Abstract
Background
Exposure to γ-radiation causes rapid hematopoietic cell apoptosis and bone marrow suppression. However, there are no approved radiation countermeasures for the acute radiation syndrome. In this study, we demonstrated that natural δ-tocotrienol, one of the isomers of vitamin E, significantly enhanced survival in total body lethally irradiated mice. We explored the effects and mechanisms of δ-tocotrienol on hematopoietic progenitor cell survival after γ-irradiation in both in vivo and in vitro experiments.
Design and Methods
CD2F1 mice and human hematopoietic progenitor CD34+ cells were treated with δ-tocotrienol or vehicle control 24 h before or 6 h after γ-irradiation. Effects of δ-tocotrienol on hematopoietic progenitor cell survival and regeneration were evaluated by clonogenicity studies, flow cytometry, and bone marrow histochemical staining. δ-tocotrienol and γ-irradiation-induced signal regulatory activities were assessed by immunofluorescence staining, immunoblotting and short-interfering RNA assay.
Results
δ-tocotrienol displayed significant radioprotective effects. A single injection of δ-tocotrienol protected 100% of CD2F1 mice from total body irradiation-induced death as measured by 30-day post-irradiation survival. δ-tocotrienol increased cell survival, and regeneration of hematopoietic microfoci and lineage−/Sca-1+/ckit+ stem and progenitor cells in irradiated mouse bone marrow, and protected human CD34+ cells from radiation-induced damage. δ-tocotrienol activated extracellular signal-related kinase 1/2 phosphorylation and significantly inhibited formation of DNA-damage marker γ-H2AX foci. In addition, δ-tocotrienol up-regulated mammalian target of rapamycin and phosphorylation of its downstream effector 4EBP-1. These alterations were associated with activation of mRNA translation regulator eIF4E and ribosomal protein S6, which is responsible for cell survival and growth. Inhibition of extracellular signal-related kinase 1/2 expression by short interfering RNA abrogated δ-tocotrienol-induced mammalian target of rapamycin phosphorylation and clonogenicity, and increased γ-H2AX foci formation in irradiated CD34+ cells.
Conclusions
Our data indicate that δ-tocotrienol protects mouse bone marrow and human CD34+ cells from radiation-induced damage through extracellular signal-related kinase activation-associated mammalian target of rapamycin survival pathways.
Keywords: γ-tocotrienol, radioprotection, Erk, mTOR
Introduction
More than 50% of cancer patients receive radiotherapy, which often results in side effects due to radiation damage to normal tissue,1 such as bone marrow failure syndrome.2 The hematopoietic system is among the most radiation-sensitive organs.3 A dose of ionizing radiation above 1 Gy in humans poses a risk of injury to the bone marrow and hematopoietic system, which leads to long-term compromised immune function and increased susceptibility to infection and internal and external hemorrhage.4 The mechanism of ionizing radiation-induced bone marrow failure is not well understood and there are no FDA-approved pharmaceuticals to counter acute radiation syndrome.
There are eight distinct analogs of vitamin E, designated α-, β-, γ-, and δ-tocopherol and α-, β-, γ-, and δ-tocotrienol (DT3).5,6 Previous studies have been conducted with tocopherols, the most commonly used vitamin E supplement and the most abundant vitamin E isoform in human and animal tissue. During the last decade, tocotrienol research has gained substantial momentum. Tocotrienols have shown neuroprotective, anticancer, anti-oxidative, and cholesterol-decreasing effects that are often not exhibited by tocopherols.5–7 The present study evaluated the mechanism of radioprotection by DT3.
We found DT3-induced extracellular signal-regulated kinase 1/2 (Erk1/2) and mammalian target of rapamycin (mTOR) activation in human hematopoietic CD34+ cells (in vitro) and mouse bone marrow (in vivo) was associated with increased cell survival, and decreased formation of the radiation-induced DNA-damage marker γ-H2AX foci.
Erk1/28 activity is a component of the pro-survival mitogen-activated protein kinase (MAPK) pathway, which induces expression of DNA repair and cell growth factors.9–11 mTOR is a member of the phosphatidylinositol 3-kinase (PI3K)-related kinase family and plays a central role in regulating protein synthesis, ribosomal protein translation, and cap-dependent translation,12 which are involved in cell proliferation, cell cycle progression, DNA damage checkpoints, and cell survival and growth.13 Major upstream signaling components that control mTOR activity are the PI3K/Akt and Erk/MAPK pathways,14,15 and the immediate mTOR downstream targets are eukaryotic initiation factor 4E (eIF4E)-binding protein-1 (4EBP-1) and the ribosomal protein S6 kinase (S6K).16 4EBP1 and S6K activity are controlled by mTOR, which phosphorylates and inactivates 4EBP1 (the inhibitor of eIF4E), and phosphorylates and activates S6K, resulting in stimulation of cap-dependent mRNA translation in eukaryotic cells. Most mRNA are translated in mammalian cells through a cap-dependent mechanism,17 and translational control allows for rapid protein regulation in response to positive or negative stimuli including cell growth, cell cycle progression, and apoptosis.18 In the present study, we investigated the effects of DT3 as a radiation countermeasure and the mechanisms of DT3-induced Erk1/2 and mTOR signaling.
