Abstract
Insulin resistance has long been associated with obesity. More than 40 years ago, Randle and colleagues postulated that lipids impaired insulin-stimulated glucose use by muscles through inhibition of glycolysis at key points. However, work over the past two decades has shown that lipid-induced insulin resistance in skeletal muscle stems from defects in insulin-stimulated glucose transport activity. The steatotic liver is also resistant to insulin in terms of inhibition of hepatic glucose production and stimulation of glycogen synthesis. In muscle and liver, the intracellular accumulation of lipids—namely, diacylglycerol—triggers activation of novel protein kinases C with subsequent impairments in insulin signalling. This unifying hypothesis accounts for the mechanism of insulin resistance in obesity, type 2 diabetes, lipodystrophy, and ageing; and the insulin-sensitising effects of thiazolidinediones.
Introduction
Obesity is now a pandemic that is largely caused by a combination of our genetics, evolutionary pressures that favour metabolic efficiency,1 and a modern environment in which highly palatable, calorie-dense food is widely available and inexpensive.2 There are now more overweight than underweight people worldwide, and children are increasingly at risk of becoming obese.3–5 In tandem with the obesity epidemic, the prevalence of related disorders, such as metabolic syndrome, non-alcoholic fatty liver disease, and type 2 diabetes mellitus, is also rising. Insulin resistance plays a crucial part in the pathogenesis of all these disorders, yet the cellular mechanisms are still poorly understood. Here, we review studies in human beings and rodents that have informed our current understanding of the mechanistic links between lipid accumulation and insulin resistance. We first discuss some of the pioneering studies in this specialty.
Glucose-fatty-acid cycle
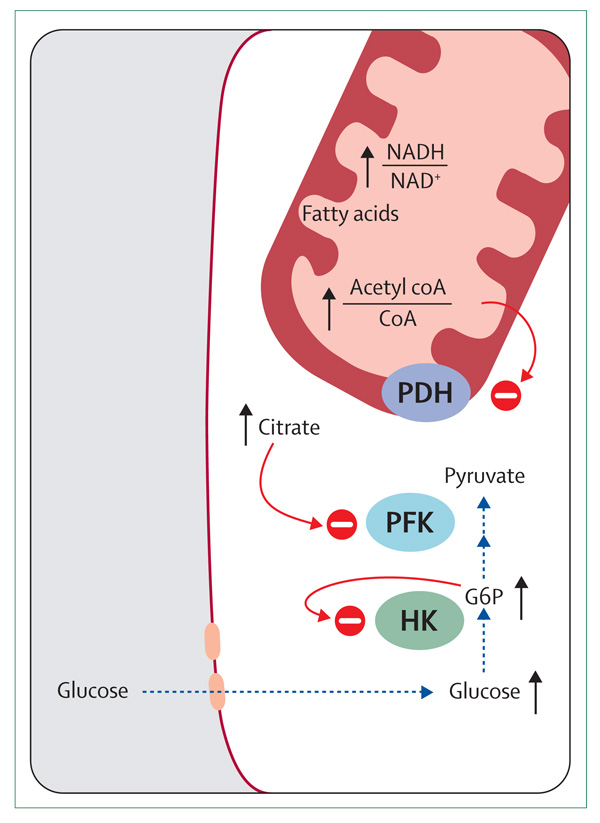
Randle and colleagues6 postulated a mechanism more than 40 years ago by which fatty acids could impair insulin-stimulated glucose oxidation in muscle. They reported that incubation of preparations of the rat heart with fatty acids increased intracellular concentrations of glucose-6-phosphate (G6P) and glucose, and incubation of preparations of diaphragm increased intracellular concentrations of glycogen (figure 1). According to Randle and colleagues’ theory, fat oxidation increased the ratios of acetyl coenzyme A to coenzyme A and NADH to NAD+ in the mitochondria, which in turn resulted in the inactivation of pyruvate dehydrogenase. Accumulation of citrate inhibits phosphofructokinase and thus increases intracellular concentrations of G6P, promoting glycogen synthesis and inhibiting hexokinase. The resulting intracellular accumulation of glucose prevents further glucose uptake. Thus, in their model, the availability of lipids as a source of fuel generated metabolic signals that impaired the use of glucose through inhibition of the key glycolytic enzymes.
Figure 1. Glucose-fatty-acid cycle proposed by Randle and colleagues.
CoA=coenzyme A. PDH=pyruvate dehydrogenase. PFK=phosphofructokinase. G6P=glucose-6-phosphate. HK=hexokinase. Red circle with minus sign represents inhibition. Black line with arrowhead represents increase or accumulation of substrate. Blue dotted line with arrowhead indicates a pathway that is inhibited.
Testing Randle and colleagues’ hypothesis
Investigation of the association between fatty acids and insulin resistance is difficult in individuals who are already obese or diabetic because of the confounding effects of other co-morbidities. These effects are avoided by investigation of the mechanisms of insulin resistance in the offspring of patients with type 2 diabetes mellitus, who are young, lean, and insulin resistant. When Perseghin and colleagues7 compared such individuals with controls matched for age and body-mass index (BMI), they noted an inverse correlation between plasma concentrations of fatty acids and insulin sensitivity. However, insulin sensitivity was more tightly associated with intramyocellular lipid content (assessed non-invasively by use of proton [1H]-magnetic resonance spectroscopy [MRS]) in lean offspring of patients with type 2 diabetes mellitus,8,9 and with intramuscular triglyceride content in muscle biopsy samples from non-diabetic male Pima Indians.10
Does insulin resistance alter the intramyocellular concentration of G6P and glycogen in human beings? In Randle and colleagues’ proposed theory about the glucose-fatty-acid cycle, muscle glycogen content was predicted to be high as a result of the accumulation of intracellular glucose and G6P. Measurement of these metabolites in vivo is difficult; repeated muscle biopsies are needed to measure a rate of change, and metabolite concentrations are affected by even brief periods of ex-vivo hypoxia.11 Use of MRS circumvents these difficulties, allowing non-invasive and real-time, sequential measurement of these metabolites in situ and thus avoiding the confounding effects of hypoxia. 13-carbon [13C]-MRS and 31-phosphorus [31P]-MRS were used to non-invasively measure insulin-stimulated changes in muscle glycogen, and in intramyocellular G6P in patients with type 2 diabetes mellitus and non-diabetic first-degree relatives.12–14 When measured by use of 13C–MRS, the rates of insulin-stimulated glucose uptake and glycogen synthesis were more than 50% lower in the patients with diabetes than in the control individuals,13 and was associated with a reduction in concentrations of G6P in muscle.14 Similarly, insulin-stimulated concentrations of G6P and rates of muscle glycogen synthesis were reduced in lean, normoglycaemic, insulin-resistant first-degree relatives of patients with type 2 diabetes mellitus.12
Could these changes be induced in healthy, otherwise insulin-sensitive individuals? Lipid infusions combined with heparin to activate lipoprotein lipase, raised plasma concentrations of fatty acids, promoted accumulation of muscle lipids,15 and impaired oral glucose tolerance16 and insulin-stimulated glucose disposal in healthy individuals.17,18 Roden and colleagues17 combined lipid infusions with the normoglycaemic-hyperinsulinaemic clamp in healthy individuals while monitoring synthesis of muscle glycogen with 13C–MRS, and concentrations of G6P with 31P–MRS. After about 3 h of hyperlipidaemia, muscle G6P concentrations were actually reduced, followed by a decrease in insulin-stimulated rates of glucose disposal. These findings contrasted with Randle and colleagues’ hypothesis and results of previous studies in which an increase was reported in intramyocellular concentrations of G6P in muscle biopsy samples during similar conditions.19 Thus, in healthy individuals, exposure to high concentrations of plasma fatty acids caused insulin resistance associated with an induced defect in either glucose transport or phosphorylation activity, not an impairment in glycolysis.
