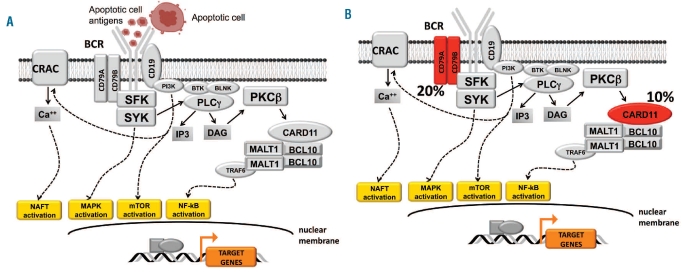
The B-cell receptor (BCR) is the hallmark of mature B cells and is expressed by many B-cell malignancies. BCR involvement in B-cell neoplasia occurs through two major mechanisms: (i) constitutive activation through somatically acquired genetic lesions of BCR signaling components; and (ii) BCR signaling induced by foreign or auto-antigens providing proliferative and anti-apoptotic signals to neoplastic B cells (Figure 1). These two mechanisms of BCR involvement preferentially associate with aggressive and indolent B-cell malignancies, respectively. Genetic lesions targeting BCR signaling components, namely CD79A, CD79B and CARD11, are detectable in a substantial fraction of diffuse large B-cell lymphomas, whereas they are exceptional in indolent disorders, including chronic lymphocytic leukemia (CLL).1 Evidence for selection and stimulation of the B-cell clone through the BCR has been gained for several indolent B-cell malignancies, including CLL, whereas such evidence is scant in aggressive B-cell lymphoma.2
Figure 1.
Mechanisms of BCR involvement in different types of B-cell neoplasia. The mechanisms of BCR activation in B-cell neoplasia are different in indolent versus aggressive B-cell neoplasms. (A) In CLL, as well as in other indolent B-cell disorders, the contribution of BCR signaling to tumor growth is conceivably due to receptor stimulation by foreign or auto-antigens. In the case of many CLL with stereotyped BCR, autoantigens are frequently derived from apoptotic cells. Genetic alterations of BCR components are rare or absent. (B) In aggressive B-cell malignancies, best exemplified by the case of diffuse large B-cell lymphoma, BCR activation proceeds through genetic lesions altering specific components of the BCR itself. In the case of activated B-cell-like diffuse large B-cell lymphoma, somatically acquired mutations of CD79A/CD79B and of CARD11 are found in 20% and 10% of the cases, respectively. These genetic lesions are responsible for tonic BCR signaling, which provides mitogenic signals to the tumor clone.
Historically, mucosa-associated lymphoid tissue (MALT) lymphoma has represented the prototype of an antigen-driven B-cell neoplasm. During the last decade, the pathogenic role of tumor cell interactions with the microenvironment through the BCR has emerged predominantly also in the context of CLL. The dissection of CLL immunogenetics has highlighted the role of the BCR in promoting CLL, based on: (i) a skewed immunoglobulin heavy chain variable (IGHV) gene repertoire utilized in CLL; (ii) identification of two CLL subgroups with markedly different prognoses according to IGHV gene mutation status; and, more recently, (iii) discovery of restricted and nearly identical BCR sequences, termed stereotyped BCR, in approximately 30% of cases of CLL.
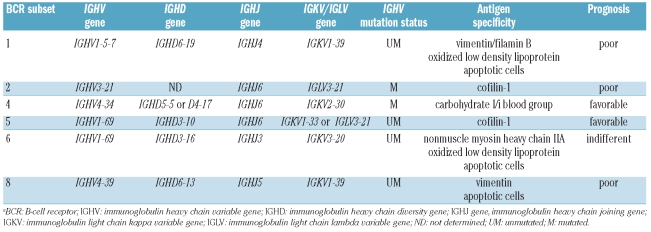
Combination diversity due to IGHV-D-J rearrangement and somatic hypermutation allows the potential synthesis of more than 1012 different immunoglobulins. In spite of this heterogeneity, the BCR of one third of CLL display nearly identical or highly homologous complementarity determining region 3 (CDR3) regions, which have been grouped into different subsets of stereotyped BCR, each conventionally identified by a sequential number.3–6 The fact that one out of three CLL patients express a BCR that is almost identical to that of another CLL patient strongly supports a role for BCR triggering by specific antigens in this leukemia. Consistently, molecular structures present on apoptotic cells or infectious antigens have been identified as antigens bound by stereotyped BCR (Table 1).7–9 In addition to increasing knowledge on the pathogenesis of CLL, the identification of stereotyped BCR has also improved the potential for a molecular prediction of the prognosis of the disease.
