Abstract
Objectives:
This is a cross-sectional, observational study to determine the frequency and associated features of HIV-associated neurocognitive disorders (HAND) in a large, diverse sample of infected individuals in the era of combination antiretroviral therapy (CART).
Methods:
A total of 1,555 HIV-infected adults were recruited from 6 university clinics across the United States, with minimal exclusions. We used standardized neuromedical, psychiatric, and neuropsychological (NP) examinations, and recently published criteria for diagnosing HAND and classifying 3 levels of comorbidity (minimal to severe non-HIV risks for NP impairment).
Results:
Fifty-two percent of the total sample had NP impairment, with higher rates in groups with greater comorbidity burden (40%, 59%, and 83%). Prevalence estimates for specific HAND diagnoses (excluding severely confounded cases) were 33% for asymptomatic neurocognitive impairment, 12% for mild neurocognitive disorder, and only 2% for HIV-associated dementia (HAD). Among participants with minimal comorbidities (n = 843), history of low nadir CD4 was a strong predictor of impairment, and the lowest impairment rate on CART occurred in the subset with suppressed plasma viral loads and nadir CD4 ≥200 cells/mm3 (30% vs 47% in remaining subgroups).
Conclusions:
The most severe HAND diagnosis (HAD) was rare, but milder forms of impairment remained common, even among those receiving CART who had minimal comorbidities. Future studies should clarify whether early disease events (e.g., profound CD4 decline) may trigger chronic CNS changes, and whether early CART prevents or reverses these changes.
GLOSSARY
- ANI
= asymptomatic neurocognitive impairment;
- CART
= combination antiretroviral therapy;
- CHARTER
= CNS HIV Antiretroviral Therapy Effects Research;
- CIDI
= Composite International Diagnostic Interview;
- CLIA
= Clinical Laboratory Improvement Amendments;
- CPE
= CNS penetration effectiveness;
- HAD
= HIV-associated dementia;
- HAND
= HIV-associated neurocognitive disorder;
- IADL
= instrumental activities of daily living;
- LP
= lumbar puncture;
- MND
= mild neurocognitive disorder;
- NP
= neuropsychological;
- PAOFI
= Patient's Assessment of Own Functioning Inventory.
A growing armamentarium of potent antiviral drugs that target multiple steps in the HIV life cycle has led to vast improvements in HIV disease management. Combining these drugs (combination antiretroviral therapy [CART]) has greatly reduced medical morbidity and mortality, but neurologic complications remain common, manifested by HIV-associated neurocognitive disorders (HAND) and distal sensory polyneuropathy.1–3 Although there appears to be a disconnection between the medical and neurologic benefits of CART, lack of large-scale comprehensive neurologic studies has made accurate estimates of the prevalence of HAND and its relationship to disease and treatment factors difficult.
The CNS HIV Antiretroviral Therapy Effects Research (CHARTER) study was commissioned by the National Institute of Mental Health and the National Institute of Neurological Diseases and Stroke to examine a diverse group of HIV-infected persons broadly reflective of patients at university-affiliated HIV treatment centers in the United States. CHARTER was designed with broad inclusion criteria, and a large sample size so as to afford ascertainment of the frequency and severity of HAND, as well as the specific contributions of HIV vs other factors (comorbidities) to neurocognitive impairment.
Here we present the baseline CHARTER neurobehavioral and neuromedical findings, including the relationships between HAND and CART, disease history and current severity, and functional outcomes. We used recently published international expert consensus guidelines “Frascati Criteria”4 to classify the participants with respect to 3 levels of HIV-related neurocognitive impairment.
METHODS
Subjects.
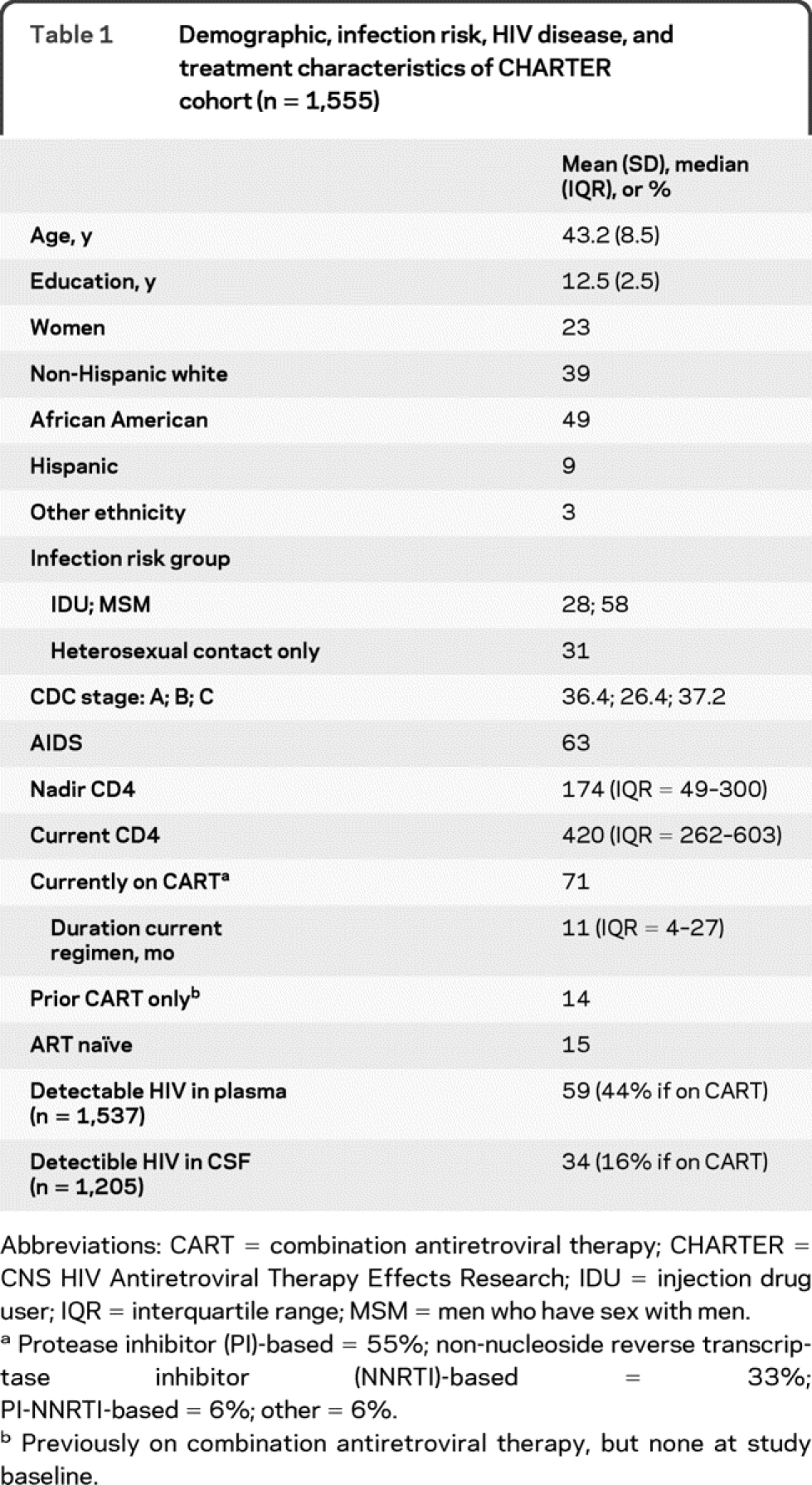
The 1,555 participants in this study were HIV infected (HIV+) and were drawn from 6 participating university centers: Johns Hopkins University (Baltimore, MD, n = 230); Mt. Sinai School of Medicine (New York, NY, n = 271); University of California at San Diego (San Diego, CA, n = 262); University of Texas Medical Branch (Galveston, TX, n = 261); University of Washington (Seattle, WA, n = 262); and Washington University (St. Louis, MO, n = 269). Subject recruitment began in September 2003 and ended in August 2007. Demographic, HIV disease, and treatment characteristics of the total sample are summarized in table 1.