To discern which of these two possible effects takes place, Dresner and colleagues20 used a novel 13C–MRS method to measure intramyocellular concentrations of free glucose in healthy people under similar conditions of high and low plasma concentrations of fatty acids. If there was a block at the hexokinase step, the concentration of intramyocellular glucose would be expected to increase. Instead, raising plasma fatty acid concentration attenuated the accumulation of intracellular glucose, implying that insulin-stimulated glucose transport activity was blunted. Cline and colleagues21 studying individuals with type 2 diabetes mellitus and healthy controls during matched hyperinsulinaemic-normoglycaemic conditions, reported that intramyocellular concentrations of glucose were much lower than predicted had hexokinase been controlling the rate of insulin-stimulated glucose uptake by muscle. This result suggested that in people with type 2 diabetes mellitus, impairment of insulin-stimulated glucose use by muscle was also largely due to reductions in insulin-stimulated glucose transport.
In parallel with these studies, there were complementary advances in knowledge about glucose transporter (GLUT) proteins. The GLUT family of proteins (also called solute carriers 2A [SLC2A]) is diverse, with 13 isoforms.22 However, GLUT4 is distinguished from the others as a high-affinity, insulin-responsive transporter that is highly expressed in muscle and adipose tissue.23 Insulin-mediated translocation of GLUT4 to the muscle sarcolemmal membrane was impaired in patients with type 2 diabetes mellitus.24,25 Thus, a defect in glucose transport, not glycolysis, was implicated as the cause of reduced insulin-mediated glucose metabolism in patients with type 2 diabetes mellitus in two independent studies.24,25 Moreover, the results of these studies suggested that ectopic accumulation of lipid within the muscle might be the cause of insulin resistance.
Diacylglycerol-induced insulin resistance
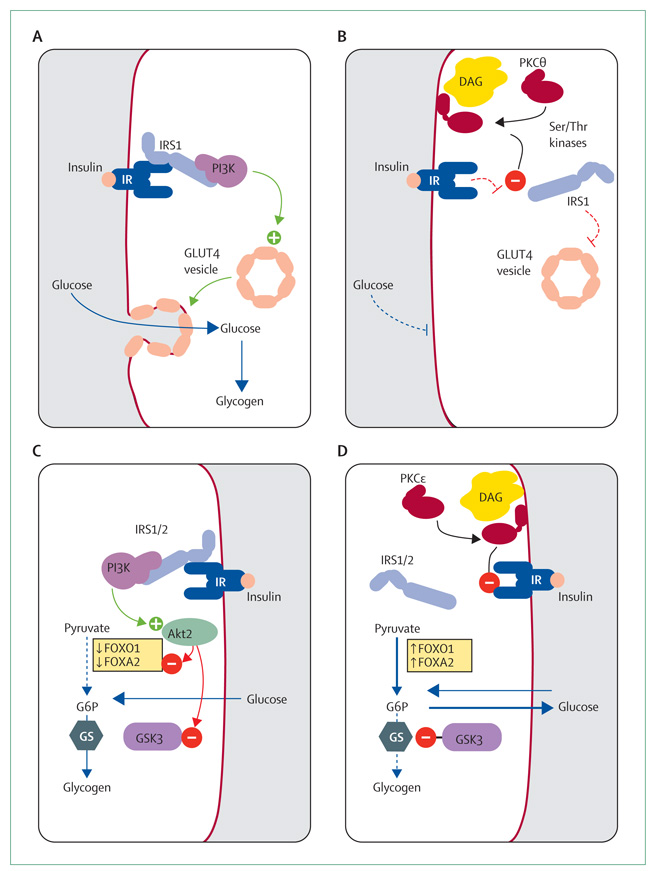
The coordinated intracellular response to insulin requires an intricate relay of signals. In skeletal muscle, insulin binds to its receptor, activating the receptor tyrosine kinase activity, with subsequent phosphorylation and activation of insulin-receptor substrate 1 (IRS1; figure 2). When phosphorylated, IRS1 activates 1-phosphatidylinositol 3-kinase (PI3K). This enzyme, through signalling intermediates, activates Akt2, which phosphorylates and inactivates AS160, a protein that prevents translocation of GLUT4 through its interaction with Rab proteins.26 Thus, insulin promotes the docking and fusion of GLUT4-containing vesicles to the plasma membrane. The tyrosine phosphorylation of IRS1 and associated activation of PI3K were impaired in rodent models of insulin resistance.27,28 Similarly, IRS1-associated PI3K activity was greatly reduced in the muscles of individuals being given lipid infusions, indicating that the lipid-induced reduction in insulin-stimulated glucose transport was attributable to a defect in insulin signalling.20 But what connects lipid accumulation with impaired insulin action in muscle? This answer is related to the family of protein kinase C (PKC) serine-threonine kinases.
Figure 2. Mechanisms of insulin sensitivity and resistance in muscle and liver.
(A) Insulin-sensitive muscle. (B) Insulin-resistant muscle. (C) Insulin-sensitive liver. (D) Insulin-resistant liver. IRS=insulin-receptor substrate. IR=insulin receptor. PI3K=1-phosphatidylinositol 3-kinase. GLUT4=glucose transporter 4. DAG=diacylglycerol. PKC=protein kinase C. Ser=serine. Thr=threonine. FOX01=forkhead box O1. FOXA2=forkhead box A2. G6P=glucose-6-phosphate. GS=glycogen synthase. GSK=glycogen synthase kinase. Green circle with plus sign represents activation. Red circle with minus sign represents inactivation. Solid line with arrowhead represents increase or accumulation of substrate. Dotted line indicates inhibition of pathway.
The PKC family consists of three main groups: conventional (α, βI, βII, and γ), novel (δ, ε, η, and θ), and atypical (ζ and λ).29 Classic PKCs become activated when calcium binds to the C2 domain, increasing the affinity of the C1 domain for diacylglycerol, which then removes a pseudosubstrate from the catalytic domain. By contrast, the C1 domains of novel PKCs intrinsically have much greater affinity for diacylglycerol,30 though subtle structural differences alter this affinity and suggest different thresholds for activation.31 The potential role of PKCs in regulating insulin action has long been recognised. Phorbol esters potently and non-selectively activate PKCs, and impair activation of the insulin receptor in vitro.32–35 Thus, the accumulation of diacylglycerol within the cells might similarly activate PKCs.
In rodents, a high-fat diet increased the concentration of diacylglycerol in muscles and activated novel PKCs.36 Similarly, infusion of lipid and heparin or heparin for 5 h caused insulin resistance in muscles that was associated with accumulation of intracellular diacylglycerol and specific activation of PKCθ.37 In this model, insulin resistance was attributed to lipid-induced defects in the insulin signalling pathway that originated from a reduction in tyrosine phosphorylation of IRS1.38 By contrast, phosphorylation of IRS1 at the serine-307 residue was increased, preventing IRS1 from interacting with the insulin receptor.39 The importance of activation of novel PKCs and serine phosphorylation of IRS1 for the development of insulin resistance was shown in mice without PKCθ,40 and in those with serine-to-alanine mutations in key residues of IRS1 (thereby preventing serine phosphorylation).41 Though lipid accumulation in muscle was similar to that in wild-type mice, those with the serine-to-alanine mutations were protected from fat-induced insulin resistance in muscle. Consistent with the results of these studies, Itani and colleagues reported activation of both muscle PKCβ2 and PKCδ after lipid or heparin infusions,42 and PKCθ in patients with type 2 diabetes mellitus.43 Increased serine phosphorylation of IRS1 has also been noted in the muscles of individuals who are insulin-resistant.44,45 However, still not known is how the activation of novel PKCs might relate to serine phosphorylation of IRS1, and which kinases (ie, jun-N terminal kinase, Iκ kinase β) might have a role in the pathway.
Diacylglycerol hypothesis
Insulin resistance develops with the accumulation of fatty-acid metabolites (namely diacylglycerols) within insulin-responsive tissues.46 Genetic murine models have been invaluable in establishing this theory—eg, tissue-specific overexpression of lipoprotein lipase promotes tissue-specific lipid accumulation and selective insulin resistance.47 By contrast, prevention of lipid entry into muscle by removal of lipoprotein lipase,48 or other proteins involved in fat transport (CD3649,50 or FATP151) prevents lipid accumulation in muscles and protects against insulin resistance. Increasing energy expenditure also protects against lipid accumulation, as shown in mice overexpressing muscle-specific uncoupling protein 352 or not expressing acetyl coenzyme A carboxylase 2.53 Hoehn and colleagues54 described a different ACC2 knockout mouse that, despite the expected increases in fat oxidation, did not have an increased energy expenditure, and gained similar amounts of weight when given a high-fat diet. Together, these data suggest that a shift in substrate preference, without an increase in total energy expenditure, will not protect against fat-induced insulin resistance.
