Abstract
Recent studies using magnetic resonance spectroscopy have shown that decreased insulin-stimulated muscle glycogen synthesis due to a defect in insulin-stimulated glucose transport activity is a major factor in the pathogenesis of type 2 diabetes. The molecular mechanism underlying defective insulin-stimulated glucose transport activity can be attributed to increases in intramyocellular lipid metabolites such as fatty acyl CoAs and diacylglycerol, which in turn activate a serine/threonine kinase cascade, thus leading to defects in insulin signaling through Ser/Thr phosphorylation of insulin receptor substrate (IRS)-1. A similar mechanism is also observed in hepatic insulin resistance associated with nonalcoholic fatty liver, which is a common feature of type 2 diabetes, where increases in hepatocellular diacylglycerol content activate protein kinase C-ε, leading to reduced insulin-stimulated tyrosine phosphorylation of IRS-2. More recently, magnetic resonance spectroscopy studies in healthy lean elderly subjects and healthy lean insulin-resistant offspring of parents with type 2 diabetes have demonstrated that reduced mitochondrial function may predispose these individuals to intramyocellular lipid accumulation and insulin resistance. Further analysis has found that the reduction in mitochondrial function in the insulin-resistant offspring can be mostly attributed to reductions in mitochondrial density. By elucidating the cellular and molecular mechanisms responsible for insulin resistance, these studies provide potential new targets for the treatment and prevention of type 2 diabetes. Diabetes 55 (Suppl. 2):S9–S15, 2006
Type 2 diabetes is rapidly emerging as one of the greatest global health challenges of the 21st century. The World Health Organization estimates that by the year 2030, ~366 million people will be afflicted with diabetes (1). This looming epidemic is also expected to trigger a steep rise in the complications associated with diabetes, such as ischemic heart disease, stroke, neuropathy, retinopathy, and nephropathy.
Developing better treatments and novel prevention strategies for type 2 diabetes is therefore a matter of great urgency. To accomplish this goal, it is necessary to better understand the pathogenesis of type 2 diabetes. Although the underlying cause remains unknown, recent studies have clearly demonstrated that insulin resistance plays a critical role in the development of type 2 diabetes (2–5). This brief review will focus on recent magnetic resonance spectroscopy (MRS) studies that have shed new light on the cellular and molecular mechanisms of insulin resistance in humans.
EARLY DEFECTS IN THE PATHOGENESIS OF TYPE 2 DIABETES
Over the past 2 decades, our group has extensively used in vivo MRS to noninvasively probe the cellular and molecular mechanisms of insulin resistance in humans. This technique is ideal for human studies, since it is capable of monitoring particular intracellular metabolite concentrations and kinetics noninvasively and in real time (6–22). Furthermore, in contrast to most other comparable imaging modalities such as positron emission tomography, MRS accomplishes this without the use of ionizing radiation.
Applying 13C MRS to examine rates of insulin-stimulated muscle glycogen synthesis in humans, we were able to demonstrate that skeletal muscle accounts for the majority of insulin-stimulated glucose uptake in both patients with type 2 diabetes as well as age- and weight-matched nondiabetic volunteers (6). Furthermore, we found that the rate of insulin-stimulated muscle glycogen synthesis was decreased by over 50% in patients with type 2 diabetes and that this was the major factor responsible for their insulin resistance under these hyperinsulinemic (~80 µU/ ml)-hyperglycemic (10 mmol/l) clamp conditions (6). To determine the rate-controlling step responsible for this reduced insulin-stimulated rate of muscle glycogen synthesis, we applied 13C and 31P MRS to monitor intracellular glucose, glucose-6-phosphate concentrations, and intramuscular glycogen synthesis under similar conditions (7,8). Using this multinuclear MRS approach, we were able to determine that glucose transport was the rate-controlling step for insulin-stimulated muscle glycogen synthesis in type 2 diabetes, rather than hexokinase. Therefore, glucose transport represents the best target to correct insulin resistance in skeletal muscle in patients with type 2 diabetes. The corollary to these findings is that hexokinase and glycogen synthase activators are probably not very good targets to reverse insulin resistance in skeletal muscle. Indeed, mice with overexpression of hexokinase in skeletal muscle (23) and rats treated with a glycogen synthase kinase-3 inhibitor to activate glycogen synthase (24) were not protected from fat-induced insulin resistance in skeletal muscle.
To gain further insights into the pathogenesis of insulin resistance in skeletal muscle, we examined insulin-stimulated muscle glucose uptake in young lean nonsmoking insulin-resistant offspring of parents with type 2 diabetes. Warram et al. (2) previously demonstrated that these individuals have a high predisposition for developing type 2 diabetes and that insulin resistance is the best predictor for them developing type 2 diabetes. The advantage of studying this cohort of individuals is that they have none of the other confounding factors that may contribute to insulin resistance, such as obesity or hyperglycemia, and the pathogenesis of type 2 diabetes can be examined at its earliest time points. Using similar 13C/31P MRS methods, we found that, like their parents with type 2 diabetes, defective insulin-stimulated muscle glycogen synthesis due to defects in insulin-stimulated glucose transport/ phosphorylation was the major factor responsible for their skeletal muscle insulin resistance (9,10). These data suggest that reduced insulin-stimulated glucose transport/ phosphorylation activity is a very early event in the pathogenesis of type 2 diabetes.
To search for mechanisms responsible for reduced insulin-stimulated glucose transport/phosphorylation activity, we screened young lean offspring of type 2 diabetic parents and found that fasting plasma fatty acid concentrations were the best predictor for insulin resistance in this otherwise young healthy cohort (25). Subsequently, we and others have found that intramyocellular lipid content assessed by 1H MRS was an even better predictor for insulin resistance in skeletal muscle in both adults (11–13) and children (14). These data are consistent with a previous muscle punch biopsy study in Pima Indians that found a strong relationship between insulin resistance and intramuscular triglyceride content (26).
