Contextual setting
Although abnormal glucose metabolism defines type 2 diabetes and accounts for many of its symptoms and complications, efforts to understand the pathogenesis of type 2 diabetes are increasingly focussed on disordered lipid metabolism. Here we review recent human studies exploring the mechanistic links between disorders of fatty acid-/ lipid metabolism and insulin resistance. As “Mouse Models of Insulin Resistance” were comprehensively reviewed in Physiological Reviews by Nandi et al in 2004 (67), we will concentrate on human studies involving the use of isotopes and/ or magnetic resonance spectroscopy, occasionally drawing on mouse models which provide additional mechanistic insight.
Introduction
Insulin resistance is a key element in the pathogenesis of the metabolic syndrome and type 2 diabetes, both of which have reached epidemic proportions worldwide (130). Although the pathogenesis of type 2 diabetes remains poorly understood, most investigators agree on the following:
Insulin resistance, which can be defined as a state of reduced responsiveness to normal circulating levels of insulin, plays a major role in the development of type 2 diabetes. This conclusion is based upon the following observations: i) cross sectional studies demonstrating the consistent presence of insulin resistance in patients with type 2 diabetes (37, 56); ii) the presence of insulin resistance in non-diabetic offspring of patients with type 2 diabetes (118); iii) prospective studies demonstrating the usefulness of insulin resistance as a predictive marker of the future development of type 2 diabetes (56, 118); and iv) prevention of diabetes by insulin sensitizing agents (16).
Early in the disease, β-cells secrete sufficient insulin to compensate for insulin resistance and maintain euglycemia. Ultimately however, relative or absolute insulin deficiency supervenes precipitating hyperglycemia and overt diabetes. β-cell dysfunction is therefore a sine qua non of the diabetic state but need not be the primary abnormality (8).
Type 2 diabetes is a heterogeneous cluster of conditions rather than a uniform entity. The spectrum includes individuals with maturity-onset diabetes of the young or MODY, manifesting predominantly β-cell dysfunction caused by mutations in genes involved in β-cell function (29), and people with Donohue’s syndrome due to insulin receptor mutations, whose phenotype is dominated by insulin resistance (52).
The current rise in prevalence of type 2 diabetes and the metabolic syndrome is believed to be a result of increasingly sedentary lifestyles combined with ready access to energy rich food sources in genetically susceptible individuals. Healthy humans respond to positive energy balance primarily by storing excess energy as triglyceride in adipose tissue. While this response enables humans to cope efficiently with fluctuating energy supplies, it predisposes persistently over-nourished individuals to weight gain and ultimately obesity. It also appears to induce lipid accumulation in “ectopic sites” such as the liver and skeletal muscle, and even in pancreatic β-cells and possibly the kidney (115). The simple explanation being that in obese states, energy intake exceeds the storage capacity of adipose tissue leading to energy “overflow” to ectopic sites. This notion is supported by the almost universal finding of ectopic lipid accumulation in mice and humans with generalized lipodystrophy1, an extreme example of limited adipose tissue storage capacity in the face of excess calorie ingestion (food intake tends to be increased in subjects with generalised lipodystrophy secondary to hypoleptinemia). One way to reduce ectopic lipid deposition in lipodystrophic mice is to transplant adipose tissue from wild type mice, a procedure which dramatically improved insulin sensitivity (35, 48). Another way in which ectopic lipid deposits can be reduced in lipodystrophic mice and humans is by replacing leptin, an anorexogenic adipocyte-derived hormone (71, 101). This leads to a significant reduction in energy intake and dramatic improvements in insulin-stimulated liver and muscle carbohydrate metabolism (80). The notion of energy intake exceeding adipose tissue storage capacity is further supported by the finding that weight loss induced by large scale liposuction fails to improve the metabolic status of obese humans (49) – in effect this procedure simply reduces adipose tissue storage capacity in the face of unchanged energy intake and could potentially exacerbate lipid accumulation in liver and skeletal muscle. On the other hand, relatively small reductions in weight due to dieting and or exercise can substantially improve insulin sensitivity (77, 78, 110). These observations, together with a growing awareness of the molecular interplay between lipid and carbohydrate metabolism have led to what might be termed a “lipocentric” view of the pathogenesis of insulin resistance and type 2 diabetes.
Here, after briefly describing the use of magnetic resonance spectroscopy in humans, we begin by reviewing human studies utilising this technique in combination with stable isotope measurements to determine the key rate-controlling steps in insulin-stimulated glucose disposal in muscle and the effects of insulin on hepatic glucose production in normal volunteers. We then consider abnormalities in these processes observed in insulin resistant type 2 diabetics and in insulin resistant offspring of type 2 diabetics, before going on to consider the notion that “ectopic” lipid accumulation and disorders of fatty acid-lipid metabolism might cause insulin resistance. We also review recent insights into the mechanisms of ectopic lipid accumulation and briefly allude to mechanistic insights into the pathogenesis of type 2 diabetes obtained from studies using thiazolidinediones. Finally we consider the potential impact of inflammatory pathways on the insulin signaling cascade and the notion that inflammation in adipose tissue may be involved in inducing systemic insulin resistance in obese states (a topic recently reviewed by Wellen and Hotamisligil (120)).
Basic principles of magnetic resonance spectroscopy
The basic principles of magnetic resonance spectroscopy (MRS) have been described in detail in a number of reviews (89, 94, 106). In short, some nuclei possess magnetic properties (referred to as the magnetic moment or “spin”). Within a strong, static magnetic field generated by a nuclear magnetic resonance (NMR) spectrometer, the nuclei spin around their own axis with a characteristic frequency in order to align with or against the magnetic field. Stimulation of nuclei by an additional oscillating magnetic field at their frequency of precession transiently swings these nuclei out of alignment. Return to the low energy-state within the static magnetic field is associated with emission of energy in the form of radiowaves which are detected by a receiver coil. Under standard experimental conditions, resonant waves from various nuclei are superimposed, generating a picture of oscillating amplitudes in an intensity vs. time display (free induction decay, FID). Fourier transformation is used to convert the FID into a display of signal intensities vs. frequencies, thereby enabling one to distinguish compounds with characteristic peak frequencies. The area under the particular peak corresponds to its tissue concentration. This result can be converted into molar terms by comparison with data obtained from a phantom containing a known amount of that compound. It can also be compared with the area under the peak of an intrinsic compound with a known concentration e.g. water peak in muscle.
Despite a low natural abundance of 1.1%, 13C can be used to measure hepatic glycogen and muscle glycogen. 13C spectroscopy can also be used to trace 13C incorporation into glycogen during infusion or ingestion of [1-13C]-enriched glucose, which can increase the sensitivity of the method by up to 100-fold. Sequential infusions of 13C-enriched and unlabelled glucose (13C pulse-12C chase experiments) have facilitated measurements of rates of glycogen synthesis and simultaneous glycogenolysis in humans. In these experiments, the increment in total hepatic glycogen over time during infusion of [1-13C] glucose gives the flux through glycogen synthase. The [1-13C] glucose infusate is then switched to an unenriched glucose infusate. In order to obtain an estimate of glycogenolysis, the change in [1-13C] glycogen concentration is compared with the predicted increment, assuming constant flux through glycogen synthase and no glycogen breakdown. Glycogenolysis can then be estimated from the difference between predicted and observed glycogen concentrations. The ratio of glycogen breakdown to glycogen synthesis provides relative rates of glycogen turnover (79, 86, 87, 104).
Proton (1H) MRS is now widely used to measure hepatic and muscle triglycerides. One of the major problems with direct measurements of TG in muscle biopsies is the need to carefully dissect off fat surrounding the myotubules. Fortunately in vivo MRS can identify two sets of resonances from methylene and methyl protons of TG acyl chains within muscle, shifted in frequency from each other by 0.2 ppm. It turns out that these signals originate from two distinct compartments, namely an extramyocellular adipocyte pool and intramyocellular TG. Magnetic susceptibility differences between compartments and the geometric arrangement of the tissue in musculature might cause the observed frequency shift. This technique has been well validated against biochemical TG measurements (109).
Phosphorus (31P) MRS can be used to measure the rate of ATP synthesis by direct observation of 31P-magnetisation transfer between inorganic phosphate (Pi) and ATP. The steady state intramyocellular Pi magnetization is measured in the presence of selective irradiation of the γ resonance of ATP and then compared to the equilibrium Pi magnetization in a control spectrum (without irradiation of γ ATP) (55, 75). To date, this technique has been used to measure the rate of ATP synthesis in skeletal muscle and brain in humans.
Insulin resistance and muscle glucose metabolism
How do healthy individuals dispose of glucose loads?
Ingested glucose can either be oxidized or stored as glycogen, or to a lesser extent as fat (via de novo lipogenesis). Early studies using indirect calorimetry in combination with femoral vein catheterisation and the euglycemic-insulin clamp suggested that non-oxidative glucose metabolism was the major pathway for glucose disposal in healthy subjects (25). Ex vivo glycogen measurements in sequential muscle biopsy studies taken in the presence of high plasma glucose concentrations (peak 20 mmol/l) suggested that over half of an infused glucose load was stored as muscle glycogen (6, 69). 13C MRS provided the first opportunity to directly assess small sequential changes in muscle glycogen concentration during hyperglycemic-hyperinsulinemic clamps (105). Indirect calorimetry was used concurrently to calculate whole body non-oxidative glucose disposal. These data suggested that during a hyperglycemic-hyperinsulinemic clamp skeletal muscle accounts for the vast majority of glucose uptake in normal humans and that over 80% of this glucose is then stored as muscle glycogen (105). Direct 13C MRS measurements of muscle glycogen have also been undertaken in healthy subjects following standard meals. In this situation, muscle glycogen synthesis accounts for ~30% of the ingested glucose (122). Glycogen concentrations peak at ~100 mmol/L around 5 hours after a meal, declining thereafter (18, 113).
