Abstract
Objectives:
Circulating endothelial progenitor cells (EPC) are markers of vascular injury and their numbers decrease in acute stroke. However, the relation of EPC levels to stroke severity has not been quantified. MRI measurements of lesion volume provide an objective method for stroke severity assessment and outcome prediction. This cross-sectional study aims to determine whether EPC are correlated with lesion volume at baseline, lesion growth, and final lesion volume.
Methods:
Seventeen patients (median age 63 years, NIH Stroke Scale score 7) were selected from 175 patients with imaging-confirmed acute ischemic stroke. EPC were quantified by flow cytometry using CD34, CD133, and VEGFR2 surface markers. Brain MRI was performed at baseline and at days 1 and 5 after the stroke onset. Stroke lesion volumes were quantified.
Results:
Larger lesion volumes measured on diffusion-weighted images (DWI) at baseline were associated with low EPC levels, while smaller lesion volumes and less lesion growth were linked with high levels of EPC subsets (CD34+CD133+, CD133+VEGFR2+, and CD34+ CD133+VEGFR2+). Similar results were observed with DWI lesion volumes and EPC (CD34+CD133+) on day 1. Lesion growth volume, represented as a difference between final lesion volume and baseline DWI, was larger in patients with lower day 1 EPC (CD133+VEGFR2+). After adjustments for age and admission glucose (model 1), mean arterial pressure and white blood cells (model 2), INR and hematocrit (model 3), the CD34+CD133+ subset remained predictive of baseline and day 1 lesion volumes, while CD133+VEGFR2+ predicted baseline lesion volume and growth of lesion volume.
Conclusions:
Higher EPC levels were indicative of smaller volumes of acute lesion, final lesion, and lesion growth, and may serve as markers of acute phase stroke severity. However, a larger prospective study is needed to confirm our findings.
GLOSSARY
- DBP
= diastolic blood pressure;
- DWI
= diffusion-weighted images;
- EPC
= endothelial progenitor cells;
- FLAIR
= fluid-attenuated inversion recovery;
- Hb
= hemoglobin;
- INR
= international normalized ratio;
- LSN
= last seen normal;
- NIHSS
= NIH Stroke Scale score;
- WBC
= white blood cells;
- WHC
= Washington Hospital Center.
Endothelial progenitor cells (EPC) were proposed as markers of vascular injury in acute myocardial infarction,1 limb ischemia,2 and stroke.3 EPC are immature hematopoietic cells circulating in peripheral blood, capable of differentiating into mature endothelial cells to aid endothelial recovery.2 In stroke patients, EPC numbers are decreased and inversely correlate with age,3 cardiovascular risk factors,3 stroke type,4 and functional stroke outcome.5
Acute6 and final7 lesion volumes measured on MRI and lesion growth volume provide objective, quantitative measurement of stroke severity and outcome. Recently, an inverse association between EPC-colony forming units and lesion volume growth was found5; however, a relationship between acute lesion volume and EPC has not been demonstrated.3 The aim of this study was to establish the associations between circulating EPC numbers and baseline lesion, lesion growth, and infarct volumes.
METHODS
Study population.