Further insights were gained with animal models in which obesity and triglyceride content were disassociated from insulin action—eg, the ob/ob adiponectin transgenic mouse is leptin deficient (ob/ob) and overexpresses adiponectin, an adipokine that improves insulin sensitivity. These mice are much heavier than ob/ob mice, but have lower liver content of triglycerides and diacylglycerol, and as a result are more insulin sensitive, which is consistent with the diacylglycerol hypothesis.55 In human beings, plasma adiponectin concentrations are directly related to insulin sensitivity and inversely related to ectopic lipid accumulation.56–60 Thus, adiponectin might play an important part in mediating ectopic fat accumulation, and the development of insulin resistance.
Diacylglycerol acyl transferase (DGAT) 1 transfers a fatty acid from a fatty acyl coenzyme A to diacylglycerol to make a triglyceride. Mice overexpressing DGAT1 in skeletal muscles (MCK-DGAT1)61 accumulate tri glycerides in their muscles, but are protected from fat-induced muscle insulin resistance. These mice model the paradox of elite endurance athletes who are insulin sensitive but who have increased triglyceride content in their muscles.62,63 In the MCK-DGAT1 mice, though muscle triglyceride content was increased, diacylglycerol content was low, consistent with protection from fat-induced insulin resistance. In man, a single bout of exercise was sufficient to induce similar changes, in which increased DGAT1 expression in muscle was associated with increased concentrations of triglyceride, reduced concentrations of diacylglycerol, and improved insulin sensitivity.64 Diacylglycerol can also be converted into phosphatidic acid, a major membrane lipid, by diacylglycerol kinases.65 Expression of diacylglycerol kinase δ was decreased in skeletal muscles of rodents with hyperglycaemia and patients with poorly controlled diabetes.65 In mice, haploinsufficiency of this enzyme increased the muscle content of diacylglycerol, but not of triglycerides, and resulted specifically in muscle insulin resistance. By disassociation of diacylglycerol and triglyceride content, the results of these studies support the hypothesis that diacylglycerol also plays a part in causing muscle insulin resistance in individuals with poorly controlled type 1 diabetes.
Diacylglycerol-induced insulin resistance in muscle and liver can readily be explained in most forms of obesity, in which increased delivery of fatty acids overwhelms the capacity of cells to oxidise fat or convert diacylglycerols to triacylglycerols. The increases in intramyocellular lipid content in healthy, lean, insulin-resistant offspring of parents with type 2 diabetes mellitus,9 and in healthy lean, elderly individuals have not been completely accounted for.66 One possibility is a reduction in lipid oxidation.44 The oxidative capacity of muscles can be quantified in vivo by measurement of the rates of flux in the citric acid cycle (with 13C–MRS in combination with an infusion of 13C–acetate), and the rates of ATP synthesis (with 31P–MRS).67 In lean, insulin-resistant offspring of parents with type 2 diabetes mellitus, the rate of ATP synthesis was reduced by 30% compared with controls matched for age and BMI.68 Befroy and colleagues69 noted that the flux of the citric acid cycle in muscle was reduced by 30% in a similar group of individuals. This decrease in flux in the citric acid cycle and in ATP synthesis was similar to the 38% reduction in mitochondrial density.44 Thus, at least in this cohort, a reduction in mitochondrial content probably accounted for the reduced rate of oxidative phosphorylation in mitochondria. Whether this reduction in mitochondrial density is a primary cause of lipid oxidation or acquired as a result of the lipid oxidation is unknown. Although results of some microarray studies have indicated a reduction in expression of the peroxisome proliferator-activated receptor (PPAR) γ coactivator 1α in the muscles of patients with type 2 diabetes mellitus,70,71 they were not replicated in lean, insulin-resistant first-degree relatives of patients with type 2 diabetes mellitus, which suggests that other factors bring about the reduction in mito chondrial content in these individuals.44 Irrespective of whether the reduction in mitochondrial content in the muscles is a primary defect, at the very least it reduces the mitochondrial fatty acid oxidation that promotes the accumulation of diacylglycerol within the muscle and contributes to the development of skeletal muscle insulin resistance in young, lean, insulin-resistant offspring of parents with type 2 diabetes mellitus.
Although the offspring of patients with type 2 diabetes mellitus have an inherited predisposition towards intramyocellular lipid accumulation and insulin resistance, almost all people manifest similar changes with increasing age. Results of population-based studies have clearly shown an association between ageing and insulin resistance.72–74 As in the young insulin-resistant individuals, the presence of peripheral insulin resistance in the elderly was associated with accumulation of intramyocellular lipids.66,75,76 To investigate the potential role of decreased oxidative and phosphorylation activities in this process, Petersen and colleagues66 used 13C–MRS and 31P–MRS to measure muscle mitochondrial function in a group of young and old people carefully matched not only for BMI, but also for body composition and activity. They noted that age-related decreases in the oxidative and phosphorylation activities were associated with increases in intramyocellular content of triglycerides and insulin resistance. These findings along with those from the studies of the insulin-resistant offspring of patients with type 2 diabetes mellitus, suggest that impaired mitochondrial function, with an impaired capacity to oxidise fatty acids, predisposes muscle to intramyocellular fat accumulation and reduced insulin sensitivity. By use of similar 13C–MRS techniques, Boumezbeur and colleagues77 reported similar reductions in neuronal mitochondrial activity in healthy elderly individuals, which suggests that these age-associated reductions in mitochondrial activity might occur in several organs. Because mitochondria have a key role in the regulation of glucose-stimulated β-cell insulin secretion, age-associated reductions in mitochondrial function might also play a part in the impairment of β-cell function associated with ageing, and promote progression to impaired glucose tolerance and type 2 diabetes mellitus in elderly people.78
Mechanisms of hepatic insulin resistance
Ectopic lipid accumulation in the liver is now widely known as non-alcoholic fatty liver disease. Formerly thought of as benign steatosis, this liver disease is now the most common chronic cause of raised serum concentrations of liver-derived enzymes in adults and children,79 and it is closely associated with obesity, insulin resistance, and type 2 diabetes mellitus.80–82 Insulin action in the liver has many similarities with insulin action in muscle. In the liver, insulin activates the insulin receptor kinase, which phosphorylates IRS1 and IRS2, leading to activation of PI3K and ultimately Akt2 (figure 2).83 At this point, Akt2 activation promotes glycogen synthesis and inhibits gluconeogenesis.
Many investigators have suggested that non-alcoholic fatty liver disease develops in the setting of insulin resistance.84–88 Increased hepatic steatosis was reported in genetic mouse models of muscle insulin resistance, specifically mice with muscle-specific deletions of the insulin receptor (MIRKO)87 and the GLUT4 transporter.88 Petersen and colleagues89 compared postprandial de-novo lipogenesis in lean healthy, young insulin-sensitive and insulin-resistant individuals matched for age, BMI, percentage of fat mass, blood pressure, and physical activity. They postulated that insulin resistance in skeletal muscles, by altering the distribution of postprandial energy storage, would lead to an atherogenic dyslipidaemia that arises in metabolic syndrome. Specifically, insulin resistance in muscle would impair storage of carbohydrate as glycogen in muscle. Instead, carbohydrates would be redirected to the liver, and become substrates for hepatic de-novo lipogenesis. After a carbohydrate challenge, the insulin-resistant group had substantial elevations in plasma insulin but glycogen synthesis in muscle was 61% lower than in the controls, as measured with 13C–MRS. Furthermore, insulin-resistant individuals had a large increase in liver triglyceride content, attributable to a roughly two-fold increase in hepatic de-novo lipogenesis. These changes were associated with a 60% increase in concentration of fasting plasma triglycerides and a roughly 20% decrease in plasma concentration of HDL. Notably, visceral fat mass, as measured with abdominal MRI, was identical in these cohorts of normal-weight, insulin-sensitive and insulin-resistant individuals, which suggests these features of the metabolic syndrome can develop independently of increased visceral adiposity. Stefan and colleagues90 also noted that, in a cohort of obese people, insulin-sensitive and insulin-resistant individuals were distinguished on the basis of lipid accumulation in the muscles and livers, but not subcutaneous or visceral adiposity. Fabbrini and colleagues91 similarly reported that intrahepatic triglyceride content, not visceral adiposity, was associated with insulin resistance and increased triglyceride secretion.