How is this altered in insulin resistant type 2 diabetics and in insulin resistant diabetic offspring?
Baseline muscle glycogen concentrations were significantly lower in type 2 diabetics than in matched controls (18, 105) and the rate of glycogen synthesis in skeletal muscle was approximately 50% lower in diabetic subjects than in normal volunteers during hyperglycemic-hyperinsulinemic clamps (105). Postprandial increments in muscle glycogen were also significantly lower than those in normal volunteers (18). First-degree relatives of type 2 diabetics have a ~40% lifetime risk of developing diabetes (51). Insulin resistance is the best predictor of the development of diabetes in these offspring (56, 118) and probably plays an important role in its pathogenesis. Baseline muscle glycogen concentrations (~70 mmol/L) were similar to those of healthy controls, but insulin-stimulated rates of muscle glycogen synthesis were reduced by 63% in these individuals (74).
What are the rate-controlling steps in glucose disposal?
Under the influence of insulin, glucose is transported into myocytes via GLUT 4 (glucose transporter 4), where it is phosphorylated by hexokinase. Glucose 6-phosphate is then either utilized in the glycolytic pathway or incorporated into glycogen by glycogen synthase.
13C and 31P MRS were used together to monitor both intracellular glucose-6-phosphate concentration and intramuscular glycogen synthesis during hyperinsulinemic-hyperglycemic clamps (93). Glucose-6-phophate is an intermediate between glucose transport into the cell and its subsequent phosphorylation by hexokinase, and glycogen synthesis. The fact that the increment in glucose-6-phosphate concentration was significantly reduced in type 2 diabetics suggested that glucose transport and/or phosphorylation was the rate-controlling step in insulin-stimulated glucose disposal in skeletal muscle rather than glycogen synthase (93). Similar observations were also made in lean insulin resistant offspring of type 2 diabetics (91) and in non-diabetic obese women (BMI 33 ± 1 kg/m2) (78), suggesting that this defect precedes the development of type 2 diabetes and is common to insulin resistant offspring of type 2 diabetics and obese subjects; both states significantly increase the risk of developing type 2 diabetes. Glucose transport in skeletal muscle is largely mediated by a specific insulin responsive transporter, known as GLUT4, whereas glucose phosphorylation is catalysed by hexokinase. In order to determine which of these two steps was defective, a novel 13C MRS method was used to assess intracellular free glucose in muscle (19), the idea being that if hexokinase were rate-controlling in insulin resistant type 2 diabetics intracellular glucose concentrations should increase substantially (>2mM). The fact that intracellular glucose concentrations in skeletal muscle from type 2 diabetics (during a hyperinsulinemic-hyperglycemic clamp) were 1/25 what they would have been if hexokinase were the primary rate-controlling enzyme suggested that glucose transport was rate-controlling as opposed to hexokinase (19). Taken together these data indicate that glucose transport into muscle is the rate-controlling step for insulin-stimulated muscle glycogen synthesis in patients with insulin resistant type 2 diabetes. They also suggest that this defect precedes the development of type 2 diabetes in lean offspring of type 2 diabetics and in obese adults.
Insulin resistance and liver glucose metabolism
The liver plays a pivotal role in maintaining energy homeostasis during fed-fasting transitions. Whilst peripheral tissues (predominantly skeletal muscle) account for the majority of postprandial insulin-stimulated glucose disposal, the liver also plays a key role in buffering ingested carbohydrate by suppressing hepatic glucose output and stimulating glucose deposition as liver glycogen. In the fasting state, hepatic glycogen stores are rapidly mobilised in order to maintain circulating glucose concentrations. In addition, the liver, aided to some extent by the kidney, converts lactate, glycerol and amino acids to glucose by gluconeogenesis.
Early human studies of hepatic glucose metabolism required invasive procedures such as liver biopsy and/ or indirect measurements of hepatic gluconeogenesis based on sampling from arterial and hepatic vein catheters. 13C MRS has made non-invasive, real-time and repetitive measurements of hepatic glycogen concentrations possible, enabling investigators to monitor net rates of hepatic glycogen synthesis and glycogenolysis in vivo.
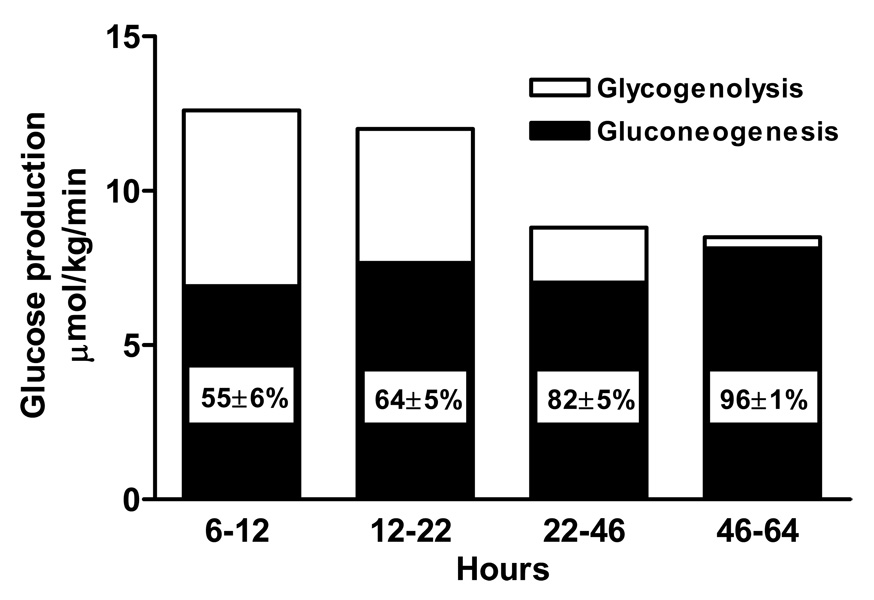
Fasting state (Fig. 1)
Figure 1. Relative contribution of gluconeogenesis and glycogenolysis to glucose production during fasting.
(Data derived from Rothman DL et al. Science 254: 573–576, 1991; and Petersen KF et al. Am. J. Physiol. 270: E186–E191, 1996.)
Rothman et al (92) combined 13C MRS measurements of liver glycogen with magnetic resonance imaging of liver volume and infusion of [3-3H]glucose which was used to determine net hepatic glucose production, to trace the relative contributions of hepatic gluconeogenesis and hepatic glycogenolysis during a prolonged fast (68 hours). In the first 22 hours after a modest 650 kcal meal, liver glycogen decreased in an almost linear fashion from 396 ± 29 mmol/l at 4 hours to 251 ± 30 mmol/l at 15 hours [the latter concentration is in agreement with that of approximately 270 mmol/l obtained by needle biopsy following a similar fasting period (70) and findings in a subsequent MRS study (33)]. Net rates of glycogenolysis were calculated to be 4.3 ± 0.6 µmol/kg/min, which only accounted for 36% of whole body glucose production, suggesting that gluconeogenesis must be the dominant contributor to whole body glucose production even in the early period of fasting. As this observation contrasted with earlier studies suggesting that the contribution of gluconeogenesis to whole body glucose production was less than 38% after an overnight (12–14 hour) fast (22, 70, 117), net glycogenolysis was followed between 5 and 12 hours after a large 1000 kcal evening meal, which was designed to fully replete liver glycogen content (81). In that study [6,6-2H] glucose was used to determine whole body glucose production. Here again, net hepatic glycogenolysis (5.8 ± 0.8 µmol/kg/min) contributed only 45% of whole body glucose production. These data imply that gluconeogenesis contributes as much as 50% of whole body glucose production in the early hours following a mixed meal. These findings were later confirmed by Landau et al (54) who used a novel D2O method to directly assess the contribution of gluconeogenesis to whole body glucose production in healthy volunteers following an overnight fast. Healthy subjects drank D2O and after 14, 22 and 42 hours of fasting the enrichments of deuterium in the hydrogens bound to carbons 2, 5 and 6 of blood glucose and in body water were determined. Enrichment of the deuterium bound to carbon 5 of glucose relative to that of water or to that of carbon 2 of glucose directly equals the fraction of glucose formed by gluconeogenesis. Between 22 and 46 hours of fasting, net glycogenolysis decreased to 1.7 ± 0.5 µmol/kg/min, indicating that even at this early stage gluconeogenesis accounts for as much as 64% of endogenous glucose production. Between 46 and 64 hours the rate of net hepatic glycogenolysis fell to 0.3 ± 0.6 µmol/kg/min (92). After 64 hours, liver glycogen content had fallen by 83% and gluconeogenesis was almost solely responsible (96%) for endogenous glucose production (92). By this point, fat oxidation and ketone bodies make a substantial contribution towards meeting whole body energy requirements.