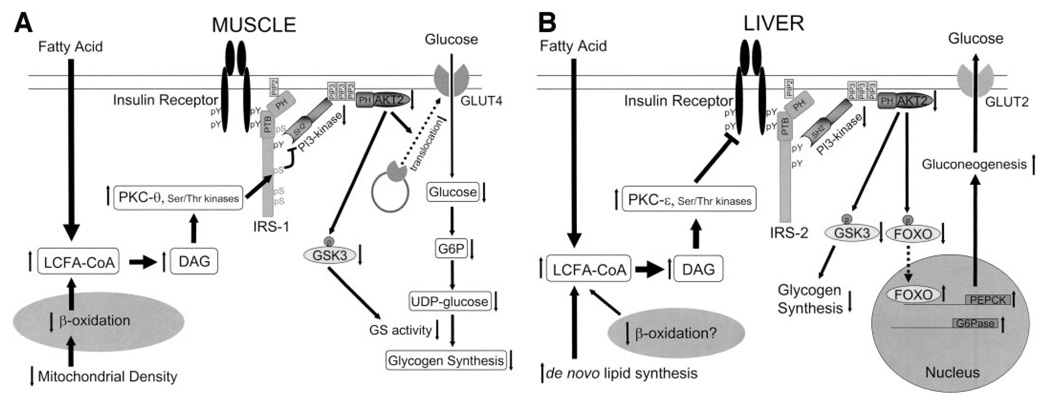
The mechanism of fat-induced insulin resistance in skeletal muscle has now been mostly elucidated (Fig. 1) (27). Insulin binds to the α subunit of the insulin receptor and activates the tyrosine kinase in the β subunit. The tyrosine kinase phosphorylates the insulin receptor substrate (IRS) proteins, and phosphotyrosine residues on IRS proteins become good targets for the p85 regulatory subunit of phosphatidylinositol (PI) 3-kinase, which in turns catalyzes phosphatidylinositol 4,5 diphosphate into phosphatidylinositol 3,4,5 triphosphate. Downstream molecules such as phosphatidylinositol-dependent kinase (PDK) and protein kinase B (PKB [AKT]) have a pleckstrin homology domain that enables these molecules to migrate toward the plasma membrane (28). In skeletal muscle, this PI 3-kinase–AKT activation is an essential step for insulin-induced GLUT4 translocation, leading to glucose uptake (29). Our group has shown that raising plasma fatty acids in both rodents (15,30) and humans (16) abolishes insulin activation of IRS-1–associated PI 3-kinase activity in skeletal muscle where IRS-1 is most prevalent. Further studies in rodents have pinpointed this defect to a block in insulin receptor tyrosine phosphorylation of IRS-1 (31).
FIG. 1.
The molecular mechanism of fat-induced insulin resistance in skeletal muscle (A) and liver (B). A: Increases in intramyocellular fatty acyl CoAs and diacylglycerol due to increased delivery from plasma and/or reduced β-oxidation due to mitochondrial dysfunction activate serine/threonine kinases such as protein kinase C (PKC-θ rodents, PKC-β and -δ humans) in skeletal muscle. The activated kinases phosphorylate serine residues on IRS-1 and inhibit insulin-induced PI 3-kinase activity, resulting in reduced insulin-stimulated AKT2 activity. Lowered AKT2 activity fails to activate GLUT4 translocation, and other downstream AKT2-dependent events, and consequently insulin-induced glucose uptake is reduced. B: Increases in hepatic diacylglycerol content due to increased delivery of fatty acids from the plasma and/or increased de novo lipid synthesis and/or reduced β-oxidation activate protein kinase C-ε (and potentially other serine kinases), leading to reduced insulin receptor kinase activity and reduced IRS-2 tyrosine phosphorylation, resulting in reduced insulin stimulation of glycogen synthase activation and decreased phosphorylation of forkhead box protein O (FOXO), leading to increased hepatic gluconeogenesis. DAG, diacylglycerol; PTB, phosphotyrosine binding domain; PH, pleckstrin homology domain; SH2, src homology domain; GSK3, glycogen synthase kinase-3.
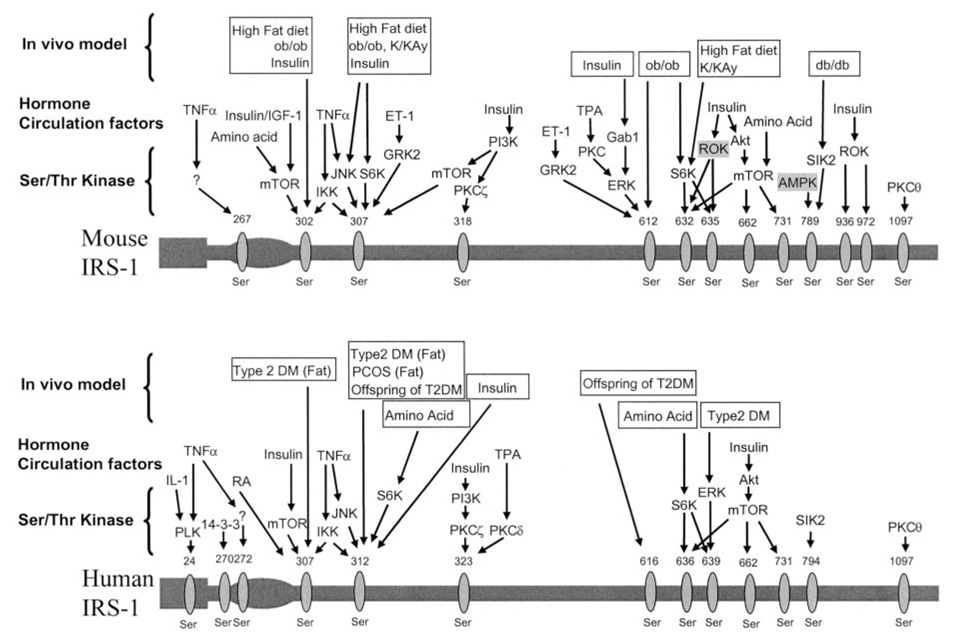
One possibility accounting for this insulin-signaling defect is serine phosphorylation on IRS-1 (Fig. 2). There are over 70 potential serine phosphorylation sites on IRS-1 (30–72), and in general, serine phosphorylation seems to negatively regulate IRS signaling, with a few exceptions (33, 55). Recent studies have demonstrated hyper-serine phosphorylation of IRS-1 on Ser302, Ser307, Ser612, and Ser632 in several insulin-resistant rodent models (Fig. 2) (30–34), as well as in lean insulin-resistant offspring of type 2 diabetic parents (17). Furthermore, high-fat diet–induced insulin resistance was abrogated in rodent models where certain Ser/Thr kinases (c-Jun NH2-terminal kinase [JNK], inhibitor of nuclear factor κB kinase β subunit [IKKβ], S6 kinase 1, and protein kinase C-θ) were either knocked down or pharmacologically inhibited (30,32–35). Further evidence for this hypothesis stems from recent studies in a muscle-specific triple serine to alanine mutant mouse (IRS-1 Ser → Ala302, Ser → Ala307, and Ser → Ala612), which has been shown to be protected from high-fat diet–induced insulin resistance in vivo (36). Based on in vitro studies, serine phosphorylation may lead to dissociation between insulin receptor/IRS-1 and/or IRS-1/PI 3-kinase, preventing PI 3-kinase activation (37–40) or increasing degradation of IRS-1 (41). Taken together, these data suggest that serine phosphorylation on key residues of IRS-1 has an important role in the pathogenesis of muscle insulin resistance. The mechanism of Ser/Thr kinase activation in vivo is still not clear but appears to be secondary to intracellular increases in long-chain fatty acyl CoAs and diacylgycerol (31). Diacylglycerol is an attractive trigger for fat-induced insulin resistance in skeletal muscle, since it has been shown to increase in muscle during both lipid infusions and fat feeding and it is a known activator of novel protein kinase C (PKC) isoforms. In this regard, PKC-θ and PKC-β and -δ have been shown to be activated during a lipid infusion in rodents (15,31) and humans (42), respectively. Furthermore PKC-θ knockout mice have been shown to be protected from fat-induced insulin resistance in skeletal muscle (30). Intracellular triglyceride (73) and ceramide (74) have also been implicated in fat-induced insulin resistance in muscle; however, recent lipid infusion studies have been able to disassociate fat-induced insulin resistance in skeletal muscle from any increases in ceramide and triglyceride, suggesting that these metabolites may represent more of a marker than the trigger for fat-induced insulin resistance (31).