Individuals of Asian-Indian ancestry are a population at risk of developing non-alcoholic fatty liver disease. Comparison of young, normal-weight men of Asian-Indian descent with eastern Asian, white, black, and Hispanic men showed that the Asian-Indian individuals had a greatly increased prevalence of insulin resistance.92 The most striking difference was a near doubling in the average liver triglyceride content in the Asian-Indian men when compared with matched white men. Though still low compared with individuals with marked obesity and type 2 diabetes mellitus, young Asian-Indian, normal-weight men were highly susceptible to hepatic steatosis associated with insulin resistance, increasing their risk of developing type 2 diabetes mellitus, steatohepatitis, and liver cirrhosis.92 Two polymorphisms (rs2854116 and rs2854117) in the apolipoprotein C3 (ApoC3) gene that seem to predispose individuals to the development of non-alcoholic fatty liver disease and insulin resistance have been identified.93 This polymorphism leads to a roughly 30% higher plasma concentration of ApoC3, and postprandial hypertriglyceridaemia. As a result, the livers of carriers of these polymorphisms can take up increased amounts of lipid from the chylomicron remnant, leading to non-alcoholic fatty liver disease and hepatic insulin resistance. Transgenic mice that overexpress ApoC3 provide genetic evidence in support of this hypothesis. When given a high-fat diet, these mice had greater accumulation of liver diacylglycerol than did wild-type mice, and the accumulation of diacylglycerol was associated with activation of PKCε and substantial hepatic insulin resistance (Lee H-Y, Yale University School of Medicine, New Haven, CT, USA, personal communication).
Hispanic adults and children are also a large ethnic group at risk of developing non-alcoholic fatty liver disease and insulin resistance.94,95 Use of genetic screening has identified a polymorphism rs738409 within patatin-like phospholipase domain containing 3 (PNPLA3 population or adiponutrin) that is prevalent in the Hispanic population, and is highly associated with non-alcoholic fatty liver disease.96 This polymorphism results in a missense mutation I148M within PNPLA3 that renders the protein incapable of triglyceride hydrolysis.97 Though the association between this polymorphism and liver triglyceride content has been noted in other populations, there is no association with worsening insulin resistance.98,99 When Kantartzis and colleagues98 analysed only patients with non-alcoholic fatty liver disease, those with the polymorphism actually seemed to have increased insulin sensitivity. The results of these studies98,99 contrast with the findings obtained with ApoC3 gene variants. Whereas individuals with ApoC3 gene variants and non-alcoholic fatty liver disease are insulin resistant, those with non-alcoholic fatty liver disease and PNPLA3 variants do not seem to have worsening insulin resistance. Further studies are needed to better define tissue-specific insulin resistance in liver and muscle, how these different polymorphisms might affect the composition and cellular localisation of intracellular lipids, and identify additional genetic factors that might affect the development of insulin resistance.
PKCε, hepatic steatosis, insulin resistance
Wild-type mice and rats develop hepatic steatosis after a few days of high-fat feeding that is associated with hepatic insulin resistance, without much change in muscle lipid content or peripheral insulin action.100 Moreover, by promotion of mitochondrial fatty acid oxidation with low doses of the mitochondrial uncoupler 2,4-dinitrophenol, rats were protected from fat-induced hepatic steatosis and hepatic insulin resistance.100 In this model, hepatic steatosis was associated with proximal defects in insulin signalling, with decreased tyrosine phosphorylation of IRS1 and IRS2 by the insulin receptor, ultimately impairing the ability of insulin to activate hepatic glycogen synthesis and suppress hepatic glucose production. This inability of insulin to regulate hepatic glycogen synthesis and glucose production has been shown in patients with type 2 diabetes mellitus.101,102 PKCs again were the logical link between hepatic steatosis and hepatic insulin resistance. Though PKCε is poorly expressed in the liver, PKCε, another novel PKC (figure 2), is expressed in high concentrations and activated in the fatty liver. If hepatic steatosis was prevented with the use of 2,4-dinitrophenol, PKCε activation was also prevented. The association between PKCε and hepatic insulin resistance has now been shown in other rodent models.53,103–106
The specific role of PKCε in the pathogenesis of hepatic insulin resistance was assessed by use of antisense oligonucleotides containing a modified 2´ -O-(2-methoxy) ethyl and phosphorothioate bond to enhance potency, stability, and cell permeability.107 They are taken up preferentially in the liver, adipose tissue, and kidney, though not in other key tissues such as muscle, brain, or β cells. The effect of a specific PKCε antisense oligonucleotide was assessed in rats fed a high-fat diet for 3 days. Though fat accumulation, and specifically diacylglycerol accumulation, was equal in all groups, the PKCε antisense oligonucleotide improved hepatic insulin sensitivity and insulin signalling. Specifically, PKCε antisense oligonucleotide prevented the impairment in insulin receptor kinase activity noted with high-fat feeding. Thus, by blockage of PKCε, hepatic insulin action was preserved despite the development of fatty liver.
Insulin resistance and lipodystrophy
One challenge in the assessment of the specific role of non-alcoholic fatty liver disease in the development of hepatic insulin resistance is the close association between obesity and non-alcoholic fatty liver disease. Thus, the changes in liver insulin action due to steatosis and those attributable to adiposity and associated changes, such as inflammation, are difficult to ascertain.108–110 The lipodystrophies offer an opportunity to assess the role of ectopic lipid deposition without any contribution from an expansion in peripheral or visceral adipose tissue mass. Lipo dystrophy and lipoatrophy are discrete genetic and acquired disorders with a lack of adipocytes, either through impaired fat-cell formation (eg, lipoatrophy) or acquired destruction of fat cells often noted in patients treated with antiretroviral drugs.111,112 Indi viduals with severe, generalised lipodystrophy have a substantial reduction in fat cells, are hypoleptinaemic, and consequentially many are hyperphagic. The lack of subcutaneous fat leads to hypertriglyceridaemia, insulin resistance, and ectopic fat deposition, including substantial hepatic steatosis.
Lipodystrophy can be modelled in mice. Moitra and colleagues113 created mice that were devoid of white adipose tissue by expressing a dominant negative protein A-ZIP/F under the control of the adipocyte specific AP-1 promoter. As in human generalised lipodystrophy, A-ZIP/F mice have no adipocytes and develop fat accumulation in the liver and skeletal muscle, and have profound peripheral and hepatic insulin resistance.114 Transplantation of fat pads into these mice from wild-type littermates rescued the phenotype and normalised the concentrations of tissue lipid, and hepatic and muscle insulin signalling and action. Shimomura and colleagues115 showed the potential to correct many of the metabolic defects associated with lipodystrophy by administering leptin to lipodystrophic mice.
Consistent with the results of the studies in mice, recombinant leptin restored plasma leptin to physiological concentrations, normalised plasma concentrations of glucose and lipids under fasting conditions, and corrected abnormalities in liver function tests in patients with severe congenital, generalised lipodystrophy.82,116 Before leptin replacement therapy, patients with lipodystrophy had higher basal rates of glucose production than did controls matched for age, weight, and sex; they also could not suppress hepatic glucose production and could not stimulate peripheral glucose uptake during hyperinsulinaemic-normoglycaemic conditions.82 After leptin replacement therapy, hepatic triglyceride content decreased by about 90% with improvements in hepatic insulin responsiveness. Similarly, the roughly 30% reduction in muscle triglyceride content was associated with a near doubling in insulin-stimulated whole-body glucose disposal. The results of these studies in patients with lipodystrophy and mouse models of severe lipodystrophy show that ectopic accumulation of lipids can lead to insulin resistance, even in the absence of peripheral and visceral adiposity.