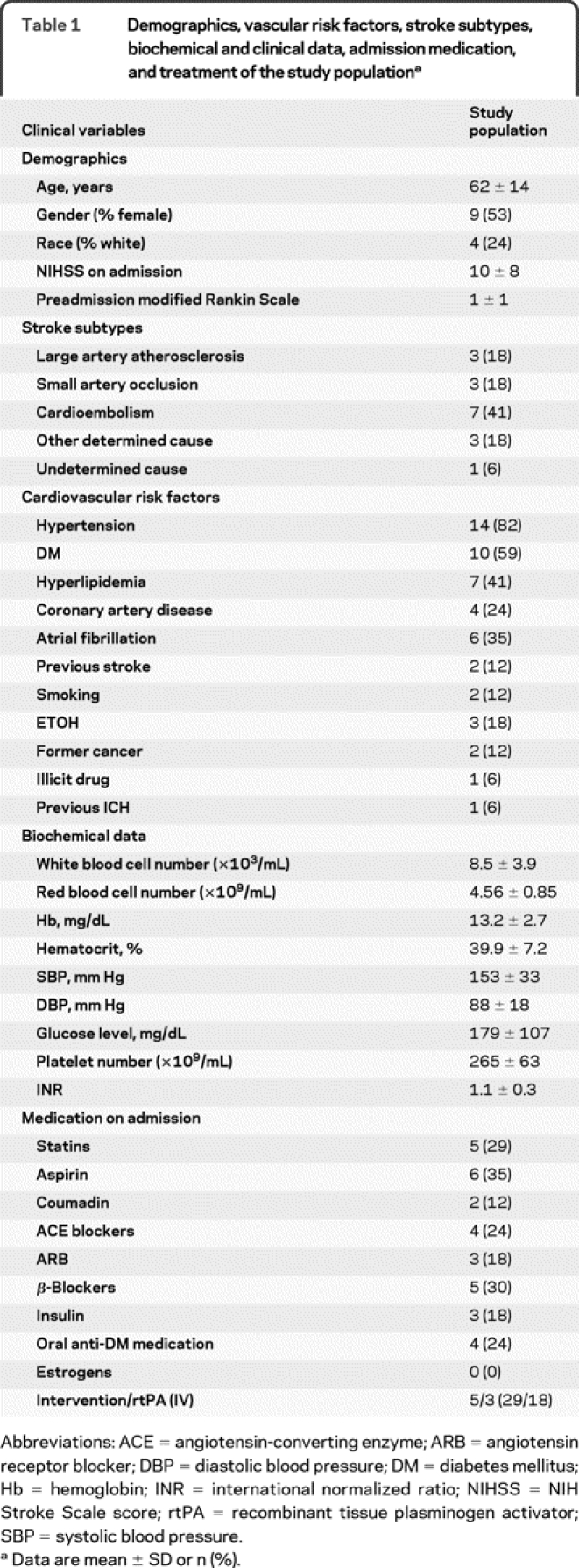
This is a prospective pilot study of patients with imaging-confirmed acute stroke admitted to the NIH Stroke Center at Washington Hospital Center (WHC) between October 2008 and May 2009. The study population consisted of 323 patients referred upon suspicion of an acute cerebrovascular event. Forty (12%) of these patients were diagnosed with hemorrhagic stroke, 13 (4%) with TIA, 96 (30%) with stroke mimics, and 175 (54%) had imaging-confirmed ischemic stroke. Forty-one (23%) of the admitted ischemic stroke patients participated in the natural history protocol. From this subgroup, patients with contraindications to MRI, known hematologic, renal, hepatic, or infectious disease, or active malignancy were excluded. The study population included 17 patients (10% of all ischemic stroke patients) and did not differ from the stroke cohort admitted to the hospital; however, the percentage of patients receiving recombinant tissue plasminogen activator was higher in the study group (table 1).
Table 1 Demographics, vascular risk factors, stroke subtypes, biochemical and clinical data, admission medication, and treatment of the study population
Standard protocol approvals, registration, and patient consent.
The protocol was approved by the NIH and WHC ethics committee. Written informed consent was obtained from all patients participating in the study.
Brain MRI and lesion volume measurements.
MRI was performed using a 3T (Philips Medical Systems) clinical scanner.8 Baseline DWI (baseline lesion volume) and MTT (mean transit time) were performed 9 ± 8 hours after last seen normal (LSN). Day 1 DWI and MTT were performed 37 ± 19 hours and fluid-attenuated inversion recovery (FLAIR) (final lesion volume) was performed at 10 ± 13 days after LSN. Lesion volumes were measured from DWI, MTT, and FLAIR series.9 Growth of lesion volume was calculated as a difference between final FLAIR and baseline DWI lesion volumes. Baseline DWI lesion volume was 17 (3–42) and MTT was 32 (4–136) mL (medians and first and third quartile). Day 1 DWI lesion volume was 20 (5–46) and MTT 32 (0.02–111) mL. FLAIR was 27 (4–76) mL, and lesion growth volume was 9 (1–39) mL.
