Abstract
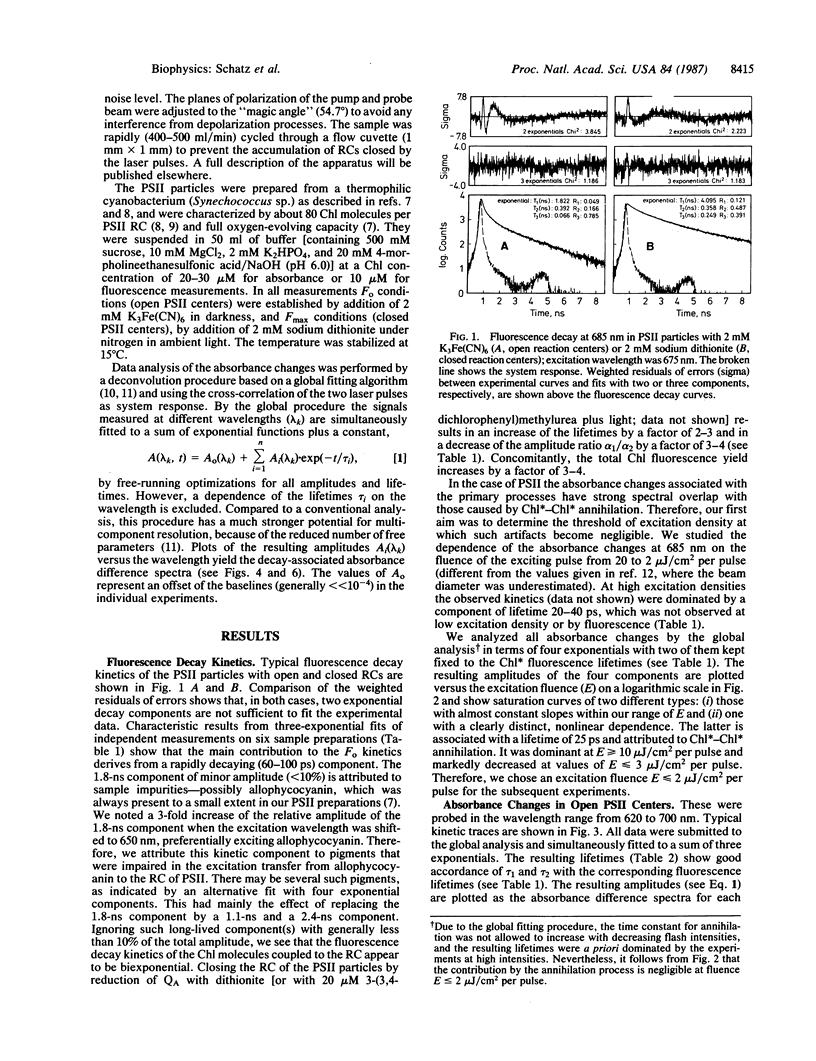
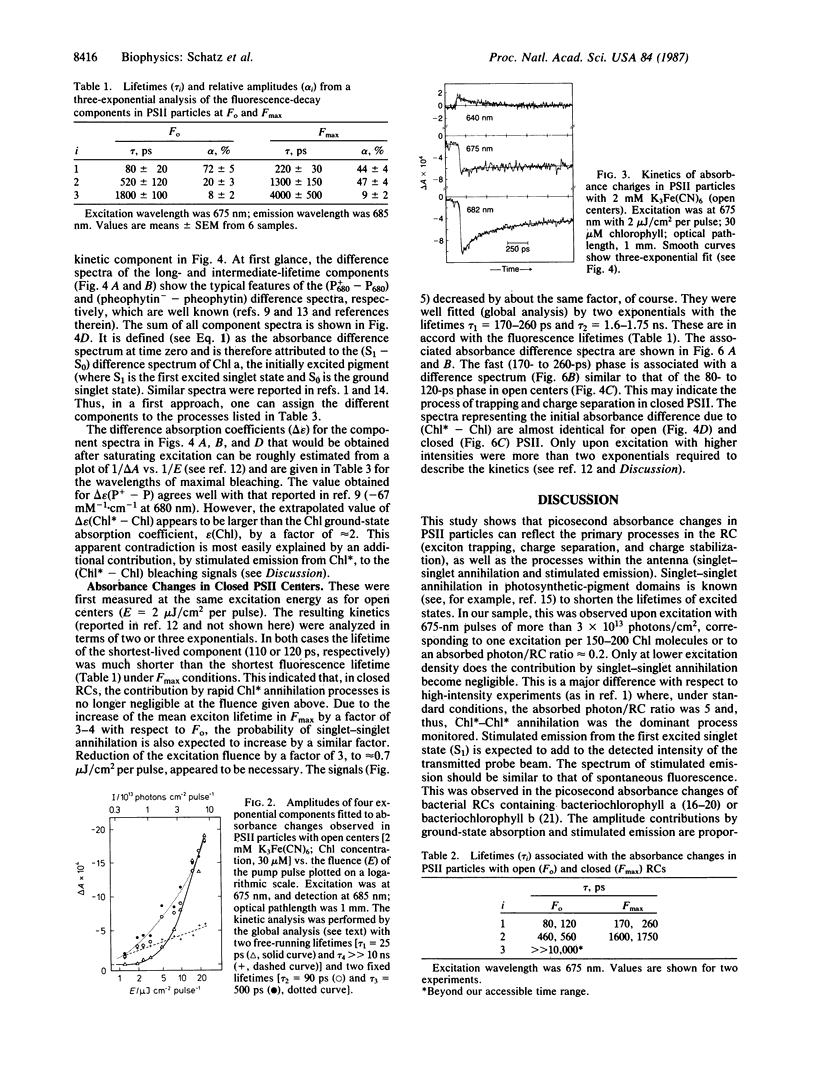
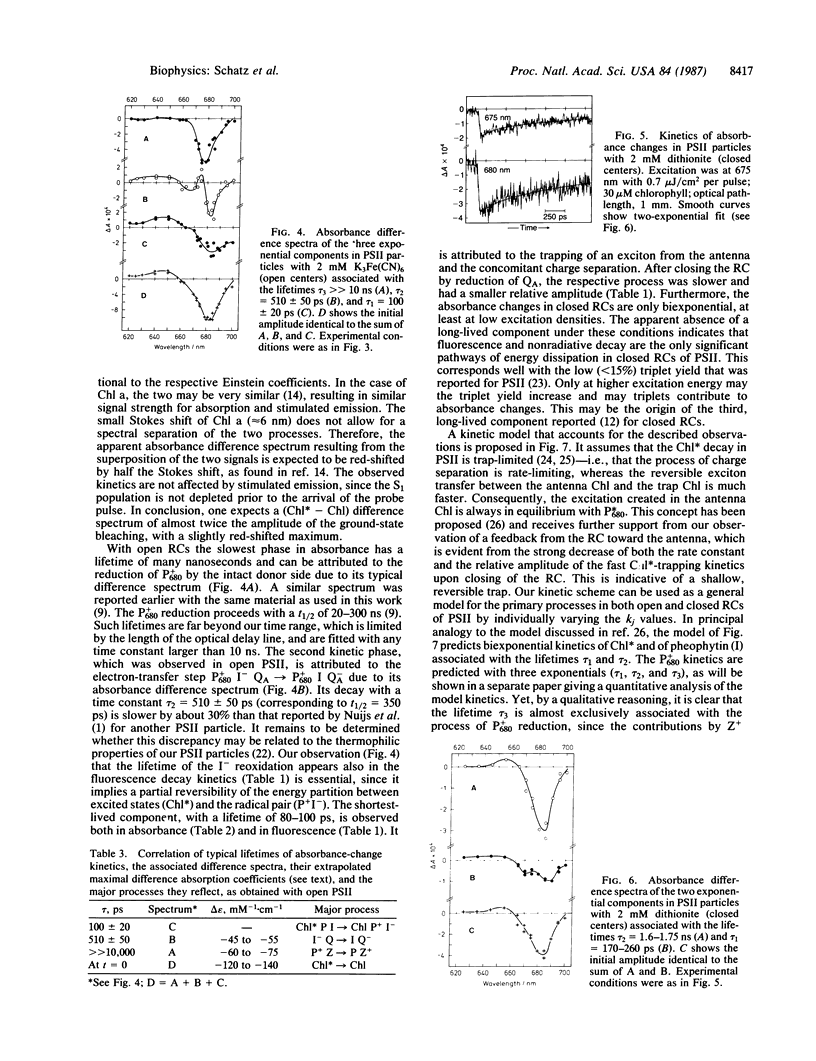
Oxygen-evolving photosystem II particles (from Synechococcus) with about 80 chlorophyll molecules per primary electron donor (P680) were used for a correlated study of picosecond kinetics of fluorescence and absorbance changes, detected by the single-photon-timing technique and by a pump-probe apparatus, respectively. Chlorophyll fluorescence decays were biexponential with lifetimes τ1 = 80 ± 20 ps and τ2 = 520 ± 120 ps in open reaction centers and τ1 = 220 ± 30 ps and τ2 = 1.3 ± 0.15 ns in closed reaction centers. The corresponding fluorescence yield ratio Fmax/Fo was 3-4. Absorbance changes were monitored in the spectral range of 620-700 nm after excitation at 675 nm with 10-ps pulses sufficiently weak (<7 × 1012 photons/cm2 per pulse) to avoid singlet-singlet annihilation. With open reaction centers, the absorbance changes could be fit to the sum of three exponentials. The associated absorbance difference spectra were attributed to (i) exciton trapping and charge separation (τ = 100 ± 20 ps), (ii) the electron-transfer step P680+ I- QA → P680+ I QA- (where I is the primary electron acceptor and QA is the first quinone acceptor) (τ = 510 ± 50 ps), and (iii) the reduction of P680+ by the intact donor side (τ > 10 ns). With closed reaction centers, the absorbance changes were biexponential with lifetimes τ1 = 170-260 ps and τ2 = 1.6-1.75 ns. The results are explained in terms of a kinetic model that assumes P680 to constitute a shallow trap. The results show that QA reduction in these photosystem II particles decreases both the apparent rate and the yield of the primary charge separation by a factor of 2-3 and increases the mean lifetime of excitons in the antenna by a factor of 3-4. Thus, we conclude that the long-lived, nanosecond chlorophyll fluorescence is not charge-recombination luminescence but rather emission from equilibrated excited states of antenna chlorophylls.