Design and Methods
Mice
Twelve- to 14-week old CD2F1 male mice (Harlan Laboratories, Indianapolis, IN, USA) were used for survival studies according to previously described methods.19 Animals were housed in an AAALAC-approved facility at the Armed Forces Radiobiology Research Institute (Bethesda, MD). The animal study protocol was approved by the Institutional Animal Care and Use Committee.
Human CD34+ cells
Human hematopoietic CD34+ cells were provided by the National Hematopoietic Cell Processing Core, Fred Hutchinson Cancer Research Center (Seattle, WA, USA). CD34+ cells were cultured in serum-free medium consisting of Iscove’s modified Dulbecco’s medium (IMDM) supplemented with BIT 9500 (Stem Cell Technologies, Tukwila, WA, USA) and penicillin/streptomycin. Recombinant human (rh) stem cell factor (SCF, 100 ng/mL), rh flt-3 ligand (FL, 100 ng/mL), and rh interleukin-3 (IL-3, 25 ng/mL) were added. All cytokines were purchased from PeproTech, Inc. (Rocky Hill, NJ, USA). CD34+ cells were incubated at 37°C with 5% CO2.
Drug and irradiation
DT3 was purchased from Yasoo Health Inc. (Johnson City, TN, USA). The drug was dissolved in PEG-400 and 5% Tween-80 for in vivo studies, and in ethanol for in vitro studies. A vehicle control consisting of PEG-400 and 5% Tween-80 was used for animal studies. Ethanol was used as a control in in vitro studies.
DT3 (400 mg/kg) or vehicle was administered as a single subcutaneous (sc) dose to mice 24 h before (−24 h) or 6 h after (+6 h) total body irradiation (TBI) at doses of 0 (sham-irradiation), 5.0, or 8.75 Gy, at a dose rate of 0.6 Gy/min in the Institute’s cobalt facility. Sham-irradiated mice were treated exactly the same way as the γ-irradiated animals except the cobalt-60 source was not raised from the shielding water pool. After irradiation, mice were returned to their home cages. The day of irradiation was regarded as day 0. Survival was monitored for 30 days post-irradiation.
For the in vitro study, DT3 (2 μM) or vehicle control (alcohol) was added to the human CD34+ cell culture 24 h before exposure to γ-irradiation at doses of 0, 2, or 4 Gy (0.6 Gy/min).20 After irradiation, cells were washed once and cultured in fresh culture medium without DT3. For the +6 h treatment groups, the same dose of DT3 or vehicle was added post-irradiation. Total survival cell number after irradiation was counted by trypan blue staining.
Pathology of mouse bone marrow
Mouse sterna were fixed in Z-Fix (formaldehyde, methanol, ionized zinc buffer, Anatech Ltd., Battle Creek, MI, USA) for at least 24 h. Samples were decalcified (Cal-EX for 3 h) and sectioned longitudinally for hematoxylineosin (HE) staining. Slides were first examined at 20x. Bone marrow cellularity was measured by a subjective analysis of 8–14 adjacent low power (200x) microscopic fields for each sectioned sternum (4–6 sternebrae). Megakaryocytes were evaluated by a subjective analysis of three adjacent high power (600x) microscopic fields for each sternebra.
Mouse bone marrow myeloid cell viability and cell phenotype analysis
Bone marrow cells were collected from mouse femora and humeri 1, 8 and 13 days after TBI. After erythrocytes were lysed by erythrocyte lysis buffer (Qiagen GmbH, Hilden), total bone marrow myeloid cell viability for pooled samples from each mouse was measured by trypan blue staining and by BD FACSCalibur flow cytometry analysis after labeling with annex-in-V (apoptotic cell marker) and 7-aminoactinomycin D (7AAD, a death marker). Total live myeloid cell numbers from individual mice were measured on days 1, 8 and 13 after TBI or sham-irradiation. Phenotypes of murine bone marrow cells were quantified using the BD FACSCalibur. Cells were gated for 7AAD-positive dead cells and negative live cells. Mouse lineage, c-kit, and Sca-1 antibodies were used for phenotype determination in 7AAD-negative populations. All antibodies and dyes were purchased from BD Biosciences (San Jose, CA, USA).