Table 1 Demographic, infection risk, HIV disease, and treatment characteristics of CHARTER cohort (n = 1,555)
Procedures.
For their baseline assessment, all subjects completed a venipuncture, neuromedical assessment, comprehensive neuropsychological (NP) testing, detailed substance use history, structured psychiatric interviews for detecting lifetime and current diagnoses of substance use disorders and affective disorders, a measure of current mood, and self-report assessments of cognitive symptoms, vocational functioning, and independence with instrumental activities of daily living. For those who consented (n = 1,205), CSF was withdrawn by lumbar puncture (LP).
Standard protocol approvals, registrations, and patient consents.
These procedures were approved by the Human Subjects Protection Committees of each participating institution. Written informed consent was obtained from all study participants.
Neuromedical examination.
This included medical history, structured neurologic and medical examination, as well as collection of blood and urine samples. These procedures were performed by physicians, nurse practitioners, or trained nurses and research associates. The staff performing CHARTER neuromedical and NP assessments were certified by the coordinating center (San Diego).
Laboratory assessment.
HIV infection was diagnosed by ELISA with Western blot confirmation. Routine clinical chemistry panels, complete blood counts, rapid plasma reagin, hepatitis C virus antibody, and CD4+ T cells (flow cytometry) were performed at each site's Clinical Laboratory Improvement Amendments (CLIA)–certified, or CLIA equivalent, medical center laboratory. HIV RNA levels were measured centrally in plasma and CSF by reverse transcriptase PCR (Roche Amplicor, v. 1.5, lower limit of quantitation 50 copies/mL).
Neurobehavioral examination.
All participants completed a comprehensive neurocognitive test battery, covering 7 cognitive domains known to be commonly affected by HIV-associated CNS dysfunction (administration time = 2–2.5 hours; see table e-1 on the Neurology® Web site at www.neurology.org for listing of specific tests). The best available normative standards were used, which correct for effects of age, education, sex, and ethnicity, as appropriate. Test scores were automatically converted to demographically corrected standard scores (t scores) using available computer programs. To classify presence and severity of neurocognitive impairment, we applied a published objective algorithm that has been shown to yield excellent interrater reliability in previous multisite studies.5 This algorithm conforms to the Frascati criteria for diagnosing HAND,4 which requires presence of a least mild impairment in at least 2 of the 7 ability domains.
Psychiatric examination.
Psychiatric diagnoses were assessed using the computer-assisted Composite International Diagnostic Interview (CIDI),6 a structured instrument widely used in psychiatric research. The CIDI classifies current and lifetime diagnoses of mood disorders and substance use disorders, as well as other mental disorders. Current mood was assessed with the Beck Depression Inventory II.7
Functional impairment in everyday life.
Reports of cognitive difficulties in everyday life were assessed using the Patient's Assessment of Own Functioning Inventory (PAOFI8). Increased dependence in performing instrumental activities of daily living (IADLs) was assessed with a modified version of the Lawton and Brody Scale.9 We also administered an employment questionnaire that asks about any decreases in work productivity, accuracy/quality of work, increased effort required to do one's usual job, and increased fatigue in association with the usual workload.
HAND classifications.
See Antinori et al.4 for details of the Frascati criteria for HAND and algorithms for establishing those criteria. In brief, asymptomatic neurocognitive impairment (ANI) and mild neurocognitive disorder (MND) both require the presence of at least mild neuropsychological impairment that involves 2 or more ability domains, and is not readily attributable to comorbid conditions. ANI is asymptomatic in the sense that specified criteria for establishing at least mild negative effects on everyday functioning have not been met. In the case of MND, functional decline is established by at least 2 types of evidence regarding decreased everyday functioning. The third HAND diagnosis, HIV-associated dementia (HAD), requires overall neuropsychological impairment of at least moderate severity that is not readily attributable to comorbid conditions. In addition, “major” functional decline must be established by evidence of at least 2 types of everyday functioning problems that are of greater severity than with MND.
Classification of comorbid conditions.
All subtypes of HAND require a determination that the neurocognitive impairment and functional disability are believed to be due to effects of HIV on the brain, and are not readily attributable to comorbid conditions. This determination requires not only detailed information about the comorbid conditions themselves, but also clinical judgment about their severity, their likely impact on neurocognition and everyday functioning, and their timing in relation to the course of HIV disease and any functional limitations in everyday life.
To facilitate interrater reliability of these determinations, we utilized the online supplement to the Antinori et al.4 report, which provided detailed guidelines for classifying the most commonly encountered comorbid conditions with respect to whether they should be considered incidental, contributing, or confounding. In the current study, a senior neuropsychologist (R.K.H.) used the Antinori et al.4 guidelines, with all available historical and testing data, to rate the comorbidity status of participants in all 6 centers. As a check on the reliability of this process, a senior neurologist and principal investigator of the Washington University site (D.B.C.) independently rated his patients (n = 269). Seventy-four percent of the independent ratings were identical. After discussion, only 7% (19 of 269) of the original ratings changed, and less than 5% required a change in HAND classification (change to or from the confounded group).