Table 1.
Immunogenetic and clinical features of BCR subsets recognizing auto-antigens in CLL.a
Stereotyped B-cell receptors as markers of poor prognosis in chronic lymphocytic leukemia
CLL patients with BCR subset 2 represent a distinct clinical subgroup characterized by a progressive phenotype regardless of IGHV mutational status.4–6,10–12 BCR subset 2 utilizes the IGHV3-21 gene and is characterized by a distinctively short VH CDR3 matching, or differing only slightly from, the consensus motif DANGMDV.10–12 Subset 2 BCR cases also display a highly homologous lambda CDR3, due to restricted usage of the IGLV3-21 light chain gene.
The unfavorable clinical outcome of patients with BCR subset 2 CLL might be explained by a distinctive transcriptome and genome profile characterized by up-regulation of genes involved in cell cycle control, leading to increased proliferation and, consequently, poor outcome.12,13 Also, patients with BCR subset 2 CLL frequently carry del11q22-q23, which affects the ATM gene and predicts poor outcome with many, although not all, therapeutic regimens used for CLL.
The role of selective antigenic pressure in promoting growth of BCR subset 2 CLL is supported by molecular, epidemiological, and immunological evidence. Although IGHV sequences of BCR subset 2 are generally less mutated than those of other IGHV3 genes, the observed mutations are frequently shared among cases and very precisely targeted, indicating selection by specific antigens.4–6,10–12 In addition, IGHV sequences of BCR subset 2 CLL show a strong tendency to retain the germline configuration in the binding motif for prototypic superantigens. The epidemiology of BCR subset 2 displays a regional gradient, suggesting variations in exposure to specific antigens in different parts of the world.4–6,10–12 The contribution of antigenic pressure to BCR subset 2 CLL is also documented by the tumor clone production of antibodies displaying a restricted reactivity against cofilin-1, a cytoskeleton-associated protein binding actin exposed during bleb formation in the later stages of apoptosis.2
Stereotyped B-cell receptors and transformation of chronic lymphocytic leukemia to aggressive lymphoma
Over time, some patients with CLL develop Richter’s syndrome, most commonly represented by diffuse large B-cell lymphoma. The cumulative incidence of Richter’s syndrome exceeds 15% at 10 years after the diagnosis of CLL.14 Richter’s syndrome is generally considered an incurable disease, with the possible exception of cases with limited tumor load. Early recognition of Richter’s syndrome is, therefore, clinically relevant and might be facilitated by the availability of predictors of development of the syndrome at the time of the initial diagnosis of CLL.14 Patients with stereotyped BCR subset 8 CLL, characterized by IGHV4-39 usage, have a 17-fold increase in the relative risk of Richter’s syndrome transformation (5-year probability of Richter’s syndrome ~70%), which is independent of other risk factors.15 This observation, beside being clinically meaningful, points to the role of antigen stimulation in Richter’s syndrome, in addition to CLL.
Stereotyped BCR subset 8 binds the cytoskeleton protein vimentin exposed on apoptotic cell blebs, for which subset 8 shows the highest affinity among BCR subsets.9 At variance with the majority of CLL that express IgM/IgD, cases belonging to BCR subset 8 are IgG-switched. Notably, class switch recombination occurs in CLL cells expressing high levels of activation-induced deaminase, which predisposes to genetic instability, and high levels of the proliferation marker c-MYC, which is frequently activated by translocation or amplification in Richter’s syndrome.14 Furthermore, BCR subset 8 closely associates with trisomy 12, which identifies the CLL cytogenetic subgroup with highest risk of developing Richter’s syndrome.