Mouse bone marrow cell and human CD34+ cell clonogenic assay
Clonogenicity of mouse bone marrow cells and human CD34+ cells was quantified in standard semisolid cultures in triplicate using 1 mL of Methocult GF+ system for either mouse cells or human cells (Stem Cell Technologies) according to the manufacturer’s instructions, as described previously.20 Briefly, mouse bone marrow cells from pooled samples or CD34+ cells from liquid culture were washed twice with IMDM and seeded at 1–5×104 cells/dish (mouse cells) or 1–5×103 cells/dish (CD34+ cells) in 35-cm cell culture dishes (BD Biosciences). Plates were scored for erythroid, granulocyte-macrophage, and mixed-lineage colonies after culturing for 10 days (for mouse colonies) or 14 days (for human colonies) at 37°C in 5% CO2.
Immunoblotting
Cells (1–5×106) from each sample were harvested, washed, and lysed with 1x Laemmli sample buffer. Protein concentrations were determined using a BCA protein assay kit (Pierce, Rockford, IL, USA). Immunoblotting was performed following standard procedures with an enhanced chemiluminescence kit (Thermo Scientific, Rockford, IL, USA) and Kodak X-ray film. Antibodies for Erk1/2, phosphorylated Erk1/2 (p-Erk1/2), Akt, p-Akt, mTOR, p-mTOR, 4EBP1, p-4EBP1, eIF4E, p-eIF4E, S6, and p-S6 were from Cell Signaling (Danvers, MA, USA).
Extracellular signal related kinase short interfering RNA transfection
SignalSilence Erk1 and Erk2 short interfering RNA (siRNA) from siGENOME SMARTpool (1.5 μg each, Dharmacon Inc., Lafayette, CO, USA) or 1.5 μg of maxGFP siRNA (positive control provided in the siRNA Test Kit, amaxa Inc.) were transfected into 3×106 human CD34+ cells using the Human CD34 Cell Nucleofector Kit and Nucleofector II (amaxa Inc., Gaithersburg, MD, USA) with program A-27 according to the manufacturer’s protocol as described previously.20 After transfection, cells were cultured with and without DT3 at 37°C with 5% CO2 until irradiation on the next day (24 h after siRNA transfection). Western blots and colony assays were performed 24 h post-irradiation (48 h after siRNA transfection).
Immunofluorescence staining
Sectioned mouse sterna from DT3- and vehicle control-treated mice 24 h after TBI, and cytospin slides from DT3- and vehicle control-treated human CD34+ cells 4 h post-irradiation were processed for immunostaining. Specimens were incubated in blocking buffer (5% bovine serum albumin in phosphate-buffered saline), followed by primary antibodies (anti-p-Erk1/2 and anti-γ-H2AX), and FITC-conjugated anti-rabbit IgG and rhodamine-conjugated anti-goat IgG second antibodies. Slides were rinsed in high salt phosphate-buffered saline (0.4M NaCl in phosphate-buffered saline), counterstained for DNA with 4, 6-diamidino-2-phenylindole (DAPI) mounting medium, and coverslipped. Slide images were examined with a Zeiss fluorescence microscope using the AxioVision MTB2004 configuration.
Statistical analysis
The difference in 30-day survival of mice was analyzed using Fisher’s exact test. Differences between means were compared by ANOVA and Student’s t tests. P values less than 0.05 were considered statistically significant. Results are presented as means ± standard deviations or standard errors of the mean as indicated.
Results
δ-tocotrienol enhanced mouse survival after γ-irradiation and protected mouse bone marrow hematopoietic progenitors
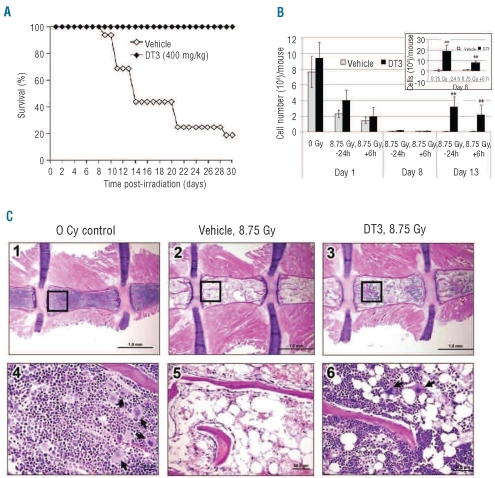
Figure 1A shows the results of a typical survival study.19 A single subcutaneous (sc) injection of DT3 (400 mg/kg) 24 h before TBI (8.75 Gy, Cobalt 60 γ-radiation, 0.6 Gy/min) protected CD2F1 mice from radiation-induced mortality with 100% 30-day survival. In contrast, in control vehicle-injected mice, radiation-induced deaths started 8 days post-irradiation and the 30-day survival after TBI was only 18% (n=16). The time course for vehicle controls shown in Figure 1A is similar to that which we observed in other studies, and is consistent with mortality due largely to hematopoietic injury.19,21
Figure 1.
DT3 protects mouse bone marrow hematopoietic cells after γ-irradiation. (A) Protective effect of DT3 on the survival of irradiated CD2F1 mice (n =16). DT3 or vehicle sc 24 h before TBI (8.75 Gy at a dose rate of 0.6 Gy/min). The difference