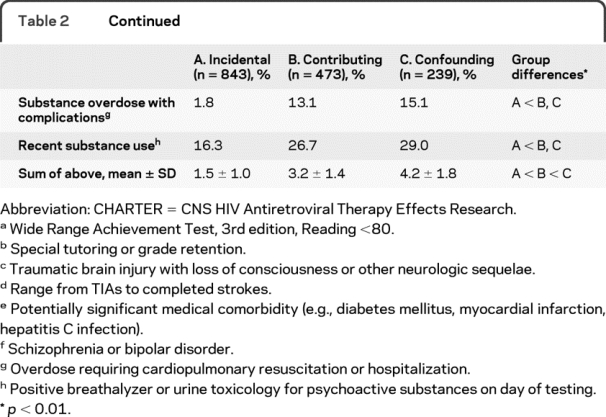
A majority of the CHARTER participants (54.2%; n = 843) was classified as having only incidental comorbidities, and 30.4% (n = 473) had contributing conditions; 15.4% (n = 239) had confounding comorbidities that precluded a HAND diagnosis (see table 2 for details concerning rates of major comorbidities found in these three groups).
Table 2 Frequency of general classes of comorbid conditions within CHARTER cohort comorbidity subgroups
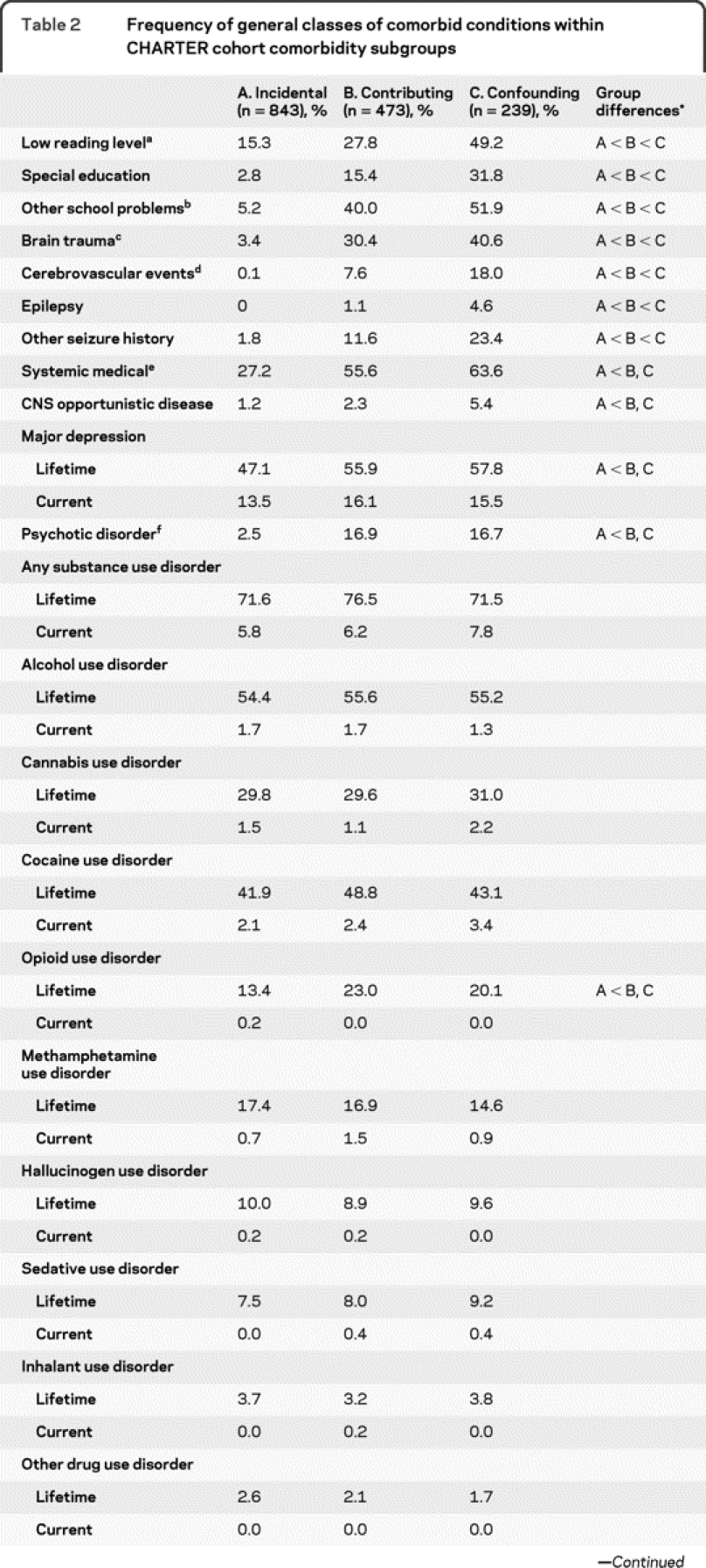
Table 2 Continued
RESULTS
Comorbidity groups and NP impairment.
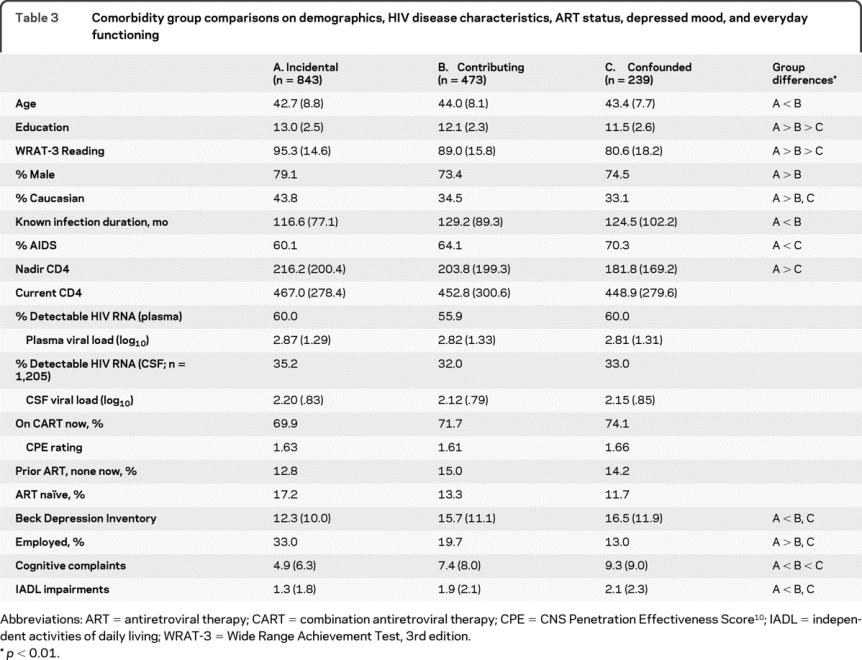
Table 3 describes the 3 comorbidity groups with respect to demographics, HIV disease and treatment characteristics, depressed mood, and everyday functioning. See table e-2 for a summary of raw scores on the neuropsychological test battery.