Stereotyped B-cell receptors as markers of clinical stability in chronic lymphocytic leukemia
BCR subset 4 utilizes IgG-switched somatically mutated IGHV4-34 genes.4–6 The characteristic clinical picture of CLL patients with BCR subset 4 is young age at disease onset (median, 43 years) and a very indolent course.4–6 A peculiarity of BCR subset 4 CLL is the coupling of autoreactivity and anergy. Subset 4 CLL conceivably originate from cells with strong autoreactivity against DNA or apoptotic bodies, which must, however, undergo modifications attenuating self-reactivity in order to be selected into peripheral B cells and, subsequently, transform into CLL.4–6 Somatic hypermutation, which extensively characterizes BCR subset 4, reduces the intrinsic strong autoreactivity against DNA of natural IGHV4-34 antibodies, and possibly renders cells less responsive to BCR engagement.4–6,16
Although CLL cells belonging to BCR subset 4 are relatively anergic upon BCR cross-linking, antigen interactions still have a role in promoting growth. In fact, BCR subset 4 is the sole CLL subgroup showing high levels of intraclonal diversification within immunogloblin genes, suggesting that ongoing interactions with antigens play a role in sustaining the CLL clone. Also, BCR subset 4 patients frequently harbor a latent/persistent infection by cytomegalovirus and Epstein-Barr virus, which induce an increase of IGHV4-34 antibodies by the immune system.17 These observations suggest that subset 4 CLL are strongly dependent on BCR stimulation, although the “anergic” state of BCR subset 4 might account for the low proliferative behavior of this CLL group, thus preventing the accumulation of genomic aberrations and leading to a favorable clinical course.
Consistent with this model, in this issue of the Journal Marincevic et al. report that BCR subset 4 CLL are characterized by a gene expression profile that may partly explain the low proliferative potential of this CLL subset.18 Interestingly, the gene expression profile of BCR subset 4 CLL differs significantly from that of the rarer BCR subset 16 CLL, although both CLL subsets utilize the same IGHV4-34 gene.18 This observation reiterates and corroborates the notion that stereotyped BCR, rather than mere usage of the same IGHV gene, are important for the identification of biologically and clinically homogeneous CLL groups. The genome wide expression analysis of 25 samples of IGHV4-34 CLL performed by Marincevic et al. significantly contributes to the clarification of the biological basis for the indolent course of BCR subset 4 CLL, since the analysis revealed that genes important for cell cycle regulation, namely TLK1, and apoptosis control, namely RPS27L, are down-regulated in BCR subset 4 CLL.18 Gene ontology enrichment analysis also disclosed several gene networks that are differentially expressed in BCR subset 4 compared to subset 16 CLL, including pathways involved in cellular growth and proliferation, humoral and cell-mediated immune responses, and hematopoiesis. In BCR subset 4 CLL, the low expression level of genes of the PI3K, AKT and NF-κB pathways provides further biological grounds for the very indolent course of this CLL subgroup.18 The results of the study by Marincevic et al., together with those of an earlier study exploring genomic aberrations in BCR subset 4 CLL,19 provide a convincing model suggesting that stereotyped BCR, gene expression profile, and pattern of genetic lesions are closely linked and interact in determining the clinical phenotype of CLL. Following the model of BCR subset 4 CLL18,19 and, previously, of BCR subset 2 CLL,10–12 it is desirable that a comprehensive biological characterization will also be gained for CLL belonging to other BCR subsets.