FIG. 2.
Serine/threonine phosphorylation on IRS-1. IRS-1 contains ~70 potential serine/threonine sites. Shown here are the individual residues on mouse IRS-1 and human IRS-1. In addition, the serine/threonine kinases, hormone, and circulating factors that lead to the phosphorylation on IRS-1 at specific sites are shown. The top row illustrates reported animal or human models in which these phosphorylation sites have been confirmed in vivo. Each residue is drawn corresponding to the position based on homology between the humans and mice. PCOS, polycystic ovarian syndrome; T2DM, type 2 diabetes.
Recent studies have revealed a similar mechanism for fat-induced insulin resistance in liver (Fig. 1B), where accumulation of intracellular lipid metabolites activate a serine kinase cascade involving PKC-ε, leading to decreased insulin receptor kinase activity resulting in 1) lower insulin-stimulated IRS-2 tyrosine phosphorylation, 2) lower IRS-2–associated PI 3-kinase activity, and 3) lower AKT2 activity (43). These fat-induced defects in insulin signaling in turn result in reduced insulin stimulation of glycogen synthase activity, resulting in decreased insulin-stimulated hepatic glucose uptake and reduced insulin stimulation of hepatic glucose production. Furthermore, reduced activity of AKT2 results in decreased phosphorylation of forkhead box protein O (FOXO), allowing it to enter the nucleus and activate the transcription of the rate-controlling enzymes of gluconeogenesis (phospho-enolpyruvate carboxykinase, glucose-6-phosphate phos-phatase). Increased gluconeogenesis further exacerbates hepatic insulin resistance and results in fasting hyperglycemia (43,75,76). Mitochondrial glycerol-3-phosphate acyltransferase (mtGPAT) is a key enzyme in de novo fat synthesis in liver, and recent studies in mtGPAT knockout mice have clearly implicated intracellular accumulation of diacylglycerol in triggering fat-induced insulin resistance in liver through activation of PKC-ε (77). These data have important implications for the development of novel therapeutic agents to reverse and prevent hepatic insulin resistance associated with nonalcoholic fatty liver and type 2 diabetes (18).
INSULIN RESISTANCE AND MITOCHONDRIAL DYSFUNCTION
It has been known for many years that severe mitochondrial dysfunction can result in diabetes that is typically associated with severe β-cell dysfunction and neurological abnormalities (78–80). More recent MRS studies, measuring rates of mitochondrial oxidative phosphorylation activity in skeletal muscle, have found that more subtle defects in mitochondrial function may play an important role in the pathogenesis of type 2 diabetes. Using 13C/31P MRS, we directly assessed mitochondrial oxidative and phosphorylation activity in the healthy lean elderly volunteers with severe muscle insulin resistance and found that they have an ~40% reduction in rates of oxidative phosphorylation activity associated with increased intramyo-cellular and intrahepatic lipid content compared with BMI activity–matched young control subjects (19). This study suggests that an acquired loss of mitochondrial function associated with aging predisposes elderly subjects to intramyocellular lipid accumulation, which results in insulin resistance through the mechanisms described earlier (Fig. 1).
Using a similar approach, we used 31P MRS to examine mitochondrial function in young lean insulin-resistant offspring of parents with type 2 diabetes (20). Insulin resistance in these individuals could be attributed to severe defects in insulin-stimulated muscle glucose metabolism, which were associated with an ~80% increase in intramyocellular lipid content assessed by 1H MRS. Furthermore, these changes were associated with a 30% reduction in rates of mitochondrial ATP production compared with age-, weight-, and activity-matched insulin-sensitive control subjects (20). To further examine the mechanism responsible for reduced mitochondrial activity in these subjects, we recently assessed mitochondrial density by electron microscopy and found that mitochondrial density was reduced by 38% in the insulin-resistant offspring (17). Taken together, these data suggest that the reduced mitochondrial function observed in the insulin-resistant offspring may be secondary to reduced mitochondrial content and is consistent with previous studies demonstrating lower mitochondrial density in patients with type 2 diabetes (81). Ritov et al. (82) also reported the subsarcolemmal fraction was especially impaired in obese and type 2 diabetic subjects. In agreement with the finding of decreased mitochondrial density using electron microscopy, we also measured the expression of several mitochondrial proteins and found mitochondrial cytochrome-c oxidase I to be reduced by ~50% in insulin-resistant offspring and a tendency for succinate dehydrogenase and pyruvate dehydrogenase to be reduced by a similar amount (17). Taken together, these data suggest that insulin-resistant offspring of patients with type 2 diabetes may have an inherited condition that causes a reduction in mitochondrial content in skeletal muscle, which in turn may be responsible for the reduced rates of mitochondrial oxidative phosphorylation predisposing them to intramyocellular lipid accumulation.