Table 3 Comorbidity group comparisons on demographics, HIV disease characteristics, ART status, depressed mood, and everyday functioning
Fifty-two percent of the total CHARTER cohort (814/1555) were neuropsychologically impaired. NP impairment rates in the comorbidity groups were as follows: 40% of incidental; 59% of contributing; 83% of confounded.
Associations of NP impairment with HIV disease severity and treatment.
CHARTER hypothesized a priori that NP impairment would show the strongest relationships with HIV disease and treatment characteristics in participants with only minimal comorbidities (i.e., the incidental group). Table 4 summarizes these relationships for the 3 comorbidity groups. NP impairment was associated with AIDS diagnosis, and lower nadir CD4, but only in the incidental group.
Table 4 Neuropsychological impairment rates for comorbidity groups by HIV disease variables and current treatment status
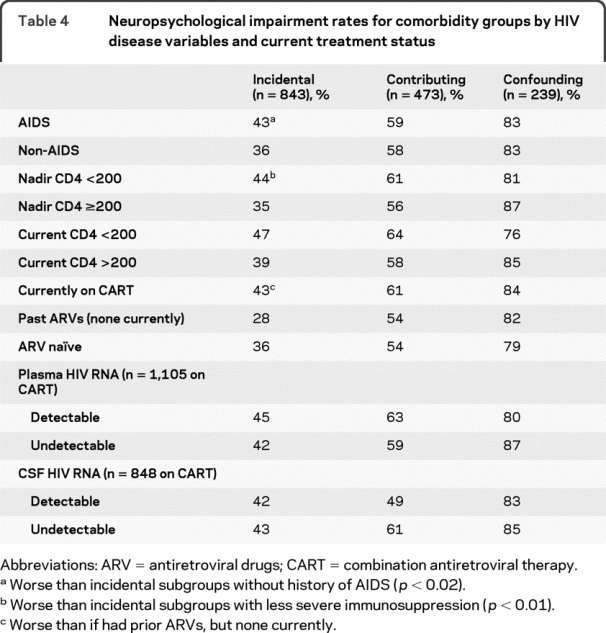
Also only in the incidental group, participants on CART had a higher rate of NP impairment than those not currently being treated. The latter finding may seem counterintuitive, especially since participants on CART were more likely to have undetectable virus in plasma (55% vs 4%, p < 0.001), but is consistent with those on CART having more advanced HIV disease (e.g., a higher proportion with AIDS diagnosis; 74% vs 27%, p < 0.01). It may be, therefore, that relationship of CART to NP outcomes depends upon multiple factors, including disease history and comorbidities, as well as the success of treatment in suppressing the virus.
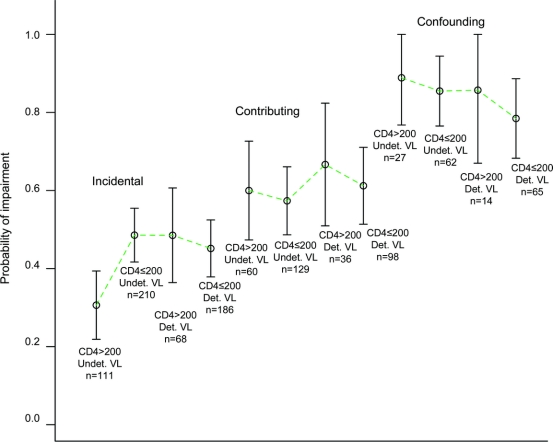
A series of multivariate logistic regression analyses was performed to identify treatment and disease characteristics that, in combination, best predicted global NP impairment (impaired/normal). The candidate predictors included comorbidity group, nadir CD4 <200 (yes/no), current CD4 <200 (yes/no), cumulative duration of ART experience, CNS penetration effectiveness (CPE) rating10 for current ART regimen, detectable HIV in plasma (yes/no), hepatitis C serostatus (yes/no), and their interactions with comorbidity group, plus interactions between nadir CD4 and detectable plasma viral load. The first analysis included all participants receiving CART having complete data on the predictor variables (n = 1,066), and revealed the following predictors: comorbidity group (p < 0.001), nadir CD4 <200 (p = 0.007), and detectable plasma viral load × nadir CD4 <200 (p = 0.037). The plot in the figure displays the size and directions of the effects, comparing the 4 nadir CD4 × detectable viral load subgroups at each comorbidity level. Follow-up logistic regressions for the 3 comorbidity groups separately revealed that, only for ARV-treated participants in the incidental group (n = 575), predictors of NP impairment included nadir CD4 <200 (p = 0.007), detectable plasma viral load (p = 0.045), and their interaction (p = 0.017). As the figure indicates, a substantially lower impairment rate was seen in treated participants with undetectable viral load and nadir CD4 >200 (30% vs 47% for the combined other Incidental subgroups, p = 0.002).
Figure Probabilities of impairment, and 95% confidence intervals, for subjects on combination antiretroviral therapy, classified by comorbidity group (incidental, contributing, and confounding), plasma HIV-1 viral load (UD = undetectable, Det = detectable), and nadir CD4 (<200 vs >200 cells/μL)
Sample sizes (N) for each group are given.
Functional disability and HAND diagnoses.
According to the Frascati criteria, HAND diagnoses cannot be made in HIV-infected people with severe comorbidities (our confounded group). Therefore, HAND diagnoses were determined in the remaining CHARTER groups with lesser comorbidities (n = 1,316) by considering the results of the NP and self-report functional measures. NP impairment was noted in 617 participants (46.9%) and of these, 430 (70%) were considered “asymptomatic”; that is, their self-reports did not suggest their NP impairment was interfering significantly with their everyday functioning. Of the remaining 185, 154 had MND and only 31 had HAD.
It is emphasized that the above distributions of HAND classifications exclude the large subgroup of HIV-infected participants who were NP-normal (n = 699, or 53.2% of the combined incidental and contributing comorbidity groups). If we consider the entire CHARTER sample without severe comorbidities (n = 1,316), we obtain the following overall rates of HAND diagnoses: 32.7% ANI, 11.7% MND, and 2.4% HAD.
Other neuropsychiatric diagnoses.
For the combined incidental and contributing comorbidity groups, NP impairment was not significantly related to either current or lifetime major depressive disorders, but people with a (mostly remote) history of any substance abuse or dependence were less likely to be NP impaired (44% vs 54%).