Fed (postprandial) state
Hepatic glucose output is suppressed within 30 minutes after an oral glucose load and the liver takes up glucose to replenish glycogen stores. Taylor et al (112) observed that liver glycogen concentrations increased from 207 ± 22 mmol/L after an overnight fast to a peak of 316 ± 19 mmol/l 5 hours after a liquid mixed meal giving a net rate of glycogen synthesis of 0.34 µmol/l/min (representing approximately 19% of the carbohydrate content of the meal). The data are consistent with estimates of 25% obtained after an oral glucose load by double tracer studies (82), splanchnic balance techniques (43) and an independent MRS study (4). Hwang et al (41) monitored liver glycogen concentrations in normal subjects during the course of a day in which three mixed meals were ingested. Liver glycogen stores increased from 274 ± 11 mmol/l, peaked just before the next meal and were maximal 4 hours after dinner (420 ± 19 mmol/l). These data suggest that net hepatic glycogenolysis is negligible in resting healthy subjects consuming three meals a day, and therefore glucose absorption from meals and possibly gluconeogenesis account for the majority of glucose production during the day, whereas net hepatic glycogenolysis only contributes to whole body glucose production during the night.
What determines net hepatic glycogen turnover?
Net glycogen synthesis is directly regulated by two enzymes, glycogen synthase and glycogen phosphorylase. A combination of 13C MRS and 13C glucose pulse-12C glucose chase techniques were used to demonstrate that glycogen synthesis and glycogenolysis occur simultaneously in the liver i.e. glycogen cycling (24, 58, 79, 86, 104). This technique was also used to assess the relative impact of glucose and insulin on glycogen turnover under hypoglucagonemic conditions (79). While hyperglycemia primarily inhibits net hepatic glycogenolysis by inhibiting glycogen phosphorylase flux, hyperinsulinemia primarily stimulates glycogen synthase flux. The net rate of glycogen synthesis depends on portal vein insulin, requiring concentrations in the 130–170 pmol/l range for half-maximal stimulation of glycogen synthesis (86). Furthermore under basal insulin concentrations, the presence and absence of glucagon was shown to have a profound effect on regulating net hepatic glycogen synthesis (86).
What happens in insulin resistant type 2 diabetes?
It is well established that fasting hyperglycemia is related to increased rates of endogenous glucose production (14, 31). This phenomenon could be a consequence of increased gluconeogenesis and/or increased glycogenolysis. Magnussen et al (59) measured rates of net hepatic glycogenolysis in poorly controlled type 2 diabetics (mean hemoglobin A1c 12 ± 1%). Gluconeogenesis was simultaneously calculated as the difference between rates of net hepatic glycogenolysis and whole-body glucose production. Baseline liver glycogen concentration was reduced in the diabetic subjects (131 ± 20 vs. 282 ± 60 mmol/l liver), in keeping with a defect in postprandial hepatic glycogen synthesis and was associated with lower rates of net hepatic glycogenolysis. More importantly the 25% increase in rates of endogenous glucose production observed in these poorly controlled type 2 diabetic subjects could entirely be attributed to increased rates of gluconeogenesis.
Fatty acid/ lipid-induced insulin resistance
Muscle
Lipid infusions designed to increase plasma fatty acid (FA) concentrations reduce insulin-stimulated glucose disposal in humans (9, 46, 88). Furthermore, the fall in insulin sensitivity during such clamp procedures only occurs 3–5 hours after elevations in FA concentrations, in keeping with the idea that fatty acid metabolite accumulation in skeletal muscle and liver is responsible for this phenomenon (8). Randle et al. originally showed that fatty acids compete with glucose for substrate oxidation in isolated rat heart muscle and rat diaphragm muscle (83). They speculated that an increase in fat oxidation might be responsible for insulin resistance. According to their proposal, increased fatty acid oxidation would cause an increase in the mitochondrial acetyl CoA:CoA and NADH:NAD+ ratios with subsequent inactivation of pyruvate dehydrogenase. This in turn would induce a rise in intracellular citrate levels, leading to inhibition of phosphofructokinase and glucose-6-phosphate accumulation. As glucose-6-phosphate inhibits hexokinase activity, this would result in intracellular glucose accumulation and decreased glucose uptake. A series of studies has recently challenged this mechanism (11, 28, 36, 88). Non-esterified fatty acid levels in healthy subjects were maintained at either high or low levels during hyperinsulinemic-euglycemic clamps. Maintaining high free fatty acid levels for 5 hours caused the expected reduction in insulin sensitivity as assessed by glucose uptake, glucose oxidation and glycogen synthesis in skeletal muscle, just as had been observed in type 2 diabetics and their insulin resistant offspring. However, rather than increasing intracellular glucose-6-phosphate levels, as predicted by the Randle mechanism (83), increasing plasma fatty acid concentrations reduced intracellular glucose-6-phosphate levels (88). This was consistent with what had been observed in patients with type 2 diabetes (91).
Fatty acid infusion could conceivably have direct effects on GLUT4 activity or it could alter insulin regulated GLUT4 trafficking between intracellular compartments and the cell membrane. To explore the latter possibility insulin signalling intermediates were examined in skeletal muscle biopsies from subjects exposed to high fatty acid levels for five hours prior to and during hyperinsulinaemic-euglycaemic clamps (28). Glucose oxidation and glycogen synthesis were 50–60% lower following the lipid infusion than with the glycerol (control) infusion, and were associated with a ~90% decrease in the increment in intramuscular glucose-6-phosphate concentration, implying diminished glucose transport or phosphorylation activity. The fact that intracellular glucose concentrations were significantly lower in the lipid infusion studies compared with those during glycerol infusion implied that glucose transport was the rate-controlling step. IRS-1 (insulin receptor substrate 1) associated PI 3-kinase (phosphoinositol 3-kinase) activity was significantly reduced under these conditions (28). Subsequent rodent and human studies suggested that this might be a consequence of serine phosphorylation of IRS-1 (28, 36, 66, 128). An important and as yet unanswered element in this proposed mechanism for insulin resistance is the precise nature of the lipid moiety responsible for fatty acid induced insulin resistance. Although triglyceride accumulation in skeletal muscle and liver clearly correlates with insulin resistance, triglycerides are generally perceived to be metabolically inert associates of more favoured candidates which include long chain acyl-coenzyme A (LCCoAs), diacylglycerol (DAG) (42, 128) and ceramides (1). Studies by Yu et al have been able to disassociated lipid-induced insulin resistance from any increases in intramuscular triglyceride or ceramide content suggesting that these lipid metabolites are not the trigger in mediating fat-induced insulin resistance in skeletal muscle (128). The fact that mtGPAT1 (mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1) knockout mice, which have elevated LCCoAs but reduced DAG and TG in liver, have improved hepatic insulin sensitivity suggests DAG may be a better candidate than LCCoA in mediating fat induced insulin resistance in liver (68). PKC is a serine/ threonine kinase known to be activated by DAGs and might account for the link between lipid accumulation and serine phosphorylation of IRS-1 in rodents (36, 98, 99). In keeping with these rodent studies, Itani et al (42) noted that DAG accumulation in human muscle during lipid/ heparin infusions was associated with increased PKC activity. If this hypothesis is true, perturbations that result in accumulation of LCCoAs, DAGs or other fatty acid derivatives within muscle and liver, either through increased delivery and/or decreased metabolism, ought to induce insulin resistance.
Liver
The effects of elevated FA concentrations on hepatic glucose metabolism are less clear. In the presence of postabsorptive insulin concentrations, plasma FA elevation increased EGP during somatostatin-insulin clamps (12, 30), but not after an overnight fast (12, 20). These apparent discrepancies may be caused by FA-induced insulin secretion counterbalancing the stimulatory effect of FAs on EGP, and/ or hepatic autoregulation2 preventing a net increase in hepatic glucose output despite increased gluconeogenesis. Roden et al (90) combined measurements of EGP using D-[6,6-2H]glucose with measurements of gluconeogenesis derived from 2H enrichments in carbons 2 and 5 of blood glucose after D2O ingestion during short term intralipid/ heparin infusions. Plasma FA elevation induced insulin secretion sufficiently to prevent any change in plasma glucose concentrations. However, if plasma insulin concentrations were maintained at fasting peripheral concentrations by a combined somatostatin-insulin infusion, FA exposure significantly increased plasma glucose primarily by increasing gluconeogenesis. Boden et al (10) lowered fasting plasma FA concentrations by administering nicotinic acid and then increased FAs by withdrawing nicotinic acid (induces a rebound increase in FAs) whilst measuring GNG and GL. They concluded that increased FAs stimulated GNG whereas reduced FA concentrations inhibited GNG.
The mechanisms by which FAs induce GNG remain poorly understood. Postulates have included activation of pyruvate carboxylase (secondary to increases in acetyl CoA) (2) and increased availability of NADH and ATP (127). Roden et al (90) excluded the possibility that elevated glycerol, a gluconeogenic substrate which is released with FAs during lipolysis, could have stimulated GNG by matching plasma glycerol levels during lipid/ heparin infusion with a glycerol infusion in controls.
Studies of hepatic insulin signalling have for obvious reasons largely been confined to animals. PKCε translocation to the plasma membrane and activity is increased in rats with isolated hepatic steatosis and hepatic insulin resistance following three days of high fat feeding (95), suggesting that PKCε may be an important mediator of fat-induced insulin resistance in liver. PKCε has also been shown to be activated in the liver in patients with type 2 diabetes (21).