MECHANISM OF REDUCED MITOCHONDRIAL BIOGENESIS
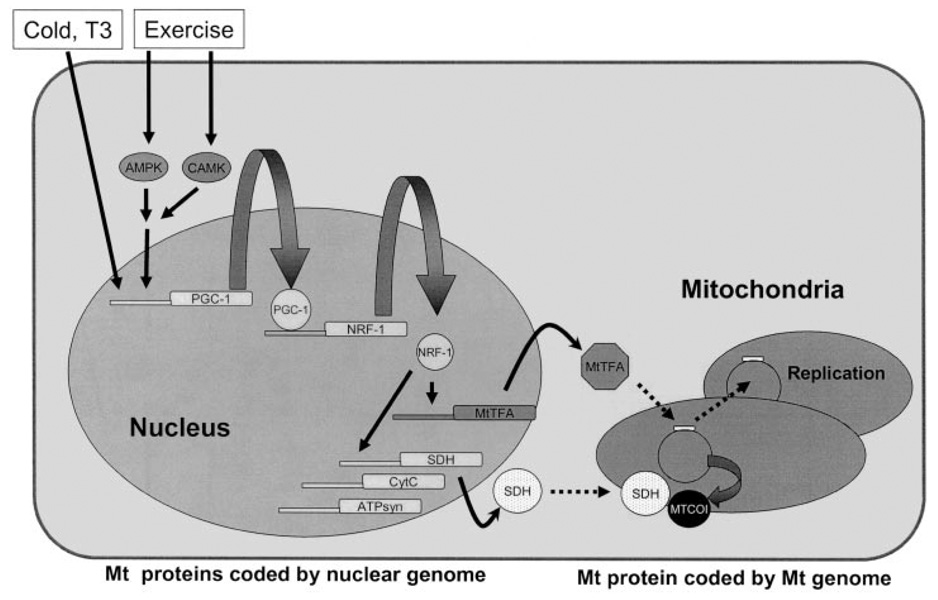
An important remaining question is the identification of the factors responsible for the lower mitochondrial density in skeletal muscle of the insulin-resistant offspring. Peroxisome proliferator–activated receptor-γ coactivator (PGC)-1α and PGC-1β are transcriptional factor co-activators that regulate mitochondrial biogenesis and potential candidates in this regard (Fig. 3). In addition AMP kinase, which is activated during exercise and ischemia by a reduction in the ATP/AMP ratio, has been shown to be an important regulator of mitochondrial biogenesis, mediating its effects through MEF2- and CREB-mediated increased PGC-1α expression (83–86). Extracellular stimuli such as cold, thyroid hormone, and exercise stimulate mitochondrial biogenesis through PGC-1 in brown fat and skeletal muscle. Increased PGC-1 protein expression leads to increases in the target genes, including nuclear respiratory factor (NRF)-1. NRF-1 is a transcription factor stimulating many nuclear-encoded mitochondrial genes such as OXPHOS genes and also mitochondrial transcription factor A (mtTFA), a key transcriptional factor for the mitochondrial genome. mtTFA can bind to the D-loop of the mitochondrial genome and increase transcription of mitochondrial genes and replication of mitochondrial DNA (Fig. 3) (87).
FIG. 3.
The molecular mechanism of mitochondrial (Mt) biogenesis. Mitochondria have their own genome, which encodes 13 proteins, 2 rRNAs, and 22 tRNAs. It is also known that most of the mitochondrial proteins are encoded by the nuclear genome and translated proteins are transported into mitochondria. Extracellular stimuli induce mitochondrial biogenesis through PGC-1 in brown fat and skeletal muscle. Increased PGC-1 protein expression leads to increases in the expression of its target genes, including NRF-1. NRF-1 is a transcription factor stimulating many nuclear-encoded mitochondrial genes such as OXPHOS genes and mtTFA, a key transcriptional factor for the mitochondrial genome. mtTFA can bind to the D-loop of the mitochondrial genome and increase transcription of mitochondrial genes and replication of mitochondrial DNA. ATPsyn, ATP synthase; CytC, cytochrome C; MTCOI, mitochondrial cytochrome c oxidase subunit 1.
Two recent DNA microarray studies found a coordinated reduction in the expression of genes encoded by PGC-1α in the skeletal muscle of type 2 diabetic patients (88,89) and nondiabetic subjects with a family history of diabetes (89). However, in contrast to these studies, we did not observe any difference in the mRNA expression levels for either PGC-1α or PGC-1β in the insulin-resistant offspring. Furthermore, we also examined the mRNA expression of NRF-1, NRF-2, and mtTFA, which PGC-1α and PGC-1β direct to initiate mitochondrial biogenesis. In contrast to these previous studies, we found no difference in the level of mRNA expression of these factors between the groups (17). These data suggest that there are other unknown factors involved in the regulation of mitochondrial biogenesis responsible for the reduced skeletal muscle mitochondrial content in lean insulin-resistant offspring. The reason for the disparity between our findings and previous studies are not clear but may be related to the fact that our insulin-resistant offspring were young, lean, and healthy, in contrast to other studies where subjects were older, obese, and diabetic (88,89), or in the case of the first-degree relatives, overweight (89). Indeed, a recent study by Ling et al. (90) demonstrated an age-dependent decrease in muscle gene expression of PGC-1α and PGC-1β in young and elderly dizygotic and monozygotic twins without known diabetes. These data suggest that age-related changes in PGC-1 can occur and may account for the disparity between previous reports and our results.