Keywords: excitation trapping, charge separation, electron transfer, pheophytin a, cyanobacterium
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breton J., Geacintov N. E. Picosecond fluorescence kinetics and fast energy transfer processes in photosynthetic membranes. Biochim Biophys Acta. 1980 Dec 22;594(1):1–32. doi: 10.1016/0304-4173(80)90011-7. [DOI] [PubMed] [Google Scholar]
- Breton J., Martin J. L., Migus A., Antonetti A., Orszag A. Femtosecond spectroscopy of excitation energy transfer and initial charge separation in the reaction center of the photosynthetic bacterium Rhodopseudomonas viridis. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5121–5125. doi: 10.1073/pnas.83.14.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzwarth A. R., Wendler J., Suter G. W. Studies on Chromophore Coupling in Isolated Phycobiliproteins: II. Picosecond Energy Transfer Kinetics and Time-Resolved Fluorescence Spectra of C-Phycocyanin from Synechococcus 6301 as a Function of the Aggregation State. Biophys J. 1987 Jan;51(1):1–12. doi: 10.1016/S0006-3495(87)83306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karukstis K. K., Sauer K. Fluorescence decay kinetics of chlorophyll in photosynthetic membranes. J Cell Biochem. 1983;23(1-4):131–158. doi: 10.1002/jcb.240230112. [DOI] [PubMed] [Google Scholar]
- Klimov V. V., Klevanik A. V., Shuvalov V. A., Kransnovsky A. A. Reduction of pheophytin in the primary light reaction of photosystem II. FEBS Lett. 1977 Oct 15;82(2):183–186. doi: 10.1016/0014-5793(77)80580-2. [DOI] [PubMed] [Google Scholar]
- Kramer H., Mathis P. Quantum yield and rate of formation of the carotenoid triplet state in photosynthetic structures. Biochim Biophys Acta. 1980 Dec 3;593(2):319–329. doi: 10.1016/0005-2728(80)90069-9. [DOI] [PubMed] [Google Scholar]
- Martin J. L., Breton J., Hoff A. J., Migus A., Antonetti A. Femtosecond spectroscopy of electron transfer in the reaction center of the photosynthetic bacterium Rhodopseudomonas sphaeroides R-26: Direct electron transfer from the dimeric bacteriochlorophyll primary donor to the bacteriopheophytin acceptor with a time constant of 2.8 +/- 0.2 psec. Proc Natl Acad Sci U S A. 1986 Feb;83(4):957–961. doi: 10.1073/pnas.83.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauzerall D. C. Evidence that the variable fluorescence in Chlorella is recombination luminescence. Biochim Biophys Acta. 1985 Aug 28;809(1):11–16. doi: 10.1016/0005-2728(85)90161-6. [DOI] [PubMed] [Google Scholar]
- Shuvalov V. A., Duysens L. N. Primary electron transfer reactions in modified reaction centers from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1690–1694. doi: 10.1073/pnas.83.6.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury N. W., Becker M., Middendorf D., Parson W. W. Picosecond kinetics of the initial photochemical electron-transfer reaction in bacterial photosynthetic reaction centers. Biochemistry. 1985 Dec 17;24(26):7516–7521. doi: 10.1021/bi00347a002. [DOI] [PubMed] [Google Scholar]