Other hypotheses
The data presented so far have supported a unifying theme—namely, that the accumulation of diacylglycerol within insulin-sensitive tissues activates novel PKCs that interfere with insulin signalling and cause insulin resistance. However, other mechanisms have been proposed to explain insulin resistance in obesity. These are only briefly discussed here, since other reviews are available.117,118
Though we have endeavoured to show how accumulation of diacylglycerol leads to insulin resistance, not all lipid species are pathogenetic—eg, omega-3 fatty acids have many beneficial effects. They might promote hepatic fat oxidation in rodents through a PPARα-dependent mechanism and increase plasma adiponectin through PPARγ activation,119 thereby preventing ectopic diacylglycerol accumulation and preserving insulin action despite a high-fat diet.120–122 Palmitoleate (C16:1n7) has also been suggested as an endogenously produced fatty acid that is secreted from adipocytes (ie, a lipokine); it might improve insulin action in liver and muscle in mice,123 and higher concentrations are associated with increased insulin sensitivity in people.124
Clinically, insulin resistance and a proinflammatory state are both associated with the metabolic syndrome. Mechanistically, inflammatory signals affect cellular pathways that intersect with insulin action.117 Specifically, inflammatory signals such as tumour necrosis factor α and interleukin 6 activate serine and threonine kinases such as Iκ kinase β and jun-N terminal kinase, which have been implicated in increased serine phosphor ylation of IRS1. The development of hepatic insulin resistance after lipid infusions was associated with activation of the Iκ kinase β-nuclear factor-κB pathway.125 Cai and colleagues126 showed the potential for inflammation to cause insulin resistance by genetically activating this pathway specifically in the livers of mice. These mice developed both hepatic and peripheral insulin resistance. Moreover, the insulin resistance was ameliorated with salicylates, which also protected against insulin resistance in rodents after acute lipid infusion,127 and reversed insulin resistance in people with type 2 diabetes mellitus.128
Inflammatory pathways might also be activated in response to endoplasmic-reticulum stress.118 These pathways protect cells from producing aberrant proteins, hence referred to as the unfolded protein response. Their activation has been shown in rodent models of obesity129 and in human obesity.130 Modulation of endoplasmicreticulum stress by genetic or chemical means ameliorates jun-N terminal kinase activation and the development of insulin resistance.131 Substantial weight loss in patients after bariatric surgery has also been associated with both improvements in insulin sensitivity and reduction in markers of endoplasmic-reticulum stress.132
Correction of hepatic steatosis
Thiazolidinediones, which are potent PPARγ agonists, can effectively reduce hepatic steatosis.133 Though PPARγ is mainly expressed in adipocytes, it has effects on hepatic and muscle insulin sensitivity. On the basis of this discordance between the site of PPARγ expression and the site of drug effects, the hypothesis was that thiazolidinediones redistribute fat from the liver and muscle into the adipocyte.46 Mayerson and colleagues133 tested this hypothesis, using rosiglitazone in patients with type 2 diabetes mellitus. Treatment for 3 months with rosiglitazone was associated with an almost 40% reduction in hepatic triglyceride content, and roughly 40% increase in extramyocellular triglyceride concentration, and improved suppression of adipocyte lipolysis.133 Though no reductions in the concentration of intramyocellular triglyceride were noted in this study, rosiglitazone improved insulin-mediated whole-body glucose disposal, consistent with improvements in muscle insulin sensitivity. This disconnection between intramyocellular triglyceride and peripheral insulin action confirms that intra myocellular triglyceride is only a crude marker for the active metabolite (putatively diacylglycerol) that causes fat-induced insulin resistance.38 Reductions in hepatic steatosis with both pioglitazone and rosig litazone ameliorate hepatic insulin resistance.134,135 These data support the hypothesis that thiazo lidinediones exert their beneficial effects through reversal of insulin resistance in patients with type 2 diabetes mellitus by shifting intracellular lipid from liver and muscle into adipose tissue.
Small amounts of weight loss can return concentrations of plasma glucose to normal in patients with type 2 diabetes mellitus.136,137 In obese patients with type 2 diabetes mellitus, after an average weight loss of 8 kg over 7 weeks on a hypocaloric (1200 kcal) low-fat diet, intramyocellular lipid concentrations or muscle insulin sensitivity did not change; however, there were substantial reductions in hepatic triglyceride concentrations (from nearly 12% to 2%), with concordant improvements in hepatic insulin sensitivity and correction of fasting hyperglycaemia.81 Additionally, fitness affects the response to calorie reduction; obese individuals with a high baseline cardiorespiratory fitness might have a greater reduction in liver fat with diet-induced weight loss.138 Thus improvement of fitness might improve the resolution of non-alcoholic fatty liver disease with weight loss.
Way forward
Achievement of sustainable weight loss, without bariatric surgery, is an enormously difficult task and, although small steps are being taken for the prevention of obesity, many hurdles remain. Until societal, political, and economic forces align to promote healthy lifestyles, the incidence of obesity, and consequently insulin resistance and type 2 diabetes mellitus, will probably increase. The development of new effective treatments for insulin resistance requires an elucidation of the underlying primary mechanism. Inflammation, endoplasmic-reticulum stress, adipokines, and lipokines have roles in the pathogenesis of insulin resistance in liver and skeletal muscle. However, because these do not change in lean insulin-resistant young and elderly individuals, they are probably secondary in nature, becoming manifest later in the course of disease, and are associated with the development of obesity. The unifying model of diacylglycerol-induced insulin resistance accounts for the insulin resistance seen in obesity, and in other disorders such as congenital and acquired lipodystrophy,82 in young lean offspring of patients with type 2 diabetes mellitus, and with increase in age.66 Moreover, reductions in intracellular diacylglycerol content account for the improvements in insulin sensitivity after weight loss81 and therapy with thiazolidinediones.133 Further studies are needed to improve our understanding of how tissue-specific diacylglycerol accumulation activates specific novel PKCs, whether the intracellular distribution of this pool differs in insulin-sensitive and insulin-resistant states, and to better understand how activation of novel PKCs impairs insulin signalling. These studies will hopefully serve the development of new treatments to correct insulin resistance and halt its progression to type 2 diabetes mellitus.
Search strategy and selection criteria.
We searched PubMed with the search terms “insulin resistance” in combination with “skeletal muscle”, “liver”, “lipids”, or “diacylglycerol” from January, 1963, until February, 2010. We also searched with “protein kinase C” and “diacylglycerol”. Papers were restricted to those published in the English language. We gave preference to recent and relevant reports, and important papers that addressed the main themes reviewed in this Seminar. Relevant review articles were selected to provide more comprehensive reference lists than were provided in this Seminar.
Acknowledgments
These studies were supported by grants from the Veterans Administration Merit Review Award, the US Public Health Service: R01 AG-23686, R01 DK-49230, P01 DK-068229, R01 DK-40936, P30 DK-45735, U24 DK-59635, UL1 RR-024139, K23 RR17404, and UL1 RR024139; and Distinguished Clinical Scientist Awards from the American Diabetes Association (KFP and GIS).
Footnotes
Contributors
All authors contributed to writing this Seminar, and have approved the final version.
Conflicts of interest
We declare that we have no conflicts of interest.
Contributor Information
Varman T Samuel, Department of Internal Medicine; Veteran’s Affairs Medical Center, West Haven, CT, USA.
Kitt Falk Petersen, Department of Internal Medicine.
Gerald I Shulman, Department of Internal Medicine; Department of Cellular and Molecular Physiology, and Howard Hughes Medical Institute, Yale University School of Medicine, New Haven, CT, USA.