Mechanisms of lipid accumulation in skeletal muscle and liver
MRS measurements of intra-myocellular lipids (IMCL) correlate more closely with insulin resistance than any other commonly measured indices including body mass index (BMI), waist-hip ratios, or total body fat (53). Non-alcoholic steatohepatitis is also increasingly recognised as a component of the insulin resistance or metabolic syndrome (60, 72, 100). Lipid accumulation in ectopic sites can occur in three ways; increased uptake of fatty acids, increased synthesis within the tissue involved and/or reduced fatty acid oxidation/disposal (103). The relative contribution of these factors to ectopic lipid accumulation may well vary in different physiological states and in different tissues. Simplistically, obesity and lipodystrophy would appear to be associated with ectopic lipid accumulation predominantly due to excess lipid influx/synthesis in the liver and muscle, whereas in lean elderly subjects (75) and lean insulin resistant offspring of type 2 diabetics (76), impaired mitochondrial fatty acid oxidation may play a major role in this process.
Obesity and lipodystrophy
Obesity is a very common cause of insulin resistance and a major risk factor for the development of type 2 diabetes. Interestingly, lipid accumulation in muscle and liver is characteristic of obesity and what might be considered its opposite extreme, lipodystrophy (77, 80). As alluded to above, it is clear that lipid/heparin infusions which increase plasma FAs can lead to lipid accumulation in skeletal muscle and short term high fat feeding elevates liver triglycerides in rats (95); in both cases insulin resistance ensues. Increased fatty acid concentrations are often said to be typical of obesity (13), most likely due to increased FA release from an expanded fat mass; providing a convenient link between obesity and ectopic lipid accumulation. Whilst suppression of FA levels by insulin is consistently impaired in insulin resistant obese subjects and at least in some forms of lipodystrophy (116), fasting plasma FA levels are not always elevated in insulin resistant obese subjects (78), nor are they typically elevated in lipodystrophics (96) (one needs to bear in mind that since triglyceride concentrations are typically increased in lipodystrophy (34), ex vivo hydrolysis is likely to produce falsely elevated FA measurements if samples are not appropriately collected and stored; one also needs to consider the fact that the normal range for fasting plasma FAs is particularly wide (of the order of 290–720 µmol/L)). The notion that obesity and lipodystrophy are associated with persistently elevated plasma FAs, which in turn result in lipid accumulation in muscle and liver is therefore probably overly simplistic. What is more likely is that chronic imbalances between energy delivery/ uptake and oxidation ultimately result in excess intracellular lipid accumulation, both at the whole body level and in individual organs or tissues. Kelley et al (44, 108) have proposed the idea that metabolic inflexibility i.e. an impaired capacity to appropriately switch between “fed” (predominantly carbohydrate oxidation) and “fasting” (predominantly lipid oxidation) metabolism, typifies obesity and insulin resistance. This mismatching between energy supply and demand may over time contribute to the accumulation of ectopic lipid. Adipose tissue plays a major role in maintaining metabolic flexibility by buffering the postprandial influx of fatty acids, a function which is disturbed in both obesity and lipodystrophy (32).
Peroxisome proliferator-activated receptor gamma (PPARγ), a nuclear hormone receptor predominantly expressed in adipocytes, appears to be a key player in the ability of adipose tissue to buffer fatty acid influx. Humans with dominant negative loss-of-function mutations in the ligand-binding domain of PPARγ manifest a stereotyped form of partial lipodystrophy and impaired postprandial fatty acid trapping (97). They also have fatty livers and are severely insulin resitant. Thiazolidinediones are commercially available PPARγ agonists which substantially improve insulin sensitivity despite promoting weight gain. Whilst their precise mode of action remains unclear, they seem to improve the ability of adipose tissue to buffer fatty acids (111), a phenomenon which is likely to be a key component of their insulin sensitising properties (50, 61). Thiazolidinediones also improve hepatic steatosis (62) and hepatic insulin sensitivity (3). Interestingly, they do not consistently lower muscle TG (62), nor is muscle TG increased in humans with loss-of-function mutations in PPARγ (97); observations which further suggest that intramyocellular triglyceride is not the trigger for fat induced insulin resistance as previously discussed. However thiazolidinediones have been shown to lower intramuscular long chain CoA’s in rodents (47).
Recent work employing stable isotopes to track the fate of ingested nutrients suggests that lipid accumulation in the liver is a result of both fatty acid re-esterification and de novo lipogenesis (27). One interesting hypothesis suggests that hyperinsulinaemia could be an important driving force for lipogenesis in the liver (and muscle) by increasing sterol regulatory element-binding protein 1c (SREBP1c) expression (102). SREBP1c is a key transcriptional regulator of de novo lipogenesis (15).
Aging associated insulin resistance and lean diabetic offspring
Together with obesity, aging is a very common cause of insulin resistance; and like obesity it too is characterised by lipid accumulation in muscle and liver (23, 75). While an increasingly sedentary lifestyle and the associated relative increase in fat mass almost certainly contributes to aging induced insulin resistance, Petersen et al (75) documented insulin resistance in elderly subjects matched to young adult controls in terms of fat mass, lean body mass and activity. Furthermore, the ability of insulin to suppress lipolysis as measured by glycerol turnover was similar to that seen in young controls, suggesting that adipose tissue dysfunction was unlikely to be a major determinant of the observed increase in liver and muscle TG in their study. In order to measure mitochondrial oxidative phosphorylation activity in skeletal muscle, MRS was used to monitor 13C labelling of carbon atoms in glutamate during an intravenous infusion of [2-13C]acetate providing a direct measure of tricarboxylic acid (TCA) cycle flux. Rates of ATP synthesis were measured using 31P MRS by the direct observation of 31P-magnetisation transfer between inorganic phosphate and ATP. Both of these measurements of mitochondrial function in muscle were impaired in the lean elderly insulin resistant subjects. Similar defects in mitochondrial function in muscle were found in lean insulin resistant offspring of type 2 diabetics, who also have elevated intramyocellular TGs. In the case of the elderly, this is likely to be a consequence of acquired mitochondrial mutations, a phenomenon known to occur with aging (64), whereas in the insulin resistant offspring, it is more likely that the reduction in mitochondrial oxidative phosphorylation is a primary genetic defect. Insulin resistant diabetic offspring were also found to have a lower ratio of oxidative type 1 to glycolytic type 2 muscle fibres in an independent study (76). Enzyme defects have also been described in isolated mitochondria derived from human skeletal muscle biopsies from type 2 diabetics (45). Mitochondria in skeletal muscle appear to be compartmentalised at a subcellular level and one report suggests that there is a disproportionately large reduction of electron transport chain activity in the subsarcolemmal mitochondrial fraction (the other fraction being the intermyofibrillar fraction which generates energy for muscle contraction) in nondiabetic obese subjects and in obese type 2 diabetics (85). Subsarcolemmal mitochondria are believed to be crucial for fatty acid oxidation, glucose transport and propogation of insulin signalling as well as several other energy-requiring processes at the cell surface (39, 85). Interestingly, the reduction in mitochondrial electron transport chain activity did not appear to be entirely attributable to a reduction in mitochondrial mass, suggesting that total cellular mitochondrial activity reflects both mitochondrial mass and the enzyme activity within the mitochondria; in other words some mitochondria may not function optimally (85). Together, these data suggest that alterations in nuclear-encoded genes that regulate mitochondrial biogenesis such as peroxisome proliferator-activated receptor gamma co-activator 1α (PGC-1α), AMP kinase (5, 131) and CAM IV kinase (124) may form the genetic basis for inheritance of at least some forms of type 2 diabetes. They also suggest that genes involved in regulating both mitochondrial enzyme activity and mitochondrial mass may be involved in the pathogenesis of type 2 diabetes. This notion is further supported by two microarray studies which revealed a reduction in PGC-1α responsive transcripts in patients with type 2 diabetes and their first-degree relatives (65, 73). PGC-1α is a key regulator of mitochondrial biogenesis (125). However, despite demonstrating that the reduction in mitochondrial function seen in insulin resistant diabetic offspring is due to reduced mitochondrial content (assessed by electron microscopy), Morino et al did not find any differences in PGC-1 mRNA or protein expression (66). These data suggest that the previously reported reduction in PGC-1 may be a secondary phenomenon. The primary factors responsible for the reduced mitochondrial content in insulin resistant diabetic offspring remain unknown (66).
Finally, one needs to remain cognisant of the fact that, to date, inherited mitochondrial disorders have tended to produce pleiotropic manifestations (107), often without diabetes. In those cases where diabetes is a feature, β cell dysfunction tends to be the primary abnormality (57, 107). One example where insulin resistance also seems to be a feature is Fredreich’s ataxia although insulin sensitivity has still to be carefully evaluated in this model (84). In our view, the mitochondrial dysfunction seen in the elderly, lean insulin resistant diabetic offspring and insulin resistant type 2 diabetics is probably milder than that observed in disorders where β cell dysfunction predominates. An important remaining question is whether mitochondrial defects, be they inherited or acquired cause the increases in intramyocellular lipid and insulin resistance, or are they secondary in nature.