Recent 31P MRS studies have also demonstrated that insulin is an important regulator of mitochondrial ATP synthesis in skeletal muscle of healthy subjects and that insulin-stimulated ATP synthesis is markedly decreased in the muscle of insulin-resistant offspring of parents with type 2 diabetes (21). These changes were associated with parallel reductions in inorganic phosphorus transport into skeletal muscle in the insulin-resistant offspring, suggesting that these two processes may be coupled. However, in contrast to the potentially inherited defects in mitochondrial biogenesis observed in these individuals, it is likely that these defects in insulin-stimulated ATP synthesis and inorganic phosphorus transport may be explained by acquired defects in insulin signaling due to the accumulation of intracellular lipids, as described above. Consistent with this hypothesis are recent studies by Roden and coworkers (22) demonstrating defects in insulin-stimulated ATP synthesis during a lipid infusion in healthy volunteers.
CONCLUSION
In summary, recent MRS studies have revealed important new insights into the pathogenesis of insulin resistance and type 2 diabetes. Specifically, they have identified defects in insulin activation of glucose transport activity as the rate-controlling step responsible for fat-induced insulin resistance in skeletal muscle and have shown that increases in intramyocellular lipid metabolites play a key role in triggering insulin resistance through activation of a serine kinase cascade leading to reduced insulin stimulation of IRS-1 tyrosine phosphorylation. Parallel studies have revealed a similar mechanism for fat-induced hepatic insulin resistance associated with nonalcoholic fatty liver, where increases in hepatocellular diacylglycerol lead to activation of protein kinase C-ε, leading to reduced insulin stimulation of IRS-2 tyrosine phosphorylation resulting in reduced insulin stimulation of glycogen synthase activation and decreased phosphorylation of forkhead box protein O (FOXO), leading to increased hepatic gluconeo-genesis. Finally, recent MRS studies have implicated defects in mitochondrial oxidative phosphorylation activity in causing insulin resistance in both the elderly and young lean insulin-resistant offspring of parents with type 2 diabetes. By elucidating the cellular and molecular mechanisms responsible for insulin resistance, these studies provide potential new targets for the treatment and prevention of type 2 diabetes.
ACKNOWLEDGMENTS
This work was supported by grants from the U.S. Public Health Service (P01 DK-063229, R01 AG-23686, P30 DK-45735, R01 DK-40936, U24 DK-59635, and M01 RR-00125). G.I.S. is the recipient of a Distinguished Clinical Scientist Award from the American Diabetes Association.
Glossary
- CREB
cAMP response element binding protein
- IRS
insulin receptor substrate
- MEF2
myocyte enhancer factor 2
- MRS
magnetic resonance spectroscopy
- mtTFA
mitochondrial transcription factor A
- NRF
nuclear respiratory factor
- PGC
peroxisome proliferator–activated receptor-γ coactivator
- PI
phosphatidylinositol
- PKC
protein kinase C
Footnotes
This article is based on a presentation at a symposium. The symposium and the publication of this article were made possible by an unrestricted educational grant from Servier.
REFERENCES
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR. Slow glucose removal rate and hyperinsulinemia precede the development of type-II diabetes in the offspring of diabetic parents. Ann Intern Med. 1990;113:909–915. doi: 10.7326/0003-4819-113-12-909. [DOI] [PubMed] [Google Scholar]
- 3.Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus: prospective studies of Pima Indians. N Engl J Med. 1993;329:1988–1992. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- 4.Azen P, Peters DS, Berkowitz MD, Kjos MD, Xiang P. TRIPOD (TRoglitazone In the Prevention Of Diabetes): a randomized, placebo-controlled trial of troglitazone in women with prior gestational diabetes mellitus. Controlled Clinical Trials. 1998;19:217–231. doi: 10.1016/s0197-2456(97)00151-7. [DOI] [PubMed] [Google Scholar]
- 5.Diabetes Prevention Program Research Group. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. 2005;54:2404–2414. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shulman GI, Rothman DL, Jue T, Stein P, Defronzo RA, Shulman RG. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990;322:223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- 7.Rothman DL, Shulman RG, Shulman GI. P-31 nuclear-magnetic-resonance measurements of muscle glucose-6-phosphate: evidence for reduced insulin-dependent muscle glucose-transport or phosphorylation activity in non-insulin-dependent diabetes-mellitus. J Clin Invest. 1992;89:1069–1075. doi: 10.1172/JCI115686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cline GW, Petersen KF, Krssak M, Shen J, Hundal RS, Trajanoski Z, Inzucchi S, Dresner A, Rothman DL, Shulman GI. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N Engl J Med. 1999;341:240–246. doi: 10.1056/NEJM199907223410404. [DOI] [PubMed] [Google Scholar]
- 9.Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, Rothman DL, Shulman GI. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med. 1996;335:1357–1362. doi: 10.1056/NEJM199610313351804. [DOI] [PubMed] [Google Scholar]