Blood collection.
Peripheral blood was collected at 31 ± 13 (day 1, n = 17) and 76 ± 29 hours (day 3, n = 9) after LSN. Samples were centrifuged at 2,000 ×g at 25°C for 25 minutes, and the isolated mononuclear cells were stored in autologous serum at −80°C until analysis.10
The expression of cell surface antigens was determined by 4-color immunofluorescence staining. Briefly, 2 × 106 cells were incubated in 120 μL buffered saline containing 2% BSA with 20 μL Fc-blocking agent (Miltenyi Biotech) for 10 minutes at 25°C. Thereafter, the cells were incubated at 4°C for 30 minutes with 20 μL CD133/AC133-PE (Miltenyi Biotech), 20 μL VEGFR2-FITC (R&D Systems), and 20 μL CD34-ECD (Beckman Coulter) in a total volume of 200 μL. The cells were washed twice before resuspension in 400 μL stain buffer (BD Biosciences). Just prior to analysis on a FACS Vantage SE (BD Biosciences), DAPI nuclear dye was added to the cell suspension to allow for viability gating that was constantly 70 ± 3%, across the 2 collection timepoints for all patients. A minimum of 1 × 106 live cells were collected, and FACS analysis was performed in triplicate for each sample. Medians of 3 measurements were calculated, and resulting EPC counts are expressed as a percentage of total mononuclear cells in each sample.
Statistical analysis.
Correlations were made using the 2-tailed Spearman rank test. Differences between groups were assessed by the Mann-Whitney test, and between repeated measurements by the Wilcoxon signed rank test. For adjustments of factors influencing EPC levels, a standard multiple regression with several models each including 3 variables was used. SPSS version 16.0 was used with the level of significance at 0.05.
RESULTS
EPC subsets in the stroke population were quantified at day 1, as follows: 0.02% (0.01–0.04) CD133+CD34+, 0.01% (0.001–0.02) CD133+ VEGFR2+, 0.002% (0.0006–0.004) CD34+ VEGFR2+, and 0.0009% (0.0003–0.0018) CD34+ CD133+VEGFR2+ (medians and first and third quartile). EPC measurements were also taken on day 3, and because no differences were observed between day 1 and 3 measurements, we used day 1 measurements for our calculations.
No differences in EPC regarding gender, ethnicity, or Rankin scale of the stoke population were found. The patients with previous stroke had increased CD34+VEGFR2+ (p = 0.03) and CD34+CD133+VEGFR2+ (p = 0.02) EPC subsets. Patients with nonactive cancer had increased subset CD133+CD34+ (p = 0.03). Neither influence of other cardiovascular risk factors on EPC levels nor differences in EPC among the stroke subtypes were found. Patients on β-blockers at admission had increased numbers of CD133+VEGFR2+ (p = 0.02) and CD34+VEGFR2+ (p = 0.04) subsets; however, no other treatment was associated with EPC changes.
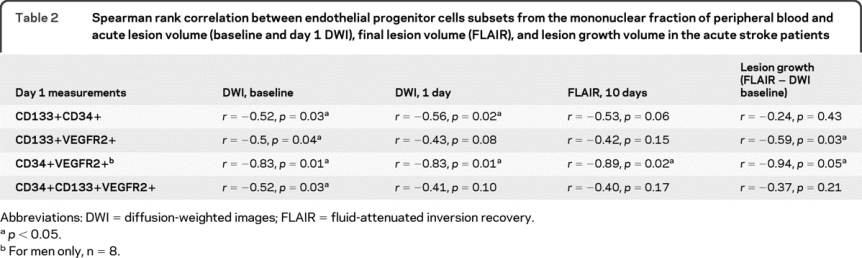
DWI lesion volumes at baseline were smaller in patients with higher numbers of CD133+ CD34+, CD133+VEGFR2+, and CD34+ CD133+VEGFR2+. DWI lesion volumes at day 1 were smaller in patients with higher CD133+CD34+ subset. Final lesion volume (FLAIR) was larger in male stroke patients with low CD34+VEGFR2+ levels. Lesion growth volume was increased in patients with low CD133+VEGFR2+ (table 2). However, no association between age, NIH Stroke Scale score (NIHSS), modified Rankin scale, systolic blood pressure, diastolic blood pressure (DBP), white blood cells (WBC), red blood cells, hemoglobin (Hb), hematocrit, glucose, platelets, international normalized ratio (INR), and MTT volumes and EPC subsets was found.
