release from subcutaneous tissue, we found that rosiglitazone treatment resulted in increased insulin suppression of peripheral lipolysis and an increase in whole-body insulin sensitivity.43 Furthermore, these changes were associated with an approximately 30% reduction in intrahepatic fat content and increase in subcutaneous fat content. There was no change in overall body weight, indicating that rosiglita-zone treatment caused a redistribution of fat from liver cells to subcutaneous fat rather than altering the amount of fat.
EFFECTS OF WEIGHT LOSS IN OBESE PATIENTS WITH TYPE 2 DIABETES
If intracellular lipid is the cause of insulin resistance in type 2 diabetes, the weight loss that results in improved insulin sensitivity should also result in a reduction of hepatic lipid content. In a recent study, 8 obese patients with type 2 diabetes were provided with a moderately hypocaloric (approximately 1,200 kcal/day), very low fat diet (3%) for 8 weeks, which reduced fasting plasma glucose from 8.8 to 6.4 mmol/L.44 During this time, weight loss was only 8 kg, amounting to approximately 8% of body weight. Despite this, there was an 81% reduction in hepatic lipid content, shifting the patients from severe hepatic steatosis and severe hepatic insulin resistance at baseline to near-normal levels of hepatic fat and hepatic insulin sensitivity by the end of the study. Furthermore, this reduction in intrahepatic lipid content was associated with the reduction in and normalization of rates of fasting hepatic glucose production. This could be attributed to a reduction in rates of gluconeogenesis, which has been identified as the major factor responsible for increased rates of glucose production in patients with poorly controlled type 2 diabetes.8
INSULIN RESISTANCE IS ASSOCIATED WITH LOSS OF MITOCHONDRIAL FUNCTION
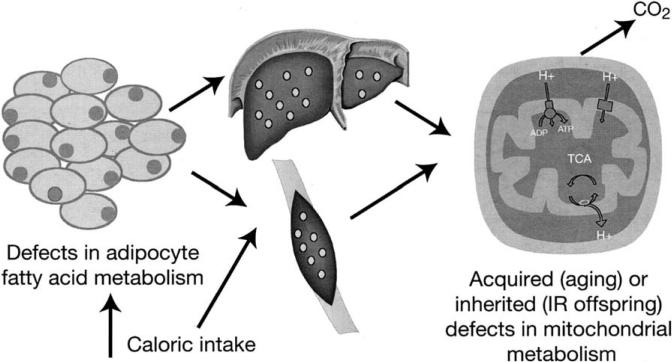
Compared with their young counterparts, lean, otherwise healthy elderly people have a marked tendency toward insulin resistance,45 and this insulin resistance is causally associated with reduced insulin-stimulated muscle glucose metabolism and increased fat accumulation in muscle and liver tissue. Notably, their mitochondrial oxidative phosphorylation activity was reduced by approximately 40% compared with BMI- and activity-matched young individuals. This loss of mitochondrial function predisposes to intramyocellular lipid accumulation, which, through the mechanisms described earlier, provides the link with insulin resistance (Figure 3). It is possible, therefore, that an age-associated decline in mitochondrial function contributes to insulin resistance in the elderly through this mechanism.
Figure 3.
Alternative causes of insulin resistance (IR) mediated via fat accumulation in skeletal muscle and liver.
Subsequent research has shown that the insulin-resistant offspring of parents with type 2 diabetes also have impaired mitochondrial function, with mitochondrial adenosine triphosphate (ATP) synthesis being reduced by approximately 30%.46 These reductions in mitochondrial function were associated with severe muscle insulin resistance and an 80% increase in intramyocellular lipid content. Because these individuals had no abnormalities of systemic or localized rates of lipolysis or plasma concentrations of tumor necrosis factor–α, interleukin-6, resistin, or adiponectin, it seems likely that the genetic factor that explains the heritability of type 2 diabetes may be connected with the loss of mitochondrial activity in these individuals. Recent studies by our group have found that the 30% reduction in mitochondrial activity in the insulin-resistant offspring could be attributed to a 38% reduction in muscle mitochondrial content.47
SUMMARY
Obesity is the most common cause of insulin resistance and type 2 diabetes. Simply being overweight (BMI >25) raises the risk of developing type 2 diabetes by a factor of 3.48
Studies of the role of fat in insulin resistance both in otherwise healthy individuals and in patients with lipodys-trophy demonstrate that the absolute quantity of fat in the body, although a useful clinical correlate of insulin resistance, is less important than how that fat is distributed. What matters more is the intracellular content of lipid in liver and skeletal muscle. Obesity, which typically occurs because caloric intake exceeds energy expenditure, leads to fat accumulation not only in adipocytes, but also in muscle and liver cells, resulting in insulin resistance in these organs.