DISCUSSION
Although CART has had a major impact on the course and long-term prognosis of HIV infection, all but the most severe CNS manifestations of infection remain very common. One important finding of our study is that profound neurocognitive impairment—HAD—has become rare. Whereas estimates of HAD prevalence in the pre-CART era ranged from about 10% to 15%,11,12 only 2% of the large CHARTER cohort met both NP and functional criteria for dementia. By contrast, 44% of CHARTER participants without severe comorbidities met criteria for milder forms of HAND, and this is consistent with pre-CART reports.13,14
Whereas Frascati criteria for HAND focus on neurocognitive impairment, higher rates of CNS abnormalities might be seen with additional consideration of noncognitive neurologic findings (saccadic eye movement, tone, reflexes, facial minima).
As stated, the present subject sample was recruited with few exclusion criteria in an effort to be broadly representative of patients being followed in the university-based clinics. The results are not necessarily generalizable to other types of clinical settings, or to people who were unwilling to volunteer. Out of 1,900 potential participants who were screened and invited to participate, 1,555 (78.1%) agreed to do so. The 435 screened individuals who declined were comparable to the study participants with respect to age (mean = 43.9 vs 43.2 years), but had slightly lower education levels (mean = 12.1 vs 12.5 years), and somewhat higher rates of female gender (30.1% vs 23.3%) and nonwhite ethnicity (73.3% vs 60.7%). Again, NP test norms were corrected for demographics, which were unrelated to impairment status in CHARTER.
The CART era differences between lower frequencies of HAD and stable or increased prevalence of milder forms of HAND have not been fully explained. Studies have demonstrated some NP benefit of instituting CART regimens in patients having HAND,15–18 but in many cases impairment persists. Relatively high percentages of CART-treated individuals continue to show some active viral replication as well; e.g., 482 of the 1,105 CHARTER participants on CART (44%) had detectable HIV RNA in plasma. Whereas rates of virologic suppression in recent clinical trials of ART typically reach 80%–90%, this is not the case in clinic-based samples, where rates of virologic suppression have been lower and similar to our findings.19,20 Extended survival with incomplete viral suppression is likely to be associated with prolonged CNS inflammatory responses that are implicated in the pathogenesis of HAND.21–24
CHARTER findings also indicate that a history of more severe immunosuppression confers an increased risk for HAND, even after CART-related immune recovery; over 70% of our participants receiving CART had a nadir CD4 <200. This raises the question of whether better neurobehavioral outcomes could be achieved by initiating CART earlier and preventing more advanced immunosuppression, rather than using declines in CD4 levels to trigger treatment. In fact, among CHARTER participants who did not have significant comorbid risks for CNS dysfunction, much lower rates of HAND were seen in those who achieved successful HIV suppression on CART and had nadir CD4 counts above 200. This was not explained by subgroup differences in other potentially influential factors, such as age or estimated duration of infection, or duration of CART, which was slightly longer in the nadir >200/undetectable group. It is possible that advanced immunosuppression reflected by low nadir CD4 is a “legacy” event whose neurologic consequences may persist. Recent reports of both neurocognitive impairment and brain imaging abnormalities in acute and early HIV infection are intriguing, and consistent with the possibility that such a “legacy” event might occur very early,25,26 indicating the importance of conducting careful longitudinal studies that focus more on early stages of HIV infection. Such studies should investigate the timing of incident neurobehavioral impairment within the context of developing immunosuppression and clinical disease, and whether earlier CART intervention might prevent or reverse CNS injury.
The findings of this study may appear to conflict with those of other recent reports indicating that neurocognitive improvement was greatest in patients treated with better CNS-penetrating CART regimens27 and that viral suppression in CSF corresponds to better neurocognitive outcomes.17 However, the latter findings are derived from longitudinal studies where the clinical outcome measurements were synchronized with the initiation and follow-up of a new CART regimen. In contrast, the data reported here are from a single cross-sectional evaluation. Cross-sectional analyses complement but do not replace those of prospective longitudinal studies and randomized clinical trials, and one cannot expect the 2 designs to yield the same results. Additionally, CART probably benefits the brain via multiple mechanisms, including immune recovery, reduced immune activation, and viral suppression—both systemically and in the CNS. Optimizing all of these parameters and possibly others as well may be needed to obtain the best neurologic protection and recovery. Randomized clinical trials targeting each of these mechanisms are needed to determine what will be the most clinically useful approach to the prevention and treatment of HAND.
CHARTER has attempted to systematically implement the recently published Frascati guidelines4 to classify comorbid conditions in a large, diverse sample of HIV-infected adults receiving care. Comorbidities in the HIV-infected population are numerous and complex, and in the past it has proven difficult to reliably determine whether they are severe enough to preclude diagnosing an HIV-related neurobehavioral disorder.5 The Frascati guidelines clearly have improved this reliability: independent neurologist and neuropsychologist raters in CHARTER agreed on more than 95% of cases concerning whether there were confounds that preclude a HAND diagnosis.
The CHARTER experience suggests that, using the Frascati guidelines, 2 criteria for HAND diagnoses can be assessed reliably: NP impairment and confounding conditions. Impairment of everyday functioning also must be documented as a criterion for symptomatic HAND, and this remains a challenge. Self-reports of functional decline are easy to obtain, but may lead to false-positive classifications (e.g., exaggerated self criticism due to depression)28 as well as false-negative ones due to lack of insight or avoidance of everyday situations that require abilities which have become impaired. Use of objective, performance-based functional assessments to supplement self-report may improve detection of symptomatic HAND,4,9,29 but such assessments are impractical in most clinical settings. Therefore, an inability to document functional decline in a person who clearly meets the other HAND criteria will continue to be a common clinical experience. Such cases deserve careful attention and monitoring, though, because they may carry a negative medical prognosis18,30,31 and the apparent lack of functional decline may change if life circumstances become more demanding.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by D.R. Franklin, Jr., Dr. Vaida, and Dr. Ake.
COINVESTIGATORS
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) group is affiliated with Johns Hopkins University, Mount Sinai School of Medicine, University of California, San Diego, University of Texas, Galveston, University of Washington, Seattle, Washington University, St. Louis, and is headquartered at the University of California, San Diego, and includes the following: Director: Igor Grant, MD; Co-Directors: J. Allen McCutchan, MD, Ronald J. Ellis, MD, PhD, Thomas D. Marcotte, PhD; Center Manager: Donald Franklin, Jr.; Neuromedical Component: Ronald J. Ellis, MD, PhD (P.I.), J. Allen McCutchan, MD, Terry Alexander, RN; Laboratory, Pharmacology and Immunology Component: Scott Letendre, MD (P.I.), Edmund Capparelli, PharmD; Neurobehavioral Component: Robert K. Heaton, PhD (P.I.), J. Hampton Atkinson, MD, Steven Paul Woods, PsyD, Matthew Dawson; Virology Component: Joseph K. Wong, MD (P.I.); Imaging Component: Christine Fennema-Notestine, PhD (Co-P.I.), Michael J. Taylor, PhD (Co-P.I.), Rebecca Theilmann, PhD; Data Management Unit: Anthony C. Gamst, PhD (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, PhD (P.I.), Florin Vaida, PhD; Protocol Coordinating Component: Thomas D. Marcotte, PhD (P.I.), Rodney von Jaeger, MPH; Johns Hopkins University Site: Justin McArthur (P.I.), Mary Smith; Mount Sinai School of Medicine Site: Susan Morgello, MD (Co-P.I.), and David Simpson, MD (Co-P.I.), Letty Mintz, NP; University of California, San Diego Site: J. Allen McCutchan, MD (P.I.), Will Toperoff, NP; University of Washington, Seattle Site: Ann Collier, MD (Co-P.I.), and Christina Marra, MD (Co-P.I.), Trudy Jones, MN, ARNP; University of Texas, Galveston Site: Benjamin Gelman, MD, PhD (P.I.), Eleanor Head, RN, BSN; and Washington University, St. Louis Site: David Clifford, MD (P.I.), Muhammad Al-Lozi, MD, Mengesha Teshome, MD.