References
- 1.Prentice AM, Hennig BJ, Fulford AJ. Evolutionary origins of the obesity epidemic: natural selection of thrifty genes or genetic drift following predation release? Int J Obes (Lond) 2008;32:1607–1610. doi: 10.1038/ijo.2008.147. [DOI] [PubMed] [Google Scholar]
- 2.Harris JL, Pomeranz JL, Lobstein T, Brownell KD. A crisis in the marketplace: how food marketing contributes to childhood obesity and what can be done. Annu Rev Public Health. 2009;30:211–225. doi: 10.1146/annurev.publhealth.031308.100304. [DOI] [PubMed] [Google Scholar]
- 3.Diet, nutrition and the prevention of chronic diseases. World Health Organ Tech Rep Ser. 2003;916:1–149. [PubMed] [Google Scholar]
- 4.Mendez MA, Monteiro CA, Popkin BM. Overweight exceeds underweight among women in most developing countries. Am J Clin Nutr. 2005;81:714–721. doi: 10.1093/ajcn/81.3.714. [DOI] [PubMed] [Google Scholar]
- 5.Sherry B, Mei Z, Scanlon KS, Mokdad AH, Grummer-Strawn LM. Trends in state-specific prevalence of overweight and underweight in 2- through 4-year-old children from low-income families from 1989 through 2000. Arch Pediatr Adolesc Med. 2004;158:1116–1124. doi: 10.1001/archpedi.158.12.1116. [DOI] [PubMed] [Google Scholar]
- 6.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 7.Perseghin G, Ghosh S, Gerow K, Shulman GI. Metabolic defects in lean nondiabetic offspring of NIDDM parents: a cross-sectional study. Diabetes. 1997;46:1001–1009. doi: 10.2337/diab.46.6.1001. [DOI] [PubMed] [Google Scholar]
- 8.Krssak M, Falk Petersen K, Dresner A, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 9.Perseghin G, Scifo P, De Cobelli F, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48:1600–1606. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 10.Pan DA, Lillioja S, Kriketos AD, et al. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997;46:983–988. doi: 10.2337/diab.46.6.983. [DOI] [PubMed] [Google Scholar]
- 11.Taylor R, Price TB, Rothman DL, Shulman RG, Shulman GI. Validation of 13C NMR measurement of human skeletal muscle glycogen by direct biochemical assay of needle biopsy samples. Magn Reson Med. 1992;27:13–20. doi: 10.1002/mrm.1910270103. [DOI] [PubMed] [Google Scholar]
- 12.Rothman DL, Magnusson I, Cline G, et al. Decreased muscle glucose transport/phosphorylation is an early defect in the pathogenesis of non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci USA. 1995;92:983–987. doi: 10.1073/pnas.92.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy [see comments] N Engl J Med. 1990;322:223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- 14.Rothman DL, Shulman RG, Shulman GI. 1P nuclear magnetic resonance measurements of muscle glucose-6-phosphate. Evidence for reduced insulin-dependent muscle glucose transport or phosphorylation activity in non-insulin-dependent diabetes mellitus. J Clin Invest. 1992;89:1069–1075. doi: 10.1172/JCI115686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brechtel K, Dahl DB, Machann J, et al. Fast elevation of the intramyocellular lipid content in the presence of circulating free fatty acids and hyperinsulinemia: a dynamic H-MRS study. Magn Reson Med. 2001;45:179–183. doi: 10.1002/1522-2594(200102)45:2<179::aid-mrm1023>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 16.Felber JP, Golay A. Pathways from obesity to diabetes. Int J Obes Relat Metab Disord. 2002;26 suppl 2:S39–S45. doi: 10.1038/sj.ijo.0802126. [DOI] [PubMed] [Google Scholar]
- 17.Roden M, Price TB, Perseghin G, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- 19.Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 1994;93:2438–2446. doi: 10.1172/JCI117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dresner A, Laurent D, Marcucci M, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cline GW, Petersen KF, Krssak M, et al. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N Engl J Med. 1999;341:240–246. doi: 10.1056/NEJM199907223410404. [DOI] [PubMed] [Google Scholar]
- 22.Joost H-G, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members. Mol Membr Biol. 2001;18:247–256. doi: 10.1080/09687680110090456. [DOI] [PubMed] [Google Scholar]
- 23.Shepherd PR, Kahn BB. Glucose transporters and insulin action— implications for insulin resistance and diabetes mellitus. N Engl J Med. 1999;341:248–257. doi: 10.1056/NEJM199907223410406. [DOI] [PubMed] [Google Scholar]
- 24.Ciaraldi TP, Abrams L, Nikoulina S, Mudaliar S, Henry RR. Glucose transport in cultured human skeletal muscle cells. Regulation by insulin and glucose in nondiabetic and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1995;96:2820–2827. doi: 10.1172/JCI118352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garvey WT, Maianu L, Zhu JH, Brechtel-Hook G, Wallace P, Baron AD. Evidence for defects in the trafficking and translocation of GLUT4 glucose transporters in skeletal muscle as a cause of human insulin resistance. J Clin Invest. 1998;101:2377–2386. doi: 10.1172/JCI1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson RT, Pessin JE. GLUT4 translocation: the last 200 nanometers. Cell Signal. 2007;19:2209–2217. doi: 10.1016/j.cellsig.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Saad MJ, Folli F, Kahn JA, Kahn CR. Modulation of insulin receptor, insulin receptor substrate-1, and phosphatidylinositol 3-kinase in liver and muscle of dexamethasone-treated rats. J Clin Invest. 1993;92:2065–2072. doi: 10.1172/JCI116803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folli F, Saad MJ, Backer JM, Kahn CR. Regulation of phosphatidylinositol 3-kinase activity in liver and muscle of animal models of insulin-resistant and insulin-deficient diabetes mellitus. J Clin Invest. 1993;92:1787–1794. doi: 10.1172/JCI116768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J. 2003;370(part 2):361–371. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dries DR, Gallegos LL, Newton AC. A single residue in the c1 domain sensitizes novel protein kinase C isoforms to cellular diacylglycerol production. J Biol Chem. 2007;282:826–830. doi: 10.1074/jbc.C600268200. [DOI] [PubMed] [Google Scholar]
- 31.Stahelin RV, Digman MA, Medkova M, et al. Diacylglycerol-induced membrane targeting and activation of protein kinase CÏμ. J Biol Chem. 2005;280:19784–19793. doi: 10.1074/jbc.M411285200. [DOI] [PubMed] [Google Scholar]
- 32.Takayama S, White MF, Kahn CR. Phorbol ester-induced serine phosphorylation of the insulin receptor decreases its tyrosine kinase activity. J Biol Chem. 1988;263:3440–3447. [PubMed] [Google Scholar]
- 33.Lewis RE, Cao L, Perregaux D, Czech MP. Threonine 1336 of the human insulin receptor is a major target for phosphorylation by protein kinase C. Biochemistry. 1990;29:1807–1813. doi: 10.1021/bi00459a020. [DOI] [PubMed] [Google Scholar]
- 34.Pillay TS, Whittaker J, Siddle K. Phorbol ester-induced downregulation of protein kinase C potentiates insulin receptor tyrosine autophosphorylation: evidence for a major constitutive role in insulin receptor regulation. Biochem Soc Trans. 1990;18:494–495. doi: 10.1042/bst0180494. [DOI] [PubMed] [Google Scholar]
- 35.Anderson CM, Olefsky JM. Phorbol ester-mediated protein kinase C interaction with wild-type and COOH-terminal truncated insulin receptors. J Biol Chem. 1991;266:21760–21764. [PubMed] [Google Scholar]
- 36.Schmitz-Peiffer C, Browne CL, Oakes ND, et al. Alterations in the expression and cellular localization of protein kinase C isozymes epsilon and theta are associated with insulin resistance in skeletal muscle of the high-fat-fed rat. Diabetes. 1997;46:169–178. doi: 10.2337/diab.46.2.169. [DOI] [PubMed] [Google Scholar]
- 37.Griffin ME, Marcucci MJ, Cline GW, et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 38.Yu C, Chen Y, Cline GW, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 39.Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in Insulin Receptor Substrate-1 Blocks Interactions with the Insulin Receptor and Inhibits Insulin Action. J Biol Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 40.Kim JK, Fillmore JJ, Sunshine MJ, et al. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest. 2004;114:823–827. doi: 10.1172/JCI22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morino K, Neschen S, Bilz S, et al. Muscle-specific IRS-1 Ser->Ala transgenic mice are protected from fat-induced insulin resistance in skeletal muscle. Diabetes. 2008;57:2644–2651. doi: 10.2337/db06-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 43.Itani SI, Pories WJ, Macdonald KG, Dohm GL. Increased protein kinase C theta in skeletal muscle of diabetic patients. Metabolism. 2001;50:553–557. doi: 10.1053/meta.2001.22512. [DOI] [PubMed] [Google Scholar]