Table 2 Spearman rank correlation between endothelial progenitor cells subsets from the mononuclear fraction of peripheral blood and acute lesion volume (baseline and day 1 DWI), final lesion volume (FLAIR), and lesion growth volume in the acute stroke patients
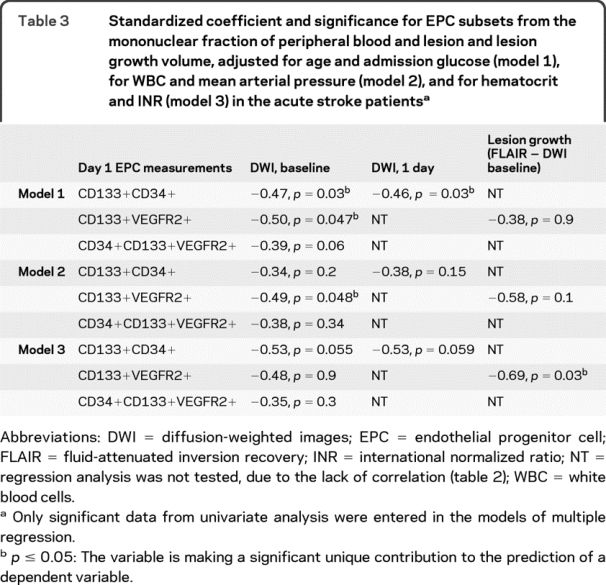
After adjustment for factors influencing EPC (age and admission glucose), the levels of CD133+CD34+ and CD133+VEGFR2+ remained the most significant predictors of lesion volume at baseline, while the number of CD133+ CD34+ was the best predictor of day 1 lesion volume (table 3, model 1). Adjustment for mean arterial pressure and admission WBC showed that the CD133+VEGFR2+ subset was a significant predictor of the baseline lesion volume (table 3, model 2). INR and hematocrit adjustments revealed CD133+VEGFR2+ cells as a significant predictor of lesion volume growth (table 3, model 3). After adjustment for NIHSS, EPC subset levels were no longer predictive of lesion volumes.
Table 3 Standardized coefficient and significance for EPC subsets from the mononuclear fraction of peripheral blood and lesion and lesion growth volume, adjusted for age and admission glucose (model 1), for WBC and mean arterial pressure (model 2), and for hematocrit and INR (model 3) in the acute stroke patients
DISCUSSION
This pilot study provides novel data regarding the link between EPC subsets and quantitative MRI measurements of lesion volume and lesion growth volume, which serve as surrogate markers of stroke severity.6,7
Acute ischemic stroke is associated with decreased EPC,4 while an increase of EPC relates to a good outcome.5 Accordingly, our study revealed that low EPC numbers indicate large lesion volumes, and higher EPC levels are linked with small ischemic lesions and smaller lesion growth. Despite the presence of various risk factors, comorbidities, and medications that could influence EPC levels in the stroke patients, higher EPC numbers were prognostic of smaller acute lesion volume, infarct volume, and lesion growth volume in acute stroke. After adjustments for major factors influencing EPC, the EPC levels remained predictive of lesion and lesion growth volume.
There were no differences in EPC levels between cardioembolic and large artery strokes. Increased EPC production influenced by estrogens in female patients may explain why the correlation between final lesion volume and EPC levels was significant only for male patients.