Open issues
Stereotyped BCR have rapidly emerged as a major player in the pathogenesis of CLL, raising many biological and clinical questions. Normal B cells carrying stereotyped BCR similar to those of CLL have been identified recently, and are a likely a source of transformation into CLL.20 Following the example of Marincevic et al., genome-wide technologies will be helpful to progressively elucidate the biology of CLL with different BCR subsets. The full prognostic significance of stereotyped BCR subsets may not be readily identifiable in CLL cohorts investigated until now, and a better understanding of this aspect will require collaborative studies of very large populations. Identification of the full complement of antigens recognized by CLL stereotyped BCR might lead to changes in our therapeutic attitude. Finally, stereotyped BCR might provide a proof of concept that extends beyond CLL and applies to other indolent B-cell malignancies.21
Footnotes
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.Lenz G, Staudt LM. Aggressive lymphomas. N Engl J Med. 2010;116 (6):953–61. doi: 10.1056/NEJMra0807082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosén A, Murray F, Evaldsson C, Rosenquist R. Antigens in chronic lymphocytic leukemia – implications for cell origin and leukemogenesis. Semin Cancer Biol. 2010 doi: 10.1016/j.semcancer.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Messmer BT, Albesiano E, Efremov DG, Ghiotto F, Allen SL, Kolitz J, et al. Multiple distinct sets of stereotyped antigen receptors indicate a role for antigen in promoting chronic lymphocytic leukemia. J Exp Med. 2004;200(4):519–25. doi: 10.1084/jem.20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stamatopoulos K, Belessi C, Moreno C, Boudjograh M, Guida G, Smilevska T, et al. Over 20% of patients with chronic lymphocytic leukemia carry stereotyped receptors: pathogenetic implications and clinical correlations. Blood. 2007;109(1):259–70. doi: 10.1182/blood-2006-03-012948. [DOI] [PubMed] [Google Scholar]
- 5.Murray F, Darzentas N, Hadzidimitriou A, Tobin G, Boudjogra M, Scielzo C, et al. Stereotyped patterns of somatic hypermutation in subsets of patients with chronic lymphocytic leukemia: implications for the role of antigen selection in leukemogenesis. Blood. 2008;111(3):1524–33. doi: 10.1182/blood-2007-07-099564. [DOI] [PubMed] [Google Scholar]
- 6.Bomben R, Dal Bo M, Capello D, Forconi F, Maffei R, Laurenti L, et al. Molecular and clinical features of chronic lymphocytic leukaemia with stereotyped B cell receptors: results from an Italian multicentre study. Br J Haematol. 2009;144(4):492–506. doi: 10.1111/j.1365-2141.2008.07469.x. [DOI] [PubMed] [Google Scholar]
- 7.Catera R, Silverman GJ, Hatzi K, Seiler T, Didier S, Zhang L, et al. Chronic lymphocytic leukemia cells recognize conserved epitopes associated with apoptosis and oxidation. Mol Med. 2008;14(11–12):665–74. doi: 10.2119/2008-00102.Catera. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu CC, Catera R, Hatzi K, Yan X-J, Zhang L, Bo Wang X, et al. Chronic lymphocytic leukemia antibodies with a common stereotypic rearrangement recognize nonmuscle myosin heavy chain IIA. Blood. 2008;112(13):5122–9. doi: 10.1182/blood-2008-06-162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu CC, Catera R, Zhang L, Didier S, Agagnina BM, Damle RN, et al. Many chronic lymphocytic leukemia antibodies recognize apoptotic cells with exposed nonmuscle myosin heavy chain IIA: implications for patient outcome and cell of origin. Blood. 2010;115(19):3907–15. doi: 10.1182/blood-2009-09-244251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobin G, Thunberg U, Johnson A, Thorn I, Soderberg O, Hultdin M, et al. Somatically mutated Ig V(H)3-21 genes characterize a new subset of chronic lymphocytic leukemia. Blood. 2002;99(6):2262–4. doi: 10.1182/blood.v99.6.2262. [DOI] [PubMed] [Google Scholar]