DISCLOSURE
Dr. Heaton serves on a scientific advisory board for the NINCDS; serves on the editorial boards of the Journal of the International Neuropsychological Society, the Journal of Clinical and Experimental Neuropsychology, and The Clinical Neuropsychologist; has received royalties from the publication of Revised Comprehensive Norms for and Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults (Psychological Assessment Resources, Inc., 1991-present), Revised Comprehensive Norms for and Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults Scoring Program (Psychological Assessment Resources, Inc., 1994-present), and Wisconsin Card Sorting Test Manual-Revised and Expanded (Psychological Assessment Resources, Inc., 1991-present); and receives research support from the NIH (P30 MH62512 [coinvestigator], P50 DA26306 [coinvestigator], P01 DA12065 [coinvestigator], R01 MH60720 [coinvestigator], R01 MH73433 [PI], N01 MH22005 [coinvestigator], R01 MH58076 [coinvestigator], R01 MH78748 [coinvestigator], R01 MH78737 [coinvestigator], U01 MH83506 [coinvestigator], R01 MH83552 [coinvestigator], and R01 MH81861 [coinvestigator]). Dr. Clifford serves/has served on scientific advisory boards for Biogen Idec, Elan Corporation, Roche, Forest Laboratories, Inc., Genentech, Inc., GlaxoSmithKline, Millennium Pharmaceuticals, Inc., Schering-Plough Corp., Bristol-Meyers Squibb, and Genzyme Corporation; received speaker honoraria and funding for travel from GlaxoSmithKline, Millennium Pharmaceuticals, Inc., and Genentech Inc.; has received research support from Pfizer Inc, Schering-Plough Corp., Bavarian Nordic, NeurogesX, GlaxoSmithKline, Tibotec Therapeutics, Boehringer Ingelheim, and Gilead Sciences, Inc.; and receives research support from the NIH (UO1 NS32228 [PI], UO1 AI69495 [PI], NIMH 22005 CHARTER Project [Site PI], NIDA RO3 DA022137 [Co-I], NIMH MH058076 [Site PI], and R21 3857-53187 [PI]). Mr. Franklin receives research support from the NIH (NIMH N01 MH22005 [Center Manager]). Dr. Woods serves as Book Review Editor for the Journal of Clinical and Experimental Neuropsychology and on the editorial board of Archives of Clinical Neuropsychology; and receives research support from the NIH (P30 MH62512 [coinvestigator], P50 DA26306 [coinvestigator], N01 MH22005 [coinvestigator], R01 MH73419 [PI], P01 DA12065 [coinvestigator], and from Uniformed Services University/Henry M. Jackson Foundation. Dr. Ake has received research support from the NIH (NIHM N01 MH22005 [statistician] and NIHM P30 MH62512 [statistician]). Dr. Vaida served on a scientific advisory board for Ardea Biosciences, Inc.; serves as Research Methodology Section Editor for the Californian Journal of Health Promotion; and receives research support from Precision Photonics Corporation and the NIH (P30 MH62512 [coinvestigator], P50 DA26306 [coinvestigator], N01 MH22005 [coinvestigator], R01 MH083552 [coinvestigator], NIH R01 AI47033 [subcontract PI], U01 AI74521 [coinvestigator], R01 MH085608 [coinvestigator], and AI068543 [coinvestigator]. Dr. Ellis serves on the editorial advisory board for the Journal of Neuroimmune Pharmacology; has served on the speakers' bureau for and received speaker honoraria from GlaxoSmithKline; and has received research support from the NIH (R01 MH058076-12 [PI], P30 MH062512-09 [coinvestigator], DA012065-10 [coinvestigator], N01 MH22005-08 [coinvestigator], MH083506-02 [coinvestigator], MH083552-02 [coinvestigator], DA026306-01 [coinvestigator], MH085608-01 [coinvestigator], and DA026146 [coinvestigator]). Dr. Letendre serves on a scientific advisory board for Tibotec Therapeutics; has received speaker honoraria from GlaxoSmithKline, Tibotec Therapeutics, and Abbott; and receives research support from Abbott, Merck Serono, Tibotec Therapeutics, Schering-Plough Corp., GlaxoSmithKline, and the NIH (N01 MH22005 [coinvestigator], R01 MH58076 [coinvestigator], P01 DA12065 [coinvestigator], R01 NS36524 [coinvestigator], P30 MH62512 [coinvestigator], U01 MH083506 [consultant], R01 MH73433 [coinvestigator], U01 AI69432 [coinvestigator], R01 MH78748 [coinvestigator], and R21 MH85610 [PI]). Dr. Marcotte receives research support from the NIH (RO1 MH78748 [PI], P30 MH62512 [coinvestigator], P50 DA26306 [coinvestigator], N01 MH22005 [coinvestigator], RO1 MH38552 [coinvestigator], RO1 MH73433 [coinvestigator], R32 AA17321 [coinvestigator], R32 MH77487 [coinvestigator], and R01 MH64907 [coinvestigator]). Dr. Atkinson serves as a consultant for Eli Lilly and Company; and receives research support from the US Department of Veterans Affairs and the NIH (NIMH P30 MH62512 [coinvestigator], P50 DA26306 [coinvestigator], U01 MH83506 [coinvestigator], NIMH R01 MH73419 [coinvestigator], NIMH R01 MH58076 [coinvestigator], NIMH N01 MH22005 [coinvestigator], NIDA P01 DA12065 [coinvestigator], NIMH R01 MH79881 [coinvestigator], and NIMH R01 MH61146 [coinvestigator]). Dr. Rivera-Mindt receives research support from the NIH (N01 MH22005 [coinvestigator] and NIMH K23MH079718 [PI]. Mr. Vigil has received research support from the NIH (NIMH N01 MH22005 [Psychiatric Coordinator]). Dr. Taylor receives royalties from the publication of Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery (Norms, Manual and Computer Program) (Psychological Assessment Resources, 2004); and receives research support from the US Veterans Administration and the NIH (NIMH P30 MH62512 [Consultant], NIMH N01 