Although obesity may cause insulin resistance regardless of genotype, it is not the only cause of increased sequestration of lipid in liver and skeletal muscle (Figure 3). Defects in adipocyte function, such as those that occur in lipodystrophy, may have similar effects and similarly lead to insulin resistance in liver and muscle. Other genetic abnormalities in the adipocyte are also likely to contribute to the accumulation of fat within muscle and liver tissue. Prime suspects include inherited defects in the PPAR-γ receptors. Another is perilipin, which coats the lipid droplets of adipocytes and protects the triglycerides within from hydrolysis by lipase.49 Mice lacking the perilipin gene have much reduced adipose tissue and develop insulin resistance.50,51
Finally, recent MRS studies have also demonstrated that both acquired and inherited defects in mitochondrial function may lead to accumulation of intramyocellular lipid and insulin resistance in skeletal muscle.
The identification of the metabolic disturbance that leads to insulin resistance should allow further study of the role of genetic factors that may contribute to the risk of developing type 2 diabetes. Furthermore, it is hoped that the results of these types of mechanistic studies will lead to the development of more rational therapies for the prevention and treatment of type 2 diabetes.
References
- 1.Williamson DF, Vinicor F, Bowman BA. Primary prevention of type 2 diabetes mellitus by lifestyle intervention: implications for health policy. Ann Intern Med. 2004;40:951–957. doi: 10.7326/0003-4819-140-11-200406010-00036. [DOI] [PubMed] [Google Scholar]
- 2.US Renal Data System [May 26, 2005];USRDS 2004 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Available at: http://www.usrds.org/atlas_2004.htm.
- 3.Ulbrecht JS, Cavanagh PR, Caputo GM. Foot problems in diabetes: an overview. Clin Infect Dis. 2004;39(suppl 2):S73–S82. doi: 10.1086/383266. [DOI] [PubMed] [Google Scholar]
- 4.Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26:917–932. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- 5.Lucioni C, Garancini MP, Massi-Benedetti M, et al. The costs of type 2 diabetes mellitus in Italy: a CODE-2 substudy. Treat Endocrinol. 2003;2:121–133. doi: 10.2165/00024677-200302020-00005. [DOI] [PubMed] [Google Scholar]
- 6.Barcelo A, Aedo C, Rajpathak S, Robles S. The cost of diabetes in Latin America and the Caribbean. Bull World Health Organ. 2003;81:19–27. [PMC free article] [PubMed] [Google Scholar]
- 7.International Diabetes Federation [June 1, 2005];Diabetes Atlas. Available at: http://www.eatlas.idf.org/.
- 8.Magnusson I, Rothman DL, Katz LD, et al. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest. 1992;90:1323–1327. doi: 10.1172/JCI115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roden M, Petersen KF, Shulman GI. Nuclear magnetic resonance studies of hepatic glucose metabolism in humans. Recent Prog Horm Res. 2001;56:219–237. doi: 10.1210/rp.56.1.219. [DOI] [PubMed] [Google Scholar]
- 10.Rossetti L, Giaccari A. Relative contribution of glycogen synthesis and glycolysis to insulin-mediated glucose uptake: a dose-response euglycemic clamp study in normal and diabetic rats. J Clin Invest. 1990;85:1785–1792. doi: 10.1172/JCI114636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shulman GI, Rothman DL, Jue T, et al. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990;322:223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- 12.Rothman DL, Shulman RG, Shulman GI. 31P nuclear magnetic resonance measurements of muscle glucose-6-phosphate. Evidence for reduced insulin-dependent muscle glucose transport or phosphorylation activity in non-insulin-dependent diabetes mellitus. J Clin Invest. 1992;89:1075. doi: 10.1172/JCI115686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cline GW, Petersen KF, Krssak M, et al. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N Engl J Med. 1999;341:240–246. doi: 10.1056/NEJM199907223410404. [DOI] [PubMed] [Google Scholar]
- 14.Warram JH, Martin BC, Krolewski AS, et al. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med. 1990;113:909–915. doi: 10.7326/0003-4819-113-12-909. [DOI] [PubMed] [Google Scholar]
- 15.Martin BC, Warram JH, Krolewski AS, et al. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet. 1992;340:925–929. doi: 10.1016/0140-6736(92)92814-v. [DOI] [PubMed] [Google Scholar]
- 16.Goldfine AB, Bouche C, Parker RA, et al. Insulin resistance is a poor predictor of type 2 diabetes in individuals with no family history of disease. Proc Natl Acad Sci U S A. 2003;100:2724–2729. doi: 10.1073/pnas.0438009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe RM, Valle T, Hauser ER, et al. the Finland–United States Investigation of NIDDM Genetics (FUSION) Study investigators Familiality of quantitative metabolic traits in Finnish families with non-insulin-dependent diabetes mellitus. Hum Hered. 1999;49:159–168. doi: 10.1159/000022865. [DOI] [PubMed] [Google Scholar]
- 18.Perseghin G, Ghosh S, Gerow K, Shulman GI. Metabolic defects in lean nondiabetic offspring of NIDDM parents: a cross-sectional study. Diabetes. 1997;46:1001–1009. doi: 10.2337/diab.46.6.1001. [DOI] [PubMed] [Google Scholar]