Inflammation and insulin resistance
While lipid accumulation in muscle and liver may be sufficient to explain the development of insulin resistance in obese subjects, obesity is also associated with a systemic chronic inflammatory response characterized by altered cytokine production and activation of inflammatory signaling pathways (121); recent reports have linked this inflammatory response to the development of insulin resistance. Almost all of this data has, to date, come from animal studies and has recently been comprehensively reviewed (120), so is not covered in detail here. To summarise, the major findings in humans include:
Increased plasma inflammatory markers [e.g. C-reactive protein (CRP)] and altered plasma adipokine concentrations have been detected in some (120), but not all, insulin resistant states. In particular, plasma levels of adiponectin, resistin, IL-6, TNFα and leptin were unchanged in both lean insulin resistant offspring of type 2 diabetics (76) and in lean insulin resistant elderly subjects (75) when compared to insulin sensitive controls, suggesting that inflammatory changes are unlikely to be the primary abnormality in these groups of subjects. Moderate weight loss in obese insulin resistant subjects also failed to significantly alter adipokine levels despite significant improvements in hepatic insulin sensitivity and normalization of fasting plasma glucose concentrations (77).
Significantly increased numbers of macrophages accumulate in adipose tissue in obese states (119, 126). Macrophages are found in the stromovascular fraction of adipose tissue in humans and appear to make a substantial contribution to gene expression within adipose tissue. These cells are derived from bone marrow precursors and appear to infiltrate adipose tissue in greater numbers in obese states. Whether this inflammatory infiltrate is responsible for the development of insulin resistance is not yet clear, although Xu et al (126) did suggest that the increase in inflammatory gene expression within adipose tissue preceded the dramatic increase in plasma insulin levels noted in high fat fed mice. They also reported down-regulation of these macrophage-derived genes in response to treatment with an insulin-sensitizing PPARγ agonist (rosiglitazone) (126). Subsequent studies in humans have suggested that the inflammatory cell infiltrate (as reflected by mRNA levels of a macrophage marker (CD68)) correlates with insulin sensitivity more closely than with BMI and that thiazolidinediones reduce CD68 mRNA levels and improve insulin sensitivity (26). Surgically-induced weight loss (bariatric) also reduces macrophage infiltration in adipose tissue of morbidly obese subjects and improves insulin sensitivity (17).
In addition to PKC, a number of serine/ threonine kinases are activated in inflammatory states. For example, Yuan et al (129) recently proposed that fatty acid induced serine phosphorylation of IRS-1 might be mediated by IKB kinase-β (IKK-β). This hypothesis has been supported by a human study demonstrating that pharmacological inhibition of IKK-β activity by high dose salicylate therapy (high doses of salicylate are required to inhibit IKK-β activity) reduced fasting hyperglycaemia and basal hepatic glucose production and, improved peripheral glucose uptake in patients with type 2 diabetes (40). Hotamisligil and co-workers have identified another inflammatory serine kinase potentially involved in inducing serine phosphorylation of IRS-1, namely Jun kinase 1 (JNK1) (38). They reported increased JNK activity in obese rodents and human adipose tissue, as well as reduced adiposity and improved insulin sensitivity in JNK1 knockout mice. Suppressor of cytokine signaling 3 (SOCS3) is another potential contributor to the links among obesity, inflammation and insulin resistance (114). One particularly intriguing aspect of the SOCS3 hypothesis is that increases in SOCS3 expression have also been observed in the hypothalamus where SOCS3 may be involved in inducing leptin resistance (7). This notion provides a potentially unifying mechanism for both insulin and leptin resistance, states which frequently co-exist in obese humans.
Conclusions
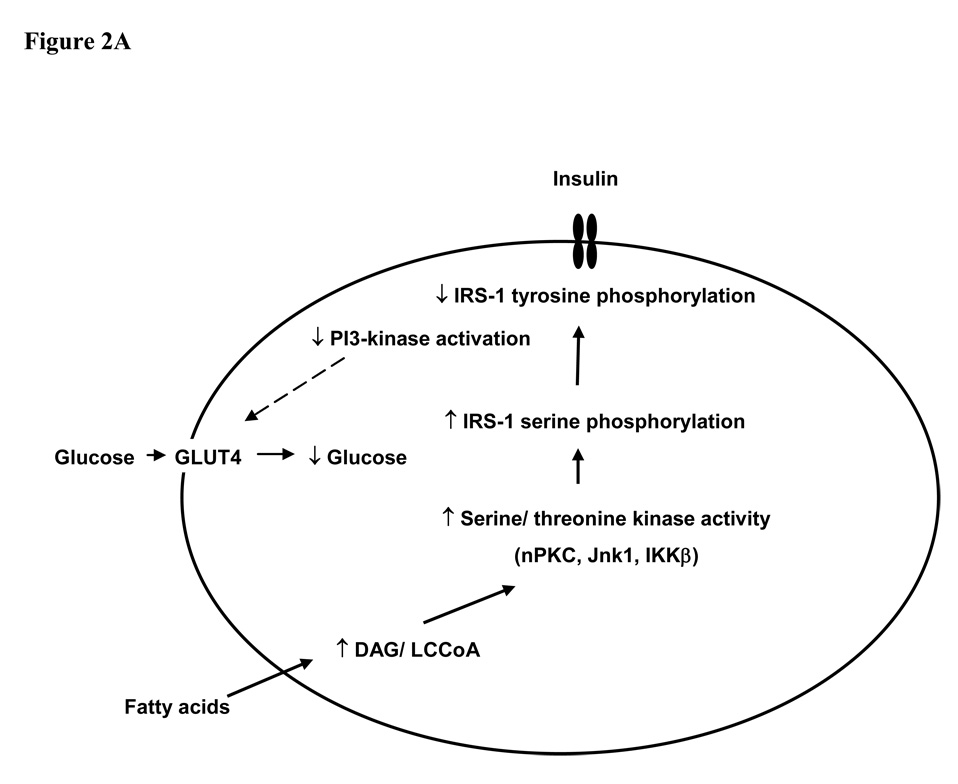
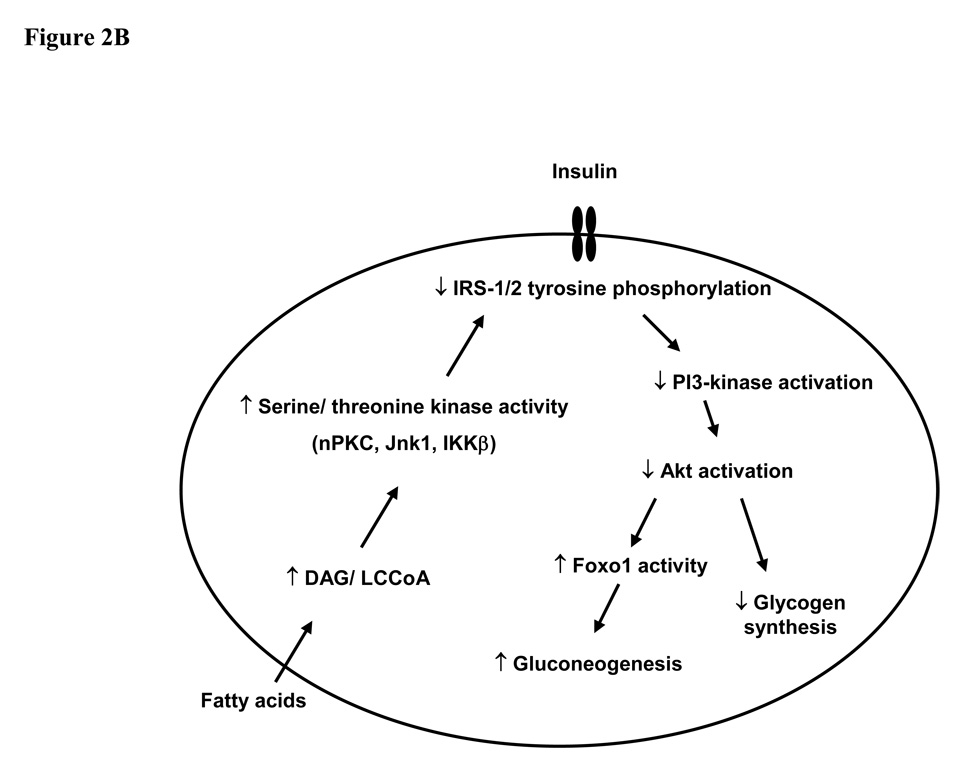
The ability to non-invasively monitor biochemical changes in living subjects in realtime has yielded a number of significant novel insights into the pathogenesis of insulin resistance and type 2 diabetes. Insulin resistance in skeletal muscle manifests primarily as a reduction in insulin-stimulated glycogen synthesis, which is in turn a consequence of reduced glucose transport. In the liver, insulin resistance seems to be somewhat paradoxically associated with a reduced ability of insulin signalling to inhibit glucose production whereas insulin stimulated lipogenesis is enhanced. Lipid accumulation within skeletal muscle is associated with serine phosphorylation on critical sites on IRS-1 and reduced tyrosine phosphorylation of IRS-1 (Fig. 2). This in turn inhibits binding and activation of PI 3-kinase. A number of different serine kinases could be responsible for serine phosphorylation of IRS-1. Candidates include members of the nPKC family, which may be activated by accumulation of lipid intermediates (particularly DAGs), as well as inflammatory intermediates such as IKKβ, JNK1 and TNFα. The latter may be activated within adipose tissue in obese states. Lipid accumulation in skeletal muscle and liver may be a result of increased delivery/synthesis of fatty acids to/in these tissues in states in which energy intake exceeds adipose tissue storage capacity (as seen in obesity and lipodystrophy), or a consequence of either acquired or inherited mitochondrial dysfunction. As well as re-inforcing the importance of life-style interventions in the management of type 2 diabetes – dietary restriction to limit the stress on energy stores and exercise to increase mitochondrial number and function - these novel ideas about the molecular pathogenesis of insulin resistance have provided several new therapeutic targets for the treatment and possible prevention of type 2 diabetes.