MH22005 [coinvestigator], NINDS R01 NS36524 [PI], and NIDA P01 DA12065 [coinvestigator]). Dr. Collier serves/has served on scientific advisory boards for Merck & Co., Inc.; Pfizer Inc, and GlaxoSmithKline; receives/has received research support from Merck & Co., Inc., Schering-Plough Corp, Boehringer Ingelheim, Gilead Sciences, Inc., Koronis Pharmaceuticals, Johnson & Johnson, and the NIH (NIAID AI 069434 [PI], NIAID AI27757 [PI on Core], NIHM N01 MH22005 [Site PI], NIAID AI069918 [coinvestigator of subcontract], NINDS RC1NS068904 [coinvestigator], NIAID P01 AI 057005 [PI on Core], and NIAID AI069438 [PI of Subcontract]); and she and an immediate family member own stock in Abbott and Bristol-Myers Squibb. Dr. Marra receives royalties from the publication of Infections of the Central Nervous System, 3d edition (Lippincott Williams & Wilkins, 2004) and from an article in UptoDate; and receives research support from the NIH (1R01 NS/AI 34235 [PI], N01 MH22005 [coinvestigator]). Dr. Gelman receives research support from the NIH/NIMH (U01MH083507 [PI], R01MH079886 [PI], and N01MH022005 [PI]). Dr. McArthur serves as a consultant to Allergan, Inc., Pfizer Inc, and Biogen Idec; receives royalties from the publication of Current Therapy in Neurologic Disease, 7th edition (Mosby, 2006); is an author on patents re: Device for thermal stimulation of small neural fibers and Immunophilin ligand treatment of antiretroviral toxic neuropathy; and receives research support from Biogen Idec, the NIH (R01 MH075673 [PI], RO1 NS44807 [PI], RO1 NS49465 [PI], and R01 MH067831 [Co-I]), the National Multiple Sclerosis Society, and the Foundation for Peripheral Neuropathy. Dr. Morgello has received research support from the NIH (U01MH083501 [PI] and N01MH22005 [PI]). Dr. Simpson has served on scientific advisory boards for Pfizer Inc, Cephalon, Inc., Eli Lilly and Company, GlaxoSmithKline, Allergan, Inc., Merz Pharmaceuticals LLC, MEDA Pharmaceuticals Ltd., Boehringer Ingelheim, Endo Pharmaceuticals, Biogen Idec, and Alpharma Inc.; serves on the editorial board of AIDS Patient Care; has served on speakers' bureaus for and received speaker honoraria from Eli Lilly and Company and GlaxoSmithKline; has served as a consultant for NeurogesX, Eli Lilly and Company, Regeneron Pharmaceuticals, Inc., GlaxoSmithKline, Allergan, Inc., Merz Pharmaceuticals LLC, and Torrey Pines; and receives research support from NeurogesX, Pfizer Inc, Allergan, Inc., Eli Lilly and Company, and the NIH (NINDS R01-NS-328-05 [coinvestigator], NINDS R24 MH59724 [coinvestigator], NIMH 00-AI-0005 [Co-PI], and NINDS R01-NS-41198 [coinvestigator]). Dr. McCutchan authors chapters on HIV for the Merck Manual; and receives research support from the NIH (P30 MH62512 [coinvestigator], U01 MH83506 [coinvestigator], CDC U2G PS00623 [PI], U01 AI69432 [coinvestigator], N01 MH22005 [coinvestigator], K30 RR22681 [coinvestigator], R01 MH58076 [coinvestigator], and U13 MH81676 [PI]). Dr. Abramson serves as an Associate Editor for the Journal of Nonparametric Statistics; and receives research support from the NIH (P30 MH62512 [coinvestigator], P50 DA26306 [coinvestigator], R01 MH61146 [coinvestigator], N01 MH22005 [coinvestigator], R01 DA21115 [coinvestigator], and R21 MH78728 [coinvestigator]). Dr. Gamst receives research support from Pfizer Inc, and the NIH (P30 MH62512 [coinvestigator], P50 DA26306 [coinvestigator], R01 HL95089 [coinvestigator], U01 MH83506 [coinvestigator], R01 HL84229 [coinvestigator], R01 MH79752 [coinvestigator], N01 MH22005 [coinvestigator], and U01 AG10483 [coinvestigator]), and from the National Science Foundation. Dr. Fennema-Notestine has received research support from the NIH (R21 NS069355 [PI], N01 MH22005 [coinvestigator], P30 MH062512 [coinvestigator], P50 DA026306 [coinvestigator], U24 RR21382 [coinvestigator], U24 RR021992 [coinvestigator], U24 RR019701 [coinvestigator], R01 MH079752 [coinvestigator], R01 AG031224 [coinvestigator], R01 AG022381 [coinvestigator], R01 MH084796 [coinvestigator], R01 AG024506 [coinvestigator]), the US Department of Veterans Affairs, and the Alzheimer's Association. Dr. Jernigan serves on the editorial advisory boards for Neuropsychology, Neurobiology of Aging: Brain Structure and Function, and Developmental Neuropsychology: Brain Imaging and Behavior; and receives research support from the NIH (NIMH P30 MH62512 [coinvestigator], P50 DA26306 [coinvestigator], NIMH R01 MH79752 [PI], NIMH N01 MH22005 [coinvestigator], NIDA P01 DA12065 [coinvestigator], R01 AA13419 [coinvestigator], R01 MH73419 [coinvestigator], NIDA RC2 DA29475 [PI]), and from the Lundbeck Foundation. Dr. Wong has served on a scientific advisory board for Abbott; serves on the editorial boards of the Journal of AIDS and AIDS Research and Therapy; has received research material support from Bristol-Myers Squibb; and has received research support from the NIH (R01 NS051132 [PI] and N01 MH22005 [Core PI], R21 MH083573 [PI], and R01 AI087145 [coinvestigator], and from the US Department of Veterans Affairs. Dr. Grant serves as an Associate Editor for the Journal of Neurovirology; and receives research support from the NIH (NIMH P30 MH62512 [PI], NIDA P50 DA26306 [PI], NIDA P01 DA12065 [PI], NIMH N01 MH22005 [PI], NIMH U01 MH83506 [PI], NIMH R01 MH78748 [coinvestigator], NIA R01 AG15301 [PI], NIMH R01 MH83552 [coinvestigator], and NIH/University of Nebraska P01 DA026146 [subcontract PI]).