AUTHOR CONTRIBUTIONS
Tanya Bogoslovsky: study design, data collection, laboratory measurements, statistics, writing of the manuscript. Aneeka Chaudhry: established the method of EPC isolation and staining, support during actual measurements, review of the manuscript. Lawrence Latour: study design and data acquisition, statistical assistance, review of the manuscript. Dragan Maric: established the method of enumeration and characterization of EPC, review of the manuscript. Marie Luby: volume measurements, review of the manuscript. Maria Spatz: study design, review of the manuscript. Joseph Frank: study design, review of the manuscript. Steven Warach: study design, review of the manuscript.
ACKNOWLEDGMENT
The authors thank the members of the NIH Stroke Program at the Washington Hospital Center who assisted with data collection and patient care and Dr. Zurab Nadareishvili for comments during preparation of the manuscript.
DISCLOSURE
Dr. Bogoslovsky, A. Chaudhry, Dr. Latour, Dr. Maric, Dr. Luby, Dr. Spatz, and Dr. Frank report no disclosures. Dr. Warach serves on the editorial boards of the Journal of Cerebral Blood Flow and Metabolism, Stroke, The Lancet Neurology, and the International Journal of Stroke and receives research support from the NIH/NINDS, Division of Intramural Research.
Address correspondence and reprint requests to Dr. T. Bogoslovsky, Center for Neuroscience & Regenerative Medicine (CNRM), Uniformed Services University of the Health Sciences, 12725 Twinbrook Parkway, Rockville, MD 20852 Tanya.Bogoslovsky.CTR@usuhs.mil
Editorial, page 2050
Study funding: Supported by the Division of Intramural Research of the NIH/NINDS and by the Finnish Cultural Foundation, the Päivikki and Sakari Sohlberg Foundation, and the Paavo Nurmi Foundation.
Disclosure: Author disclosures are provided at the end of the article.
Received January 29, 2010. Accepted in final form July 1, 2010.
REFERENCES
- 1.Shintani S, Murohara T, Ikeda H, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation 2001;103:2776–2779. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–967. [DOI] [PubMed] [Google Scholar]
- 3.Taguchi A, Matsuyama T, Moriwaki H, et al. Circulating CD34-positive cells provide an index of cerebrovascular function. Circulation 2004;109:2972–2975. [DOI] [PubMed] [Google Scholar]
- 4.Chu K, Jung KH, Lee ST, et al. Circulating endothelial progenitor cells as a new marker of endothelial dysfunction or repair in acute stroke. Stroke 2008;39:1441–1447. [DOI] [PubMed] [Google Scholar]
- 5.Sobrino T, Hurtado O, Moro MA, et al. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke 2007;38:2759–2764. [DOI] [PubMed] [Google Scholar]
- 6.Lovblad KO, Baird AE, Schlaug G, et al. Ischemic lesion volumes in acute stroke by diffusion-weighted magnetic resonance imaging correlate with clinical outcome. Ann Neurol 1997;42:164–170. [DOI] [PubMed] [Google Scholar]
- 7.Saunders DE, Clifton AG, Brown MM. Measurement of infarct size using MRI predicts prognosis in middle cerebral artery infarction. Stroke 1995;26:2272–2276. [DOI] [PubMed] [Google Scholar]
- 8.Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol 2004;56:468–477. [DOI] [PubMed] [Google Scholar]
- 9.Luby M, Bykowski JL, Schellinger PD, Merino JG, Warach S. Intra- and interrater reliability of ischemic lesion volume measurements on diffusion-weighted, mean transit time and fluid-attenuated inversion recovery MRI. Stroke 2006;37:2951–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruitenberg JJ, Mulder CB, Maino VC, Landay AL, Ghanekar SA. VACUTAINER CPT and Ficoll density gradient separation perform equivalently in maintaining the quality and function of PBMC from HIV seropositive blood samples. BMC Immunol 2006;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]