Figure 2. Mechanism of fatty acid induced insulin resistance in muscle (A) and liver (B).
Fatty acid metabolites (LCCoA, DAG), which may accumulate within myotubules and hepatocytes due to increased fatty acid delivery and/ or decreased mitochondrial oxidation, trigger a serine/threonine kinase cascade (possibly involving nPKC, IKKβ and/ or JNK-1). This ultimately induces serine/ threonine phosphorylation of critical IRS-1 sites (at least in muscle), thereby inhibiting IRS-1/2 tyrosine phosphorylation and activation of PI 3-kinase, resulting in i) reduced insulin–stimulated glucose transport in muscle; and ii) reduced glycogen synthesis and increased gluconeogenesis in liver. GLUT4, glucose transporter 4; LCCoA, long chain acylcoenzymeA; DAG, diacylglycerol; IRS, insulin receptor substrate; PI3-kinase, phosphoinositol 3-kinase; nPKCs, novel protein kinase Cs; JNK-1, Jun kinase-1; IKK β; IκB kinase-β.
Studies in lean offspring of type 2 diabetics suggest that intramyocellular lipid accumulation and muscle insulin resistance precede the development of hepatic insulin resistance and type 2 diabetes (76). We propose that insulin resistance in skeletal muscle is the earliest event in the pathogenesis of type 2 diabetes in most patients. Muscle insulin resistance is in turn associated with peripheral and portal vein hyperinsulinemia which promotes hepatic steatosis, at least in part by inducing SREBP1c mediated de novo lipogenesis and inhibiting fatty acid oxidation. Recent work by Wolfram et al (123) suggests that hyperinsulinemia induces nuclear exclusion and inhibition of Foxa2, a regulator of fatty acid oxidation. In time, this leads to lipid accumulation in the liver, hepatic insulin resistance and ultimately type 2 diabetes. Adipocyte dysfunction due to either obesity or lipodystrophy is associated with excessive and untimely delivery of FAs to the liver and skeletal muscle and probably contributes to insulin resistance in both organs, by altering the balance between FA uptake/synthesis and disposal leading to increases in intracellular lipid content. Further human studies will be required to prove or disprove this theory and are also expected to continue to unveil novel therapeutic approaches for the treatment and prevention of insulin resistance and type 2 diabetes.
Acknowledgements
G.I.S. is an investigator of the Howard Hughes Medical Institute. This work was supported by grants from the US Public Health Service: P01 DK-68829, R01 DK-40936, R01 DK-49230, R01 AG-23686, M01 RR-00125, U24 DK-59635 and a Distinguished Scientist Award from the American Diabetes Association. D.B.S. is supported by the Wellcome Trust.
Footnotes
The lipodystrophic syndromes encompass a rare group of conditions characterized by partial or complete absence of adipose tissue. The disorders may be genetic or acquired, and are further classified according to the anatomical distribution of the lipodystrophy.
Autoregulation refers to the tendency of hepatic glycogenolysis to compensate for increased gluconeogenesis, thereby preventing an increase in HGP.
References
- 1.Adams JM, 2nd, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53:25–31. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- 2.Bahl JJ, Matsuda M, DeFronzo RA, Bressler R. In vitro and in vivo suppression of gluconeogenesis by inhibition of pyruvate carboxylase. Biochem Pharmacol. 1997;53:67–74. doi: 10.1016/s0006-2952(96)00660-0. [DOI] [PubMed] [Google Scholar]
- 3.Bajaj M, Suraamornkul S, Piper P, Hardies LJ, Glass L, Cersosimo E, Pratipanawatr T, Miyazaki Y, DeFronzo RA. Decreased plasma adiponectin concentrations are closely related to hepatic fat content and hepatic insulin resistance in pioglitazone-treated type 2 diabetic patients. J Clin Endocrinol Metab. 2004;89:200–206. doi: 10.1210/jc.2003-031315. [DOI] [PubMed] [Google Scholar]
- 4.Beckmann N, Fried R, Turkalj I, Seelig J, Keller U, Stalder G. Noninvasive observation of hepatic glycogen formation in man by 13C MRS after oral and intravenous glucose administration. Magn Reson Med. 1993;29:583–590. doi: 10.1002/mrm.1910290502. [DOI] [PubMed] [Google Scholar]
- 5.Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M, Young LH, Semenkovich CF, Shulman GI. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2001;281:E1340–E1346. doi: 10.1152/ajpendo.2001.281.6.E1340. [DOI] [PubMed] [Google Scholar]
- 6.Bergstrom J, Hultman E. Synthesis of muscle glycogen in man after glucose and fructose infusion. Acta Med Scand. 1967;182:93–107. doi: 10.1111/j.0954-6820.1967.tb11503.x. [DOI] [PubMed] [Google Scholar]
- 7.Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 1999;274:30059–30065. doi: 10.1074/jbc.274.42.30059. [DOI] [PubMed] [Google Scholar]
- 8.Boden G. Pathogenesis of type 2 diabetes. Insulin resistance. Endocrinol Metab Clin North Am. 2001;30:801–815. doi: 10.1016/s0889-8529(05)70216-4. v. [DOI] [PubMed] [Google Scholar]
- 9.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- 10.Boden G, Chen X, Capulong E, Mozzoli M. Effects of free fatty acids on gluconeogenesis and autoregulation of glucose production in type 2 diabetes. Diabetes. 2001;50:810–816. doi: 10.2337/diabetes.50.4.810. [DOI] [PubMed] [Google Scholar]
- 11.Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 1994;93:2438–2446. doi: 10.1172/JCI117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boden G, Jadali F. Effects of lipid on basal carbohydrate metabolism in normal men. Diabetes. 1991;40:686–692. doi: 10.2337/diab.40.6.686. [DOI] [PubMed] [Google Scholar]
- 13.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. (Suppl 3) 2002;32:14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 14.Bogardus C, Lillioja S, Howard BV, Reaven G, Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest. 1984;74:1238–1246. doi: 10.1172/JCI111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes. 2002;51:2796–2803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 17.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumie A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clement K. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 18.Carey PE, Halliday J, Snaar JE, Morris PG, Taylor R. Direct assessment of muscle glycogen storage after mixed meals in normal and type 2 diabetic subjects. Am J Physiol Endocrinol Metab. 2003;284:E688–E694. doi: 10.1152/ajpendo.00471.2002. [DOI] [PubMed] [Google Scholar]
- 19.Cline GW, Petersen KF, Krssak M, Shen J, Hundal RS, Trajanoski Z, Inzucchi S, Dresner A, Rothman DL, Shulman GI. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N Engl J Med. 1999;341:240–246. doi: 10.1056/NEJM199907223410404. [DOI] [PubMed] [Google Scholar]
- 20.Clore JN, Glickman PS, Nestler JE, Blackard WG. In vivo evidence for hepatic autoregulation during FFA-stimulated gluconeogenesis in normal humans. Am J Physiol. 1991;261:E425–E429. doi: 10.1152/ajpendo.1991.261.4.E425. [DOI] [PubMed] [Google Scholar]
- 21.Considine RV, Nyce MR, Allen LE, Morales LM, Triester S, Serrano J, Colberg J, Lanza-Jacoby S, Caro JF. Protein kinase C is increased in the liver of humans and rats with non-insulin-dependent diabetes mellitus: an alteration not due to hyperglycemia. J Clin Invest. 1995;95:2938–2944. doi: 10.1172/JCI118001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Consoli A, Kennedy F, Miles J, Gerich J. Determination of Krebs cycle metabolic carbon exchange in vivo and its use to estimate the individual contributions of gluconeogenesis and glycogenolysis to overall glucose output in man. J Clin Invest. 1987;80:1303–1310. doi: 10.1172/JCI113206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cree MG, Newcomer BR, Katsanos CS, Sheffield-Moore M, Chinkes D, Aarsland A, Urban R, Wolfe RR. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab. 2004;89:3864–3871. doi: 10.1210/jc.2003-031986. [DOI] [PubMed] [Google Scholar]
- 24.David M, Petit WA, Laughlin MR, Shulman RG, King JE, Barrett EJ. Simultaneous synthesis and degradation of rat liver glycogen. An in vivo nuclear magnetic resonance spectroscopic study. J Clin Invest. 1990;86:612–617. doi: 10.1172/JCI114752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 26.Di Gregorio GB, Yao-Borengasser A, Rasouli N, Varma V, Lu T, Miles LM, Ranganathan G, Peterson CA, McGehee RE, Kern PA. Expression of CD68 and Macrophage Chemoattractant Protein-1 Genes in Human Adipose and Muscle Tissues: Association With Cytokine Expression, Insulin Resistance, and Reduction by Pioglitazone. Diabetes. 2005;54:2305–2313. doi: 10.2337/diabetes.54.8.2305. [DOI] [PubMed] [Google Scholar]
- 27.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, Petersen KF, Shulman GI. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fajans SS, Bell GI, Polonsky KS. Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N Engl J Med. 2001:345. doi: 10.1056/NEJMra002168. PG - 971-80. [DOI] [PubMed] [Google Scholar]