Address correspondence and reprint requests to Dr. Robert K. Heaton, 220 Dickinson Street, Suite B, San Diego, CA 92013 rheaton@ucsd.edu
Editorial, page 2052
Supplemental data at www.neurology.org
Study funding: The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) study is supported by the NIH (N01 MH22005).
The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
Disclosure: Author disclosures are provided at the end of the article.
Received January 5, 2010. Accepted in final form August 11, 2010.
REFERENCES
- 1.Giancola ML, Lorenzini P, Balestra P, et al. Neuroactive antiretroviral drugs do not influence neurocognitive performance in less advanced HIV-infected patients responding to highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2006;41:332–337. [DOI] [PubMed] [Google Scholar]
- 2.Sevigny JJ, Albert SM, McDermott MP, et al. An evaluation of neurocognitive status and markers of immune activation as predictors of time to death in advanced HIV infection. Arch Neurol 2007;64:97–102. [DOI] [PubMed] [Google Scholar]
- 3.Tozzi V, Balestra P, Bellagamba R, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr 2007;45:174–182. [DOI] [PubMed] [Google Scholar]
- 4.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007;69:1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woods SP, Rippeth JD, Frol AB, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol 2004;26:759–778. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Composite International Diagnostic Interview, version 2.1. Geneva: WHO; 1997. [Google Scholar]
- 7.Beck AT, Steer RA, Brown GK. Beck Depression Inventory: Second Edition manual. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- 8.Chelune GJ, Heaton RK, Lehman RA. Neuropsychological and personality correlates of patients complaints of disability. In: Goldstein G, Tarter RE, eds. Advances in Clinical Neuropsychology, Third Edition. New York: Plenum Press; 1986:95–126. [Google Scholar]
- 9.Heaton RK, Marcotte TD, Mindt MR, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc 2004;10:317–331. [DOI] [PubMed] [Google Scholar]
- 10.Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 2008;65:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janssen RS, Nwanyanwu OC, Selik RM, Stehr-Green JK. Epidemiology of human immunodeficiency virus encephalopathy in the United States. Neurology 1992;42:1472–1476. [DOI] [PubMed] [Google Scholar]
- 12.McArthur J, Grant I. HIV neurocognitive disorders. In: Gendelman H, Lipton S, Epstein L, Swindells S, eds. The Neurology of AIDS. New York: International Thompson Publishing; 1998:499–523. [Google Scholar]
- 13.Heaton RK, Grant I, Butters N, et al. The HNRC 500: neuropsychology of HIV infection at different disease stages: HIV Neurobehavioral Research Center. J Int Neuropsychol Soc 1995;1:231–251. [DOI] [PubMed] [Google Scholar]
- 14.White DA, Heaton RK, Monsch AU. Neuropsychological studies of asymptomatic human immunodeficiency virus-type-1 infected individuals: The HNRC Group: HIV Neurobehavioral Research Center. J Int Neuropsychol Soc 1995;1:304–315. [DOI] [PubMed] [Google Scholar]
- 15.Cohen RA, Boland R, Paul R, et al. Neurocognitive performance enhanced by highly active antiretroviral therapy in HIV-infected women. AIDS 2001;15:341–345. [DOI] [PubMed] [Google Scholar]
- 16.Ferrando S, van Gorp W, McElhiney M, Goggin K, Sewell M, Rabkin J. Highly active antiretroviral treatment in HIV infection: benefits for neuropsychological function. AIDS 1998;12:F65–F70. [DOI] [PubMed] [Google Scholar]
- 17.Letendre SL, McCutchan JA, Childers ME, et al. Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Ann Neurol 2004;56:416–423. [DOI] [PubMed] [Google Scholar]
- 18.Tozzi V, Balestra P, Galgani S, et al. Positive and sustained effects of highly active antiretroviral therapy on HIV-1-associated neurocognitive impairment. AIDS 1999;13:1889–1897. [DOI] [PubMed] [Google Scholar]
- 19.Pallella FJ, Chmiel JS, Moorman AC, Holmberg SD, Investigators tHOS. Durability and predictors of sucess of highly active antiretroviral therapy for ambulatory HIV-infected patients. AIDS 2002;16:1617–1626. [DOI] [PubMed] [Google Scholar]
- 20.Paredes R, Mocroft A, Kirk O, et al. Predictors of virological success and ensuing failure in HIV-positive patients starting highly active antiretroviral therapy in Europe: results from the EuroSIDA study. Arch Intern Med 2000;160:1123–1132. [DOI] [PubMed] [Google Scholar]
- 21.Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev 2005;48:16–42. [DOI] [PubMed] [Google Scholar]
- 22.Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci 2007;8:33–44. [DOI] [PubMed] [Google Scholar]
- 23.Gisolf EH, van Praag RM, Jurriaans S, et al. Increasing cerebrospinal fluid chemokine concentrations despite undetectable cerebrospinal fluid HIV RNA in HIV-1-infected patients receiving antiretroviral therapy. J Acquir Immune Defic Syndr 2000;25:426–433. [DOI] [PubMed] [Google Scholar]
- 24.Kaul M, Lipton SA. Mechanisms of neuroimmunity and neurodegeneration associated with HIV-1 infection and AIDS. J Neuroimmune Pharmacol 2006;1:138–151. [DOI] [PubMed] [Google Scholar]
- 25.Ances BM, Sisti D, Vaida F, et al. Resting cerebral blood flow: a potential biomarker of the effects of HIV in the brain. Neurology 2009;73:702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lentz MR, Kim WK, Lee V, et al. Changes in MRS neuronal markers and T cell phenotypes observed during early HIV infection. Neurology 2009;72:1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cysique LA, Vaida F, Letendre S, et al. Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology 2009;73:342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cysique LA, Deutsch R, Atkinson JH, et al. Incident major depression does not affect neuropsychological functioning in HIV-infected men. J Int Neuropsychol Soc 2007;13:1–11. [DOI] [PubMed] [Google Scholar]
- 29.Marcotte TD, Wolfson T, Rosenthal TJ, et al. A multimodal assessment of driving performance in HIV infection. Neurology 2004;63:1417–1422. [DOI] [PubMed] [Google Scholar]
- 30.Ellis RJ, Hsia K, Spector SA, et al. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome: HIV Neurobehavioral Research Center Group. Ann Neurol 1997;42:679–688. [DOI] [PubMed] [Google Scholar]
- 31.Mayeux R, Stern Y, Tang MX, et al. Mortality risks in gay men with human immunodeficiency virus infection and cognitive impairment. Neurology 1993;43:176–182. [DOI] [PubMed] [Google Scholar]