- 19.Phillips DI, Caddy S, Ilic V, et al. Intramuscular triglyceride and muscle insulin sensitivity: evidence for a relationship in nondiabetic subjects. Metabolism. 1996;45:947–950. doi: 10.1016/s0026-0495(96)90260-7. [DOI] [PubMed] [Google Scholar]
- 20.Szczepaniak LS, Babcock EE, Schick F, et al. Measurement of intracellular triglyceride stores by 1H spectroscopy: validation in vivo. Am J Physiol. 1999;276:E977–E989. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 21.Krssak M, Falk PK, Dresner A, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 22.Perseghin G, Scifo P, De Cobelli F, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48:1600–1606. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 23.Reaven GM, Hollenbeck C, Jeng CY, et al. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988;37:1020–1024. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- 24.Randle PJ, Garland PB, Newsholme EA, Hales CN. The glucose fatty acid cycle in obesity and maturity-onset diabetes mellitus. Ann N Y Acad Sci. 1965;131:324–333. doi: 10.1111/j.1749-6632.1965.tb34800.x. [DOI] [PubMed] [Google Scholar]
- 25.Boden G, Chen X, Ruiz J, et al. Mechanisms of fatty acid–induced inhibition of glucose uptake. J Clin Invest. 1994;93:2438–2446. doi: 10.1172/JCI117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roden M, Price TB, Perseghin G, et al. Mechanism of free fatty acid–induced insulin resistance in humans. J Clin Invest. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dresner A, Laurent D, Marcucci M, et al. Effects of free fatty acids on glucose transport and IRS-1–associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okada T, Kawano Y, Sakakibara T, et al. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J Biol Chem. 1994;269:3568–3573. [PubMed] [Google Scholar]
- 30.Griffin ME, Marcucci MJ, Cline GW, et al. Free fatty acid–induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 31.Kim JK, Fillmore JJ, Sunshine MJ, et al. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest. 2004;114:823–827. doi: 10.1172/JCI22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Previs SF, Withers DJ, Ren JM, et al. Contrasting effects of IRS-1 versus IRS-2 gene disruption on carbohydrate and lipid metabolism in vivo. J Biol Chem. 2000;275:38990–38994. doi: 10.1074/jbc.M006490200. [DOI] [PubMed] [Google Scholar]
- 33.Samuel VT, Liu ZX, Qu X, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 34.Neschen S, Morino K, Hammond LE, et al. Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA: glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab. 2005;2:55–65. doi: 10.1016/j.cmet.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Garg A. Lipodystrophies. Am J Med. 2000;108:143–152. doi: 10.1016/s0002-9343(99)00414-3. [DOI] [PubMed] [Google Scholar]
- 36.Moitra J, Mason MM, Olive M, et al. Life without white fat: a transgenic mouse. Genes Dev. 1998;12:3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JK, Gavrilova O, Chen Y, et al. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J Biol Chem. 2000;275:8456–8460. doi: 10.1074/jbc.275.12.8456. [DOI] [PubMed] [Google Scholar]
- 38.Shimomura I, Hammer RE, Ikemoto S, et al. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 39.Petersen KF, Oral EA, Dufour S, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petersen KF, Krssak M, Inzucchi S, et al. Mechanism of troglitazone action in type 2 diabetes. Diabetes. 2000;49:827–831. doi: 10.2337/diabetes.49.5.827. [DOI] [PubMed] [Google Scholar]
- 41.Maggs DG, Buchanan TA, Burant CF, et al. Metabolic effects of troglitazone monotherapy in type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:176–185. doi: 10.7326/0003-4819-128-3-199802010-00002. [DOI] [PubMed] [Google Scholar]
- 42.Braissant O, Foufelle F, Scotto C, et al. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 43.Mayerson AB, Hundal RS, Dufour S, et al. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 2002;51:797–802. doi: 10.2337/diabetes.51.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petersen KF, Dufour S, Befroy D, et al. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petersen KF, Dufour S, Befroy D, et al. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morino K, Petersen K, Dufour S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brancati FL, Wang NY, Mead LA, et al. Body weight patterns from 20 to 49 years of age and subsequent risk for diabetes mellitus: the Johns Hopkins Precursors Study. Arch Intern Med. 1999;159:957–963. doi: 10.1001/archinte.159.9.957. [DOI] [PubMed] [Google Scholar]
- 49.Londos C, Gruia-Gray J, Brasaemle DL, et al. Perilipin: possible roles in structure and metabolism of intracellular neutral lipids in adipocytes and steroidogenic cells. Int J Obes Relat Metab Disord. 1996;20(suppl 3):S97–S101. [PubMed] [Google Scholar]
- 50.Tansey JT, Sztalryd C, Gruia-Gray J, et al. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci U S A. 2001;98:6494–6499. doi: 10.1073/pnas.101042998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saha PK, Kojima H, Martinez-Botas J, et al. Metabolic adaptations in the absence of perilipin: increased beta-oxidation and decreased hepatic glucose production associated with peripheral insulin resistance but normal glucose tolerance in perilipin-null mice. J Biol Chem. 2004;279:35150–35158. doi: 10.1074/jbc.M405499200. [DOI] [PubMed] [Google Scholar]