- 30.Ferrannini E, Barrett EJ, Bevilacqua S, DeFronzo RA. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983;72:1737–1747. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fery F. Role of hepatic glucose production and glucose uptake in the pathogenesis of fasting hyperglycemia in type 2 diabetes: normalization of glucose kinetics by short-term fasting. J Clin Endocrinol Metab. 1994;78:536–542. doi: 10.1210/jcem.78.3.8126123. [DOI] [PubMed] [Google Scholar]
- 32.Frayn KN. Adipose tissue as a buffer for daily lipid flux. Diabetologia. 2002;45:1201–1210. doi: 10.1007/s00125-002-0873-y. [DOI] [PubMed] [Google Scholar]
- 33.Fried R, Beckmann N, Keller U, Ninnis R, Stalder G, Seelig J. Early glycogenolysis and late glycogenesis in human liver after intravenous administration of galactose. Am J Physiol. 1996;270:G14–G19. doi: 10.1152/ajpgi.1996.270.1.G14. [DOI] [PubMed] [Google Scholar]
- 34.Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350:1220–1234. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 35.Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, Vinson C, Eckhaus M, Reitman ML. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest. 2000;105:271–278. doi: 10.1172/JCI7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 37.Haffner SM, Stern MP, Mitchell BD, Hazuda HP, Patterson JK. Incidence of type II diabetes in Mexican Americans predicted by fasting insulin and glucose levels, obesity, and body-fat distribution. Diabetes. 1990;39:283–288. doi: 10.2337/diab.39.3.283. [DOI] [PubMed] [Google Scholar]
- 38.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 39.Hood DA. Invited Review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol. 2001;90:1137–1157. doi: 10.1152/jappl.2001.90.3.1137. [DOI] [PubMed] [Google Scholar]
- 40.Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, Shulman GI. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321–1326. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hwang JH, Perseghin G, Rothman DL, Cline GW, Magnusson I, Petersen KF, Shulman GI. Impaired net hepatic glycogen synthesis in insulin-dependent diabetic subjects during mixed meal ingestion. A 13C nuclear magnetic resonance spectroscopy study. J Clin Invest. 1995;95:783–787. doi: 10.1172/JCI117727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 43.Katz LD, Glickman MG, Rapoport S, Ferrannini E, DeFronzo RA. Splanchnic and peripheral disposal of oral glucose in man. Diabetes. 1983;32:675–679. doi: 10.2337/diab.32.7.675. [DOI] [PubMed] [Google Scholar]
- 44.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999;277:E1130–E1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 45.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 46.Kelley DE, Mokan M, Simoneau JA, Mandarino LJ. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J Clin Invest. 1993;92:91–98. doi: 10.1172/JCI116603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim JK, Fillmore JJ, Gavrilova O, Chao L, Higashimori T, Choi H, Kim HJ, Yu C, Chen Y, Qu X, Haluzik M, Reitman ML, Shulman GI. Differential effects of rosiglitazone on skeletal muscle and liver insulin resistance in A-ZIP/F-1 fatless mice. Diabetes. 2003;52:1311–1318. doi: 10.2337/diabetes.52.6.1311. [DOI] [PubMed] [Google Scholar]
- 48.Kim JK, Gavrilova O, Chen Y, Reitman ML, Shulman GI. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J Biol Chem. 2000;275:8456–8460. doi: 10.1074/jbc.275.12.8456. [DOI] [PubMed] [Google Scholar]
- 49.Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, Mohammed BS. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med. 2004;350:2549–2557. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- 50.Knouff C, Auwerx J. Peroxisome proliferator-activated receptor-gamma calls for activation in moderation: lessons from genetics and pharmacology. Endocr Rev. 2004;25:899–918. doi: 10.1210/er.2003-0036. [DOI] [PubMed] [Google Scholar]
- 51.Kobberling J. Studies on the genetic heterogeneity of diabetes mellitus. Diabetologia. 1971;7:46–49. doi: 10.1007/BF02346253. [DOI] [PubMed] [Google Scholar]
- 52.Krook A, O'Rahilly S. Mutant insulin receptors in syndromes of insulin resistance. Baillieres Clin Endocrinol Metab. 1996;10:97–122. doi: 10.1016/s0950-351x(96)80330-2. [DOI] [PubMed] [Google Scholar]
- 53.Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 54.Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC. Contributions of gluconeogenesis to glucose production in the fasted state. J Clin Invest. 1996;98:378–385. doi: 10.1172/JCI118803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lebon V, Dufour S, Petersen KF, Ren J, Jucker BM, Slezak LA, Cline GW, Rothman DL, Shulman GI. Effect of triiodothyronine on mitochondrial energy coupling in human skeletal muscle. J Clin Invest. 2001;108:733–737. doi: 10.1172/JCI11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med. 1993;329:1988–1992. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- 57.Maassen JA, LM TH, Van Essen E, Heine RJ, Nijpels G, Jahangir Tafrechi RS, Raap AK, Janssen GM, Lemkes HH. Mitochondrial diabetes: molecular mechanisms and clinical presentation. Diabetes. 2004;53 Suppl 1:S103–S109. doi: 10.2337/diabetes.53.2007.s103. [DOI] [PubMed] [Google Scholar]
- 58.Magnusson I, Rothman DL, Jucker B, Cline GW, Shulman RG, Shulman GI. Liver glycogen turnover in fed and fasted humans. Am J Physiol. 1994;266:E796–E803. doi: 10.1152/ajpendo.1994.266.5.E796. [DOI] [PubMed] [Google Scholar]
- 59.Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest. 1992;90:1323–1327. doi: 10.1172/JCI115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 61.Martin G, Schoonjans K, Staels B, Auwerx J. PPARgamma activators improve glucose homeostasis by stimulating fatty acid uptake in the adipocytes. Atherosclerosis. 1998;137 Suppl:S75–S80. doi: 10.1016/s0021-9150(97)00315-8. [DOI] [PubMed] [Google Scholar]
- 62.Mayerson AB, Hundal RS, Dufour S, Lebon V, Befroy D, Cline GW, Enocksson S, Inzucchi SE, Shulman GI, Petersen KF. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 2002;51:797–802. doi: 10.2337/diabetes.51.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- 64.Michikawa Y, Mazzucchelli F, Bresolin N, Scarlato G, Attardi G. Aging-dependent large accumulation of point mutations in the human mtDNA control region for replication. Science. 1999;286:774–779. doi: 10.1126/science.286.5440.774. [DOI] [PubMed] [Google Scholar]
- 65.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 66.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nandi A, Kitamura Y, Kahn CR, Accili D. Mouse models of insulin resistance. Physiol Rev. 2004;84:623–647. doi: 10.1152/physrev.00032.2003. [DOI] [PubMed] [Google Scholar]
- 68.Neschen S, Morino K, Hammond LE, Zhang D, Liu ZX, Romanelli AJ, Cline GW, Pongratz RL, Zhang XM, Choi CS, Coleman RA, Shulman GI. Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab. 2005;2:55–65. doi: 10.1016/j.cmet.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 69.Nilsson LH, Hultman E. Liver and muscle glycogen in man after glucose and fructose infusion. Scand J Clin Lab Invest. 1974;33:5–10. doi: 10.3109/00365517409114190. [DOI] [PubMed] [Google Scholar]
- 70.Nilsson LH, Hultman E. Liver glycogen in man--the effect of total starvation or a carbohydrate-poor diet followed by carbohydrate refeeding. Scand J Clin Lab Invest. 1973;32:325–330. doi: 10.3109/00365517309084355. [DOI] [PubMed] [Google Scholar]
- 71.Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 72.Pagano G, Pacini G, Musso G, Gambino R, Mecca F, Depetris N, Cassader M, David E, Cavallo-Perin P, Rizzetto M. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology. 2002;35:367–372. doi: 10.1053/jhep.2002.30690. [DOI] [PubMed] [Google Scholar]
- 73.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, Rothman DL, Shulman GI. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med. 1996;335:1357–1362. doi: 10.1056/NEJM199610313351804. [DOI] [PubMed] [Google Scholar]
- 75.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Petersen KF, Hendler R, Price T, Perseghin G, Rothman DL, Held N, Amatruda JM, Shulman GI. 13C/31P NMR studies on the mechanism of insulin resistance in obesity. Diabetes. 1998;47:381–386. doi: 10.2337/diabetes.47.3.381. [DOI] [PubMed] [Google Scholar]
- 79.Petersen KF, Laurent D, Rothman DL, Cline GW, Shulman GI. Mechanism by which glucose and insulin inhibit net hepatic glycogenolysis in humans. J Clin Invest. 1998;101:1203–1209. doi: 10.1172/JCI579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, Shulman GI. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petersen KF, Price T, Cline GW, Rothman DL, Shulman GI. Contribution of net hepatic glycogenolysis to glucose production during the early postprandial period. Am J Physiol. 1996;270:E186–E191. doi: 10.1152/ajpendo.1996.270.1.E186. [DOI] [PubMed] [Google Scholar]
- 82.Radziuk J, McDonald TJ, Rubenstein D, Dupre J. Initial splanchnic extraction of ingested glucose in normal man. Metabolism. 1978;27:657–669. doi: 10.1016/0026-0495(78)90003-3. [DOI] [PubMed] [Google Scholar]