- 11.Thorsélius M, Kröber A, Murray F, Thunberg U, Tobin G, Bühler A, et al. Strikingly homologous immunoglobulin gene rearrangements and poor outcome in VH3-21-using chronic lymphocytic leukemia patients independent of geographic origin and mutational status. Blood. 2006;107(7):2889–94. doi: 10.1182/blood-2005-06-2227. [DOI] [PubMed] [Google Scholar]
- 12.Bomben R, Dal Bo M, Capello D, Benedetti D, Marconi D, Zucchetto A, et al. Comprehensive characterization of IGHV3-21-expressing B-cell chronic lymphocytic leukemia: an Italian multicenter study. Blood. 2007;109(7):2989–98. doi: 10.1182/blood-2006-10-051110. [DOI] [PubMed] [Google Scholar]
- 13.Fält S, Merup M, Tobin G, Thunberg U, Gahrton G, Rosenquist R, Wennborg A. Distinctive gene expression pattern in VH3-21 utilizing B-cell chronic lymphocytic leukemia. Blood. 2005;106(2):681–9. doi: 10.1182/blood-2004-10-4073. [DOI] [PubMed] [Google Scholar]
- 14.Rossi D, Gaidano G. Richter syndrome: molecular insights and clinical perspectives. Hematol Oncol. 2009;27(1):1–10. doi: 10.1002/hon.880. [DOI] [PubMed] [Google Scholar]
- 15.Rossi D, Spina V, Cerri M, Rasi S, Deambrogi C, De Paoli L, et al. Stereotyped B-cell receptor is an independent risk factor of chronic lymphocytic leukemia transformation to Richter syndrome. Clin Cancer Res. 2009;15(13):4415–22. doi: 10.1158/1078-0432.CCR-08-3266. [DOI] [PubMed] [Google Scholar]
- 16.Guarini A, Chiaretti S, Tavolaro S, Maggio R, Peragine N, Citarella F, et al. BCR ligation induced by IgM stimulation results in gene expression and functional changes only in IgVH unmutated chronic lymphocytic leukemia (CLL) cells. Blood. 2008;112(3):782–92. doi: 10.1182/blood-2007-12-127688. [DOI] [PubMed] [Google Scholar]
- 17.Kostareli E, Hadzidimitriou A, Stavroyianni N, Darzentas N, Athanasiadou A, Gounari M, et al. Molecular evidence for EBV and CMV persistence in a subset of patients with chronic lymphocytic leukemia expressing stereotyped IGHV4-34 B-cell receptors. Leukemia. 2009;23(5):919–24. doi: 10.1038/leu.2008.379. [DOI] [PubMed] [Google Scholar]
- 18.Marincevic M, Mansouri M, Kanduri M, Isaksson A, Göransson H, Ekström Smedby K, et al. Distinct gene expression profiles in subsets of chronic lymphocytic leukemia expressing stereotyped IGHV4-34 B-cell receptors. Haematologica. 2010;95(12):2072–9. doi: 10.3324/haematol.2010.028639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marincevic M, Cahill N, Gunnarsson R, Isaksson A, Mansouri M, Göransson H, et al. High-density screening reveals a different spectrum of genomic aberrations in chronic lymphocytic leukemia patients with “stereotyped” IGHV3-21 and IGHV4-34 B cell receptors. Haematologica. 2010;95(9):1519–25. doi: 10.3324/haematol.2009.021014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forconi F, Potter KN, Wheatley I, Darzentas N, Sozzi E, Stamatopoulos K, et al. The normal IGHV1-69-derived B-cell repertoire contains stereotypic patterns characteristic of unmutated CLL. Blood. 2010;115(1):71–7. doi: 10.1182/blood-2009-06-225813. [DOI] [PubMed] [Google Scholar]
- 21.Zibellini S, Capello D, Forconi F, Marcatili P, Rossi D, Rattotti S, et al. Stereotyped patterns of B-cell receptor in splenic marginal zone lymphoma. Haematologica. 2010;95(10):1792–6. doi: 10.3324/haematol.2010.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]