- 10.Rothman DL, Magnusson I, Cline G, Gerard D, Kahn CR, Shulman RG, Shulman GI. Decreased muscle glucose transport/phosphorylation is an early defect in the pathogenesis of non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A. 1995;92:983–987. doi: 10.1073/pnas.92.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krssak M, Petersen KF, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a H-1 NMR spectroscopy study. Diabetologia. 1999;42:386. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 12.Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H–13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48:1600–1606. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 13.Szczepaniak LS, Babcock EE, Schick F, Dobbins RL, Garg A, Burns DK, McGarry JD, Stein DT. Measurement of intracellular triglyceride stores by H-1 spectroscopy: validation in vivo. Am J Physiol Endocrinol Metab. 1999;276:E977–E989. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 14.Weiss R, Dufour S, Taksali SE, Tamborlane WV, Petersen KF, Bonadonna RC, Boselli L, Barbetta G, Allen K, Rife F, Savoye M, Dziura J, Sherwin R, Shulman GI, Caprio S. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet. 2003;362:951–957. doi: 10.1016/S0140-6736(03)14364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 16.Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, Petersen KF, Shulman GI. Effects of free fatty acids on glucose transport and IRS-1–associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen KF, Dufour S, Shulman GI. Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Medicine. 2005;2:879–884. doi: 10.1371/journal.pmed.0020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brehm A, Krssak M, Schmid AI, Nowotny P, Waldhausl W, Roden M. Increased lipid availability impairs insulin-stimulated ATP synthesis in human skeletal muscle. Diabetes. 2006;55:136–140. [PubMed] [Google Scholar]
- 23.Fueger PT, Bracy DP, Malabanan CM, Pencek RR, Granner DK, Wasserman DH. Hexokinase II overexpression improves exercise-stimulated but not insulin-stimulated muscle glucose uptake in high-fat-fed C57BL/6J mice. Diabetes. 2004;53:306–314. doi: 10.2337/diabetes.53.2.306. [DOI] [PubMed] [Google Scholar]
- 24.Cline GW, Johnson K, Regittnig W, Perret P, Tozzo E, Xiao L, Damico C, Shulman GI. Effects of a novel glycogen synthase kinase-3 inhibitor on insulin-stimulated glucose metabolism in Zucker diabetic fatty (fa/fa) rats. Diabetes. 2002;51:2903–2910. doi: 10.2337/diabetes.51.10.2903. [DOI] [PubMed] [Google Scholar]
- 25.Perseghin G, Ghosh S, Gerow K, Shulman GI. Metabolic defects in lean nondiabetic offspring of NIDDM parents: a cross-sectional study. Diabetes. 1997;46:1001–1009. doi: 10.2337/diab.46.6.1001. [DOI] [PubMed] [Google Scholar]
- 26.Phillips DIW, Caddy S, Ilic V, Fielding BA, Frayn KN, Borthwick AC, Taylor R. Intramuscular triglyceride and muscle insulin sensitivity: evidence for a relationship in nondiabetic subjects. Metabol Clin Exp. 1996;45:947–950. doi: 10.1016/s0026-0495(96)90260-7. [DOI] [PubMed] [Google Scholar]
- 27.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 29.Okada T, Kawano Y, Sakakibara T, Hazeki O, Ui M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes: studies with a selective inhibitor wortmannin. J Biol Chem. 1994;269:3568–3573. [PubMed] [Google Scholar]
- 30.Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW, Liu ZX, Soos TJ, Cline GW, O’Brien WR, Littman DR, Shulman GI. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest. 2004;114:823–827. doi: 10.1172/JCI22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu CL, Chen Y, Cline GW, Zhang DY, Zong HH, Wang YL, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 32.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 33.Furukawa N, Ongusaha P, Jahng WJ, Araki K, Choi CS, Kim HJ, Lee YH, Kaibuchi K, Kahn BB, Masuzaki H, Kim JK, Lee SW, Kim YB. Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metab. 2005;2:119–129. doi: 10.1016/j.cmet.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Hirosumi J, Tuncman G, Chang LF, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 35.Yuan MS, Konstantopoulos N, Lee JS, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of IKK beta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 36.Morino K, Neschen S, Bilz S, Sono S, Tsirigotis D, Reznick RM, Samuel V, Philbrick WM, Shulman GI. IRS-1 serine phosphorylation is a key molecular event in the pathogenesis of fat-induced insulin resistance in skeletal muscle in vivo (Abstract) Diabetes. 2005;54(Suppl. 1):A339. [Google Scholar]
- 37.Mothe I, Van Obberghen E. Phosphorylation of insulin receptor substrate-1 on multiple serine residues, 612, 632, 662, and 731, modulates insulin action. J Biol Chem. 1996;271:11222–11227. doi: 10.1074/jbc.271.19.11222. [DOI] [PubMed] [Google Scholar]
- 38.Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser(307) in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 39.Moeschel K, Beck A, Weigert C, Lammers R, Kalbacher H, Voelter W, Schleicher ED, Haring HU, Lehmann R. Protein kinase C-zeta-induced phosphorylation of Ser(318) in insulin receptor substrate-1 (IRS-1) attenuates the interaction with the insulin receptor and the tyrosine phosphorylation of IRS-1. J Biol Chem. 2004;279:25157–25163. doi: 10.1074/jbc.M402477200. [DOI] [PubMed] [Google Scholar]
- 40.Li JP, Defea K, Roth RA. Modulation of insulin receptor substrate-1 tyrosine phosphorylation by an Akt/phosphatidylinositol 3-kinase pathway. J Biol Chem. 1999;274:9351–9356. doi: 10.1074/jbc.274.14.9351. [DOI] [PubMed] [Google Scholar]
- 41.Egawa K, Nakashima N, Sharma PM, Maegawa H, Nagai Y, Kashiwagi A, Kikkawa R, Olefsky JM. Persistent activation of phosphatidylinositol 3-kinase causes insulin resistance due to accelerated insulin-induced insulin receptor substrate-1 degradation in 3T3–L1 adipocytes. Endocrinology. 2000;141:1930–1935. doi: 10.1210/endo.141.6.7516. [DOI] [PubMed] [Google Scholar]
- 42.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and I kappa B-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 43.Samuel VT, Liu ZX, Qu XQ, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 44.Gao Z, Zuberi A, Quon MJ, Dong Z, Ye J. Aspirin inhibits serine phosphorylation of insulin receptor substrate 1 in tumor necrosis factor-treated cells through targeting multiple serine kinases. J Biol Chem. 2003;278:24944–24950. doi: 10.1074/jbc.M300423200. [DOI] [PubMed] [Google Scholar]