- 44.Morino K, Petersen KF, Dufour S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corbould A, Kim YB, Youngren JF, et al. Insulin resistance in the skeletal muscle of women with PCOS involves intrinsic and acquired defects in insulin signaling. Am J Physiol Endocrinol Metab. 2005;288:E1047–E1054. doi: 10.1152/ajpendo.00361.2004. [DOI] [PubMed] [Google Scholar]
- 46.Shulman G. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim JK, Fillmore JJ, Chen Y, et al. Tissue-specific overexpression of lipoprotein lipase causes tissue- specific insulin resistance. Proc Natl Acad Sci USA. 2001;98:7522–7527. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang H, Knaub LA, Jensen DR, et al. Skeletal muscle-specific deletion of lipoprotein lipase enhances insulin signaling in skeletal muscle but causes insulin resistance in liver and other tissues. Diabetes. 2009;58:116–124. doi: 10.2337/db07-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goudriaan JR, Dahlmans VEH, Teusink B, et al. CD36 deficiency increases insulin sensitivity in muscle, but induces insulin resistance in the liver in mice. J Lipid Res. 2003;44:2270–2277. doi: 10.1194/jlr.M300143-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Hajri T, Han XX, Bonen A, Abumrad NA. Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J Clin Invest. 2002;109:1381–1389. doi: 10.1172/JCI14596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim JK, Gimeno RE, Higashimori T, et al. Inactivation of fatty acid transport protein 1 prevents fat-induced insulin resistance in skeletal muscle. J Clin Invest. 2004;113:756–763. doi: 10.1172/JCI18917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi CS, Fillmore JJ, Kim JK, et al. Overexpression of uncoupling protein 3 in skeletal muscle protects against fat-induced insulin resistance. J Clin Invest. 2007;117:1995–2003. doi: 10.1172/JCI13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi CS, Savage DB, Abu-Elheiga L, et al. Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc Natl Acad Sci USA. 2007;104:16480–16485. doi: 10.1073/pnas.0706794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoehn KL, Turner N, Swarbrick MM, et al. Acute or chronic upregulation of mitochondrial fatty acid oxidation has no net effect on whole-body energy expenditure or adiposity. Cell Metab. 2010;11:70–76. doi: 10.1016/j.cmet.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim JY, van de Wall E, Laplante M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bajaj M, Suraamornkul S, Piper P, et al. Decreased plasma adiponectin concentrations are closely related to hepatic fat content and hepatic insulin resistance in pioglitazone-treated type 2 diabetic patients. J Clin Endocrinol Metab. 2004;89:200–206. doi: 10.1210/jc.2003-031315. [DOI] [PubMed] [Google Scholar]
- 57.Lindsay RS, Funahashi T, Hanson RL, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–58. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- 58.Kotronen A, Seppala-Lindroos A, Bergholm R, Yki-Jarvinen H. Tissue specificity of insulin resistance in humans: fat in the liver rather than muscle is associated with features of the metabolic syndrome. Diabetologia. 2008;51:130–138. doi: 10.1007/s00125-007-0867-x. [DOI] [PubMed] [Google Scholar]
- 59.Weiss R, Dufour S, Groszmann A, et al. Low adiponectin levels in adolescent obesity: a marker of increased intramyocellular lipid accumulation. J Clin Endocrinol Metab. 2003;88:2014–2018. doi: 10.1210/jc.2002-021711. [DOI] [PubMed] [Google Scholar]
- 60.Stefan N, Vozarova B, Funahashi T, et al. Plasma adiponectin concentration is associated with skeletal muscle insulin receptor tyrosine phosphorylation, and low plasma concentration precedes a decrease in whole-body insulin sensitivity in humans. Diabetes. 2002;51:1884–1888. doi: 10.2337/diabetes.51.6.1884. [DOI] [PubMed] [Google Scholar]
- 61.Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu YH. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest. 2007;117:1679–1689. doi: 10.1172/JCI30565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86:5755–5761. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- 63.Krssak M, Petersen KF, Bergeron R, et al. Intramuscular glycogen and intramyocellular lipid utilization during prolonged exercise and recovery in man: a 13C and 1H nuclear magnetic resonance spectroscopy study. J Clin Endocrinol Metab. 2000;85:748–754. doi: 10.1210/jcem.85.2.6354. [DOI] [PubMed] [Google Scholar]
- 64.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest. 2007;117:1690–1698. doi: 10.1172/JCI30566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chibalin AV, Leng Y, Vieira E, et al. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell. 2008;132:375–386. doi: 10.1016/j.cell.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 66.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lebon V, Dufour S, Petersen KF, et al. Effect of triiodothyronine on mitochondrial energy coupling in human skeletal muscle. J Clin Invest. 2001;108:733–737. doi: 10.1172/JCI11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Befroy DE, Petersen KF, Dufour S, et al. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes. 2007;56:1376–1381. doi: 10.2337/db06-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 71.Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abbatecola AM, Ferrucci L, Grella R, et al. Diverse effect of inflammatory markers on insulin resistance and insulin-resistance syndrome in the elderly. J Am Geriatr Soc. 2004;52:399–404. doi: 10.1111/j.1532-5415.2004.52112.x. [DOI] [PubMed] [Google Scholar]
- 73.Rodriguez A, Muller DC, Engelhardt M, Andres R. Contribution of impaired glucose tolerance in subjects with the metabolic syndrome: Baltimore Longitudinal Study of Aging. Metabolism. 2005;54:542–547. doi: 10.1016/j.metabol.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 74.Wilson PW, Anderson KM, Kannel WB. Epidemiology of diabetes mellitus in the elderly. The Framingham Study. Am J Med. 1986;80:3–9. doi: 10.1016/0002-9343(86)90532-2. [DOI] [PubMed] [Google Scholar]
- 75.Cree MG, Newcomer BR, Katsanos CS, et al. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab. 2004;89:3864–3871. doi: 10.1210/jc.2003-031986. [DOI] [PubMed] [Google Scholar]
- 76.Nakagawa Y, Hattori M, Harada K, Shirase R, Bando M, Okano G. age-related changes in intramyocellular lipid in humans by in vivo 1 H-MR spectroscopy. Gerontology. 2007;53:218–223. doi: 10.1159/000100869. [DOI] [PubMed] [Google Scholar]
- 77.Boumezbeur F, Mason GF, de Graaf RA, et al. Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 2010;30:211–221. doi: 10.1038/jcbfm.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 79.McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:521–533. doi: 10.1016/j.cld.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 80.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 81.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Petersen KF, Oral EA, Dufour S, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Previs SF, Withers DJ, Ren JM, et al. Contrasting effects of IRS-1 versus IRS-2 gene disruption on carbohydrate and lipid metabolism in vivo Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J Biol Chem. 2000;275:38990–38994. doi: 10.1074/jbc.M006490200. [DOI] [PubMed] [Google Scholar]
- 84.Utzschneider KM, Kahn SE. The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91:4753–4761. doi: 10.1210/jc.2006-0587. [DOI] [PubMed] [Google Scholar]
- 85.Marchesini G, Moscatiello S, Di Domizio S, Forlani G. Obesity-associated liver disease. J Clin Endocrinol Metab. 2008;93(11 suppl 1):S74–S80. doi: 10.1210/jc.2008-1399. [DOI] [PubMed] [Google Scholar]