- 83.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 84.Ristow M. Neurodegenerative disorders associated with diabetes mellitus. J Mol Med. 2004;82:510–529. doi: 10.1007/s00109-004-0552-1. [DOI] [PubMed] [Google Scholar]
- 85.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54:8–14. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- 86.Roden M, Perseghin G, Petersen KF, Hwang JH, Cline GW, Gerow K, Rothman DL, Shulman GI. The roles of insulin and glucagon in the regulation of hepatic glycogen synthesis and turnover in humans. J Clin Invest. 1996;97:642–648. doi: 10.1172/JCI118460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roden M, Petersen KF, Shulman GI. Nuclear magnetic resonance studies of hepatic glucose metabolism in humans. Recent Prog Horm Res. 2001;56:219–237. doi: 10.1210/rp.56.1.219. [DOI] [PubMed] [Google Scholar]
- 88.Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roden M, Shulman GI. Applications of NMR spectroscopy to study muscle glycogen metabolism in man. Annu Rev Med. 1999;50:277–290. doi: 10.1146/annurev.med.50.1.277. [DOI] [PubMed] [Google Scholar]
- 90.Roden M, Stingl H, Chandramouli V, Schumann WC, Hofer A, Landau BR, Nowotny P, Waldhausl W, Shulman GI. Effects of free fatty acid elevation on postabsorptive endogenous glucose production and gluconeogenesis in humans. Diabetes. 2000;49:701–707. doi: 10.2337/diabetes.49.5.701. [DOI] [PubMed] [Google Scholar]
- 91.Rothman DL, Magnusson I, Cline G, Gerard D, Kahn CR, Shulman RG, Shulman GI. Decreased muscle glucose transport/phosphorylation is an early defect in the pathogenesis of non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A. 1995;92:983–987. doi: 10.1073/pnas.92.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rothman DL, Magnusson I, Katz LD, Shulman RG, Shulman GI. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science. 1991;254:573–576. doi: 10.1126/science.1948033. [DOI] [PubMed] [Google Scholar]
- 93.Rothman DL, Shulman RG, Shulman GI. 31P nuclear magnetic resonance measurements of muscle glucose-6-phosphate. Evidence for reduced insulin-dependent muscle glucose transport or phosphorylation activity in non-insulin-dependent diabetes mellitus. J Clin Invest. 1992;89:1069–1075. doi: 10.1172/JCI115686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rothman DL, Shulman RG, Shulman GI. N.m.r. studies of muscle glycogen synthesis in normal and non-insulin-dependent diabetic subjects. Biochem Soc Trans. 1991;19:992–994. doi: 10.1042/bst0190992. [DOI] [PubMed] [Google Scholar]
- 95.Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004 doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 96.Savage DB, Murgatroyd PR, Chatterjee VK, O'Rahilly S. Energy expenditure and adaptive responses to an acute hypercaloric fat load in humans with lipodystrophy. J Clin Endocrinol Metab. 2005;90:1446–1452. doi: 10.1210/jc.2004-1494. [DOI] [PubMed] [Google Scholar]
- 97.Savage DB, Tan GD, Acerini CL, Jebb SA, Agostini M, Gurnell M, Williams RL, Umpleby AM, Thomas EL, Bell JD, Dixon AK, Dunne F, Boiani R, Cinti S, Vidal-Puig A, Karpe F, Chatterjee VK, O'Rahilly S. Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-gamma. Diabetes. 2003;52:910–917. doi: 10.2337/diabetes.52.4.910. [DOI] [PubMed] [Google Scholar]
- 98.Schmitz-Peiffer C, Browne CL, Oakes ND, Watkinson A, Chisholm DJ, Kraegen EW, Biden TJ. Alterations in the expression and cellular localization of protein kinase C isozymes epsilon and theta are associated with insulin resistance in skeletal muscle of the high-fat-fed rat. Diabetes. 1997;46:169–178. doi: 10.2337/diab.46.2.169. [DOI] [PubMed] [Google Scholar]
- 99.Schmitz-Peiffer C, Oakes ND, Browne CL, Kraegen EW, Biden TJ. Reversal of chronic alterations of skeletal muscle protein kinase C from fat-fed rats by BRL-49653. Am J Physiol. 1997;273:E915–E921. doi: 10.1152/ajpendo.1997.273.5.E915. [DOI] [PubMed] [Google Scholar]
- 100.Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, Goto T, Westerbacka J, Sovijarvi A, Halavaara J, Yki-Jarvinen H. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 101.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 102.Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000;6:77–86. [PubMed] [Google Scholar]
- 103.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shulman GI, Rothman DL, Chung Y, Rossetti L, Petit WA, Jr, Barrett EJ, Shulman RG. 13C NMR studies of glycogen turnover in the perfused rat liver. J Biol Chem. 1988;263:5027–5029. [PubMed] [Google Scholar]
- 105.Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990;322:223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- 106.Shulman RG, Rothman DL. 13C NMR of intermediary metabolism: implications for systemic physiology. Annu Rev Physiol. 2001;63:15–48. doi: 10.1146/annurev.physiol.63.1.15. [DOI] [PubMed] [Google Scholar]
- 107.Simon DK, Johns DR. Mitochondrial disorders: clinical and genetic features. Annu Rev Med. 1999;50:111–127. doi: 10.1146/annurev.med.50.1.111. [DOI] [PubMed] [Google Scholar]
- 108.Storlien L, Oakes ND, Kelley DE. Metabolic flexibility. Proc Nutr Soc. 2004;63:363–368. doi: 10.1079/PNS2004349. [DOI] [PubMed] [Google Scholar]
- 109.Szczepaniak LS, Babcock EE, Schick F, Dobbins RL, Garg A, Burns DK, McGarry JD, Stein DT. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol. 1999;276:E977–E989. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 110.Tamura Y, Tanaka Y, Sato F, Choi JB, Watada H, Niwa M, Kinoshita J, Ooka A, Kumashiro N, Igarashi Y, Kyogoku S, Maehara T, Kawasumi M, Hirose T, Kawamori R. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2005;90:3191–3196. doi: 10.1210/jc.2004-1959. [DOI] [PubMed] [Google Scholar]
- 111.Tan GD, Fielding BA, Currie JM, Humphreys SM, Desage M, Frayn KN, Laville M, Vidal H, Karpe F. The effects of rosiglitazone on fatty acid and triglyceride metabolism in type 2 diabetes. Diabetologia. 2005;48:83–95. doi: 10.1007/s00125-004-1619-9. [DOI] [PubMed] [Google Scholar]
- 112.Taylor R, Magnusson I, Rothman DL, Cline GW, Caumo A, Cobelli C, Shulman GI. Direct assessment of liver glycogen storage by 13C nuclear magnetic resonance spectroscopy and regulation of glucose homeostasis after a mixed meal in normal subjects. J Clin Invest. 1996;97:126–132. doi: 10.1172/JCI118379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Taylor R, Price TB, Katz LD, Shulman RG, Shulman GI. Direct measurement of change in muscle glycogen concentration after a mixed meal in normal subjects. Am J Physiol. 1993;265:E224–E229. doi: 10.1152/ajpendo.1993.265.2.E224. [DOI] [PubMed] [Google Scholar]
- 114.Ueki K, Kondo T, Kahn CR. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol. 2004;24:5434–5446. doi: 10.1128/MCB.24.12.5434-5446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Unger RH. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology. 2003;144:5159–5165. doi: 10.1210/en.2003-0870. [DOI] [PubMed] [Google Scholar]
- 116.van Wijk JP, Cabezas MC, de Koning EJ, Rabelink TJ, van der Geest R, Hoepelman IM. In vivo evidence of impaired peripheral fatty acid trapping in patients with human immunodeficiency virus-associated lipodystrophy. J Clin Endocrinol Metab. 2005;90:3575–3582. doi: 10.1210/jc.2004-2343. [DOI] [PubMed] [Google Scholar]
- 117.Wahren J, Felig P, Cerasi E, Luft R. Splanchnic and peripheral glucose and amino acid metabolism in diabetes mellitus. J Clin Invest. 1972;51:1870–1878. doi: 10.1172/JCI106989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med. 1990;113:909–915. doi: 10.7326/0003-4819-113-12-909. [DOI] [PubMed] [Google Scholar]
- 119.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Woerle HJ, Meyer C, Dostou JM, Gosmanov NR, Islam N, Popa E, Wittlin SD, Welle SL, Gerich JE. Pathways for glucose disposal after meal ingestion in humans. Am J Physiol Endocrinol Metab. 2003;284:E716–E725. doi: 10.1152/ajpendo.00365.2002. [DOI] [PubMed] [Google Scholar]
- 123.Wolfrum C, Asilmaz E, Luca E, Friedman JM, Stoffel M. Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature. 2004;432:1027–1032. doi: 10.1038/nature03047. [DOI] [PubMed] [Google Scholar]
- 124.Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, Bassel-Duby R, Williams RS. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science. 2002;296:349–352. doi: 10.1126/science.1071163. [DOI] [PubMed] [Google Scholar]
- 125.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 126.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Youn JH, Bergman RN. Enhancement of hepatic glycogen by gluconeogenic precursors: substrate flux or metabolic control? Am J Physiol. 1990;258:E899–E906. doi: 10.1152/ajpendo.1990.258.6.E899. [DOI] [PubMed] [Google Scholar]
- 128.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 129.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 130.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 131.Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]