- 45.Giraud J, Leshan R, Lee YH, White MF. Nutrient-dependent and insulin-stimulated phosphorylation of insulin receptor substrate-1 on serine 302 correlates with increased insulin signaling. J Biol Chem. 2004;279:3447–3454. doi: 10.1074/jbc.M308631200. [DOI] [PubMed] [Google Scholar]
- 46.Gual P, Gonzalez T, Gremeaux T, Barres R, Marchand-Brustel Y, Tanti JF. Hyperosmotic stress inhibits insulin receptor substrate-1 function by distinct mechanisms in 3T3–l1 adipocytes. J Biol Chem. 2003;278:26550–26557. doi: 10.1074/jbc.M212273200. [DOI] [PubMed] [Google Scholar]
- 47.Usui I, Imamura T, Babendure JL, Satoh H, Lu JC, Hupfeld CJ, Olefsky JM. G protein-coupled receptor kinase 2 mediates endothelin-1-induced insulin resistance via the inhibition of both Gαq/11 and insulin receptor substrate-1 pathways in 3T3-L1 adipocytes. Mol Endocrinol. 2005;19:2760–2768. doi: 10.1210/me.2004-0429. [DOI] [PubMed] [Google Scholar]
- 48.Mussig K, Staiger H, Fiedler H, Moeschel K, Beck A, Kellerer M, Haring HU. Shp2 is required for protein kinase C-dependent phosphorylation of serine 307 in insulin receptor substrate-1. J Biol Chem. 2005;280:32693–32699. doi: 10.1074/jbc.M506549200. [DOI] [PubMed] [Google Scholar]
- 49.Mussig K, Fiedler H, Staiger H, Weigert C, Lehmann R, Schleicher ED, Haring HU. Insulin-induced stimulation of JNK and the PI 3-kinase/mTOR pathway leads to phosphorylation of serine 318 of IRS-1 in C2C12 myotubes. Biochem Biophys Res Comm. 2005;335:819–825. doi: 10.1016/j.bbrc.2005.07.154. [DOI] [PubMed] [Google Scholar]
- 50.Weigert C, Hennige AM, Brischmann T, Beck A, Moeschel K, Schauble M, Brodbeck K, Haring HU, Schleicher ED, Lehmann R. The phosphorylation of Ser318 of insulin receptor substrate 1 is not per se inhibitory in skeletal muscle cells but is necessary to trigger the attenuation of the insulin-stimulated signal. J Biol Chem. 2005;280:37393–37399. doi: 10.1074/jbc.M506134200. [DOI] [PubMed] [Google Scholar]
- 51.Liu YF, Herschkovitz A, Boura-Halfon S, Ronen D, Paz K, LeRoith D, Zick Y. Serine phosphorylation proximal to its phosphotyrosine binding domain inhibits insulin receptor substrate 1 function and promotes insulin resistance. Mol Cell Biol. 2004;24:9668–9681. doi: 10.1128/MCB.24.21.9668-9681.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Fea K, Roth RA. Modulation of insulin receptor substrate-1 tyrosine phosphorylation and function by mitogen-activated protein kinase. J Biol Chem. 1997;272:31400–31406. doi: 10.1074/jbc.272.50.31400. [DOI] [PubMed] [Google Scholar]
- 53.Bard-Chapeau EA, Hevener AL, Long S, Zhang EE, Olefsky JM, Feng GS. Deletion of Gab1 in the liver leads to enhanced glucose tolerance and improved hepatic insulin action. Nat Med. 2005;11:567–571. doi: 10.1038/nm1227. [DOI] [PubMed] [Google Scholar]
- 54.Ozes ON, Akca H, Mayo LD, Gustin JA, Maehama T, Dixon JE, Donner DB. A phosphatidylinositol 3-kinase/Akt/mTOR pathway mediates and PTEN antagonizes tumor necrosis factor inhibition of insulin signaling through insulin receptor substrate-1. Proc Natl Acad Sci U S A. 2001;98:4640–4645. doi: 10.1073/pnas.051042298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE. 5′-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J Biol Chem. 2001;276:46912–46916. doi: 10.1074/jbc.C100483200. [DOI] [PubMed] [Google Scholar]
- 56.Li Y, Soos TJ, Li X, Wu J, DeGennaro M, Sun X, Littman DR, Birnbaum MJ, Polakiewicz RD. Protein kinase C θ inhibits insulin signaling by phosphor-ylating IRS1 at Ser1101. J Biol Chem. 2004;279:45304–45307. doi: 10.1074/jbc.C400186200. [DOI] [PubMed] [Google Scholar]
- 57.Kim J, Yeh DC, Ver M, Li Y, Carranza A, Conrads TP, Veenstra TD, Harrington MA, Quon MJ. Phosphorylation of Ser24 in the pleckstrin homology domain of insulin receptor substrate-1 by mouse pelle-like kinase/interleukin-1 receptor-associated kinase: cross-talk between inflammatory signaling and insulin signaling that may contribute to insulin resistance. J Biol Chem. 2005;280:23173–23183. doi: 10.1074/jbc.M501439200. [DOI] [PubMed] [Google Scholar]
- 58.Ogihara T, Isobe T, Ichimura T, Taoka M, Funaki M, Sakoda H, Onishi Y, Inukai K, Anai M, Fukushima Y, Kikuchi M, Yazaki Y, Oka Y, Asano T. 14-3-3 Protein binds to insulin receptor substrate-1, one of the binding sites of which is in the phosphotyrosine binding domain. J Biol Chem. 1997;272:25267–25274. doi: 10.1074/jbc.272.40.25267. [DOI] [PubMed] [Google Scholar]
- 59.Gao ZG, Hwang D, Bataille F, Lefevre M, York D, Quon M, Ye JP. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem. 2002;277:48115–48121. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- 60.Gao Z, Zhang X, Zuberi A, Hwang D, Quon MJ, Lefevre M, Ye J. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3–L1 adipocytes. Mol Endocrinol. 2004;18:2024–2034. doi: 10.1210/me.2003-0383. [DOI] [PubMed] [Google Scholar]
- 61.Werner ED, Lee J, Hansen L, Yuan M, Shoelson SE. Insulin resistance due to phosphorylation of insulin receptor substrate-1 at serine 302. J Biol Chem. 2004;279:35298–35305. doi: 10.1074/jbc.M405203200. [DOI] [PubMed] [Google Scholar]
- 62.del Rincon SV, Rousseau C, Samanta R, Miller WH. Retinoic acid-induced growth arrest of MCF-7 cells involves the selective regulation of the IRS-1/PI 3-kinase/AKT pathway. Oncogene. 2003;22:3353–3360. doi: 10.1038/sj.onc.1206485. [DOI] [PubMed] [Google Scholar]
- 63.Danielsson A, Ost A, Nystrom FH, Stralfors P. Attenuation of insulin-stimulated insulin receptor substrate-1 serine 307 phosphorylation in insulin resistance of type 2 diabetes. J Biol Chem. 2005;280:34389–34392. doi: 10.1074/jbc.C500230200. [DOI] [PubMed] [Google Scholar]
- 64.Corbould A, Kim YB, Youngren JF, Pender C, Kahn BB, Lee A, Dunaif A. Insulin resistance in the skeletal muscle of women with PCOS involves intrinsic and acquired defects in insulin signaling. Am J Physiol Endocrinol Metab. 2005;288:E1047–E1054. doi: 10.1152/ajpendo.00361.2004. [DOI] [PubMed] [Google Scholar]