- 86.Abdelmalek MF, Diehl AM. Nonalcoholic fatty liver disease as a complication of insulin resistance. Med Clin North Am. 2007;91:1125–1149. doi: 10.1016/j.mcna.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 87.Kim JK, Michael MD, Previs SF, et al. Redistribution of substrates to adipose tissue promotes obesity in mice with selective insulin resistance in muscle. J Clin Invest. 2000;105:1791–1797. doi: 10.1172/JCI8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim JK, Zisman A, Fillmore JJ, et al. Glucose toxicity and the development of diabetes in mice with muscle-specific inactivation of GLUT4. J Clin Invest. 2001;108:153–160. doi: 10.1172/JCI10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Petersen KF, Dufour S, Savage DB, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci USA. 2007;104:12587–12594. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 91.Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Petersen KF, Dufour S, Feng J, et al. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Natl Acad Sci USA. 2006;103:18273–18277. doi: 10.1073/pnas.0608537103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Petersen KF, Dufour S, Hariri A, et al. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. N Engl J Med. 2010;362:1082–1089. doi: 10.1056/NEJMoa0907295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 95.Liska D, Dufour S, Zern TL, et al. Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PLoS One. 2007;2:e569. doi: 10.1371/journal.pone.0000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.He S, McPhaul C, Li JZ, et al. A sequence variation (I148M) in PNPlA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J Biol Chem. 2010;285:6706–6715. doi: 10.1074/jbc.M109.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kantartzis K, Peter A, Machicao F, et al. Dissociation between fatty liver and insulin resistance in humans carrying a variant of the patatin-like phospholipase 3 gene. Diabetes. 2009;58:2616–2623. doi: 10.2337/db09-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kotronen A, Johansson LE, Johansson LM, et al. A common variant in PNPLA3, which encodes adiponutrin, is associated with liver fat content in humans. Diabetologia. 2009;52:1056–1060. doi: 10.1007/s00125-009-1285-z. [DOI] [PubMed] [Google Scholar]
- 100.Samuel VT, Liu ZX, Qu X, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–33253. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 101.Krssak M, Brehm A, Bernroider E, et al. Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes. 2004;53:3048–3056. doi: 10.2337/diabetes.53.12.3048. [DOI] [PubMed] [Google Scholar]
- 102.Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest. 1992;90:1323–1327. doi: 10.1172/JCI115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Savage DB, Choi CS, Samuel VT, et al. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest. 2006;116:817–824. doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang D, Liu Z-X, Choi CS, et al. Mitochondrial dysfunction due to long-chain Acyl-CoA dehydrogenase deficiency causes hepatic steatosis and hepatic insulin resistance. Proc Natl Acad Sci USA. 2007;104:17075–17080. doi: 10.1073/pnas.0707060104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Matsuzaka T, Shimano H, Yahagi N, et al. Crucial role of a long-chain fatty acid elongase, Elovl6, in obesity-induced insulin resistance. Nat Med. 2007;13:1193–1202. doi: 10.1038/nm1662. [DOI] [PubMed] [Google Scholar]
- 106.Varela GM, Antwi DA, Dhir R, et al. Inhibition of ADRP prevents diet-induced insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2008;295:G621–G628. doi: 10.1152/ajpgi.90204.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crooke ST. Progress in antisense technology. Annu Rev Med. 2004;55:61–95. doi: 10.1146/annurev.med.55.091902.104408. [DOI] [PubMed] [Google Scholar]
- 108.Gastaldelli A, Miyazaki Y, Pettiti M, et al. Separate contribution of diabetes, total fat mass, and fat topography to glucose production, gluconeogenesis, and glycogenolysis. J Clin Endocrinol Metab. 2004;89:3914–3921. doi: 10.1210/jc.2003-031941. [DOI] [PubMed] [Google Scholar]
- 109.Miyazaki Y, Glass L, Triplitt C, Wajcberg E, Mandarino LJ, DeFronzo RA. Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2002;283:E1135–E1143. doi: 10.1152/ajpendo.0327.2001. [DOI] [PubMed] [Google Scholar]
- 110.Rebrin K, Steil GM, Getty L, Bergman RN. Free fatty acid as a link in the regulation of hepatic glucose output by peripheral insulin. Diabetes. 1995;44:1038–1045. doi: 10.2337/diab.44.9.1038. [DOI] [PubMed] [Google Scholar]
- 111.Carr A, Samaras K, Chisholm DJ, Cooper DA. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998;351:1881–1883. doi: 10.1016/S0140-6736(98)03391-1. [DOI] [PubMed] [Google Scholar]
- 112.Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350:1220–1234. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 113.Moitra J, Mason MM, Olive M, et al. Life without white fat: a transgenic mouse. Genes Dev. 1998;12:3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim JK, Gavrilova O, Chen Y, Reitman ML, Shulman GI. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J Biol Chem. 2000;275:8456–8460. doi: 10.1074/jbc.275.12.8456. [DOI] [PubMed] [Google Scholar]
- 115.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 116.Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 117.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hotamisligil GKS. Role of Endoplasmic Reticulum Stress and c-Jun NH2-Terminal Kinase Pathways in Inflammation and Origin of Obesity and Diabetes. Diabetes. 2005;54 suppl 2:S73–S78. doi: 10.2337/diabetes.54.suppl_2.s73. [DOI] [PubMed] [Google Scholar]
- 119.Neschen S, Morino K, Rossbacher J, et al. Fish oil regulates adiponectin secretion by a PPAR*-dependent mechanism in mice. Diabetes. 2006;55:924–928. doi: 10.2337/diabetes.55.04.06.db05-0985. [DOI] [PubMed] [Google Scholar]
- 120.Neschen S, Moore I, Regittnig W, et al. Contrasting effects of fish oil and safflower oil on hepatic peroxisomal and tissue lipid content. Am J Physiol Endocrinol Metab. 2002;282:E395–E401. doi: 10.1152/ajpendo.00414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Neschen S, Morino K, Dong J, et al. n-3 Fatty acids preserve insulin sensitivity in vivo in a peroxisome proliferator-activated receptor-alpha-dependent manner. Diabetes. 2007;56:1034–1041. doi: 10.2337/db06-1206. [DOI] [PubMed] [Google Scholar]
- 122.Storlien LH, Jenkins AB, Chisholm DJ, Pascoe WS, Khouri S, Kraegen EW. Infl uence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes. 1991;40:280–289. doi: 10.2337/diab.40.2.280. [DOI] [PubMed] [Google Scholar]
- 123.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stefan N, Kantartzis K, Celebi N, et al. Circulating palmitoleate strongly and independently predicts insulin sensitivity in humans. Diabetes Care. 2010;33:405–407. doi: 10.2337/dc09-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Boden G, She P, Mozzoli M, et al. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-{kappa}b pathway in rat liver. Diabetes. 2005;54:3458–3465. doi: 10.2337/diabetes.54.12.3458. [DOI] [PubMed] [Google Scholar]
- 126.Cai D, Yuan M, Frantz DF, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim JK, Kim YJ, Fillmore JJ, et al. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108:437–446. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hundal RS, Petersen KF, Mayerson AB, et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321–1326. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 130.Boden G, Duan X, Homko C, et al. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 2008;57:2438–2444. doi: 10.2337/db08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gregor MF, Yang L, Fabbrini E, et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mayerson AB, Hundal RS, Dufour S, et al. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 2002;51:797–802. doi: 10.2337/diabetes.51.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Miyazaki Y, Mahankali A, Matsuda M, et al. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2002;87:2784–2791. doi: 10.1210/jcem.87.6.8567. [DOI] [PubMed] [Google Scholar]
- 135.Tiikkainen M, Hakkinen A-M, Korsheninnikova E, Nyman T, Makimattila S, Yki-Jarvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes. 2004;53:2169–2176. doi: 10.2337/diabetes.53.8.2169. [DOI] [PubMed] [Google Scholar]
- 136.Henry RR, Scheaffer L, Olefsky JM. Glycemic effects of intensive caloric restriction and isocaloric refeeding in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1985;61:917–925. doi: 10.1210/jcem-61-5-917. [DOI] [PubMed] [Google Scholar]
- 137.Henry RR, Wallace P, Olefsky JM. Effects of weight loss on mechanisms of hyperglycemia in obese non-insulin-dependent diabetes mellitus. Diabetes. 1986;35:990–998. doi: 10.2337/diab.35.9.990. [DOI] [PubMed] [Google Scholar]
- 138.Kantartzis K, Thamer C, Peter A, et al. High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut. 2009;58:1281–1288. doi: 10.1136/gut.2008.151977. [DOI] [PubMed] [Google Scholar]