- 65.Tremblay F, Krebs M, Dombrowski L, Brehm A, Bernroider E, Roth E, Nowotny P, Waldhausl W, Marette A, Roden M. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes. 2005;54:2674–2684. doi: 10.2337/diabetes.54.9.2674. [DOI] [PubMed] [Google Scholar]
- 66.Greene MW, Morrice N, Garofalo RS, Roth RA. Modulation of human insulin receptor substrate-1 tyrosine phosphorylation by protein kinase C delta. Biochem J. 2004;378:105–116. doi: 10.1042/BJ20031493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luo M, Reyna S, Wang L, Yi Z, Carroll C, Dong LQ, Langlais P, Weintraub ST, Mandarino LJ. Identification of insulin receptor substrate 1 serine/ threonine phosphorylation sites using mass spectrometry analysis: regulatory role of serine 1223. Endocrinology. 2005;146:4410–4416. doi: 10.1210/en.2005-0260. [DOI] [PubMed] [Google Scholar]
- 68.Andreozzi F, D’Alessandris C, Federici M, Laratta E, Del Guerra S, Del Prato S, Marchetti P, Lauro R, Perticone F, Sesti G. Activation of the hexosamine pathway leads to phosphorylation of insulin receptor substrate-1 on Ser307 and Ser612 and impairs the phosphatidylinositol 3-ki-nase/akt/mammalian target of rapamycin insulin biosynthetic pathway in RIN pancreatic β-cells. Endocrinology. 2004;145:2845–2857. doi: 10.1210/en.2003-0939. [DOI] [PubMed] [Google Scholar]
- 69.Bouzakri K, Roques M, Gual P, Espinosa S, Guebre-Egziabher F, Riou JP, Laville M, Marchand-Brustel Y, Tanti JF, Vidal H. Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes. 2003;52:1319–1325. doi: 10.2337/diabetes.52.6.1319. [DOI] [PubMed] [Google Scholar]
- 70.Tremblay F, Gagnon A, Veilleux A, Sorisky A, Marette A. Activation of the mammalian target of rapamycin pathway acutely inhibits insulin signaling to Akt and glucose transport in 3T3–L1 and human adipocytes. Endocrinology. 2005;146:1328–1337. doi: 10.1210/en.2004-0777. [DOI] [PubMed] [Google Scholar]
- 71.Horike N, Takemori H, Katoh Y, Doi J, Min L, Asano T, Sun XJ, Yamamoto H, Kasayama S, Muraoka M, Nonaka Y, Okamoto M. Adipose-specific expression, phosphorylation of Ser794 in insulin receptor substrate-1, and activation in diabetic animals of salt-inducible kinase-2. J Biol Chem. 2003;278:18440–18447. doi: 10.1074/jbc.M211770200. [DOI] [PubMed] [Google Scholar]
- 72.Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, Gout I, Downes CP, Lamb RF. The TSC1–2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chalkley SM, Hettiarachchi M, Chisholm DJ, Kraegen EW. Five-hour fatty acid elevation increases muscle lipids and impairs glycogen synthesis in the rat. Metabol Clin Exp. 1998;47:1121–1126. doi: 10.1016/s0026-0495(98)90287-6. [DOI] [PubMed] [Google Scholar]
- 74.Summers SA, Garza LA, Zhou H, Birnbaum MJ. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and akt kinase activity by ceramide. Moll Cell Biol. 1998;18:5457–5464. doi: 10.1128/mcb.18.9.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Savage DB, Choi CS, Samuel VT, Liu ZX, Zhang DY, Wang A, Zhang XM, Cline GW, Yu XX, Geisler JG, Bhanot S, Monia BP, Shulman GI. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by anti-sense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest. 2006;116:817–824. doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 77.Neschen S, Morino K, Hammond LE, Zhang DY, Liu ZX, Romanelli AJ, Cline GW, Pongratz RL, Zhang XM, Choi CS, Coleman RA, Shulman GI. Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA: glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab. 2005;2:55–65. doi: 10.1016/j.cmet.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 78.Moraes CT, Shanske S, Tritschler HJ, Aprille JR, Andreetta F, Bonilla E, Schon EA, DiMauro S. MTDNA depletion with variable tissue expression: a novel genetic abnormality in mitochondrial diseases. Am J Hum Genet. 1991;48:492–501. [PMC free article] [PubMed] [Google Scholar]
- 79.van den Ouweland NM, Lemkes HH, Ruitenbeck W, Sandkuijl LA, de Vijlder MF, Struyvenberg PA, van de Kamp JJ, Maassen JA. Mutation in mitochondrial tRNA (Leu) (UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat Genet. 1992;1:368–371. doi: 10.1038/ng0892-368. [DOI] [PubMed] [Google Scholar]
- 80.DiMauro S, Schon EA. Mechanisms of disease: mitochondrial respiratory-chain diseases. N Engl J Med. 2003;348:2656–2668. doi: 10.1056/NEJMra022567. [DOI] [PubMed] [Google Scholar]
- 81.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 82.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54:8–14. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- 83.Akimoto T, Ribar TJ, Williams RS, Yan Z. Skeletal muscle adaptation in response to voluntary running in Ca2+/calmodulin-dependent protein kinase IV-deficient mice. Am J Physiol Cell Physiol. 2004;287:C1311–C1319. doi: 10.1152/ajpcell.00248.2004. [DOI] [PubMed] [Google Scholar]
- 84.Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M, Young LH, Semenkovich CF, Shulman GI. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2001;281:E1340–E1346. doi: 10.1152/ajpendo.2001.281.6.E1340. [DOI] [PubMed] [Google Scholar]
- 85.Zong HH, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol. 2000;88:2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- 87.Scarpulla RC. Nuclear control of respiratory gene expression in mammalian cells. J Cell Biochem. 2006;97:673–683. doi: 10.1002/jcb.20743. [DOI] [PubMed] [Google Scholar]
- 88.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1 alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 89.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ling C, Poulsen P, Carlsson E, Ridderstrale M, Almgren P, Wojtaszewski J, Beck-Nielsen H, Groop L, Vaag A. Multiple environmental and genetic factors influence skeletal muscle PGC-1 alpha and PGC-1 beta gene expression in twins. J Clin Invest. 2004;114:1518–1526. doi: 10.1172/JCI21889. [DOI] [PMC free article] [PubMed] [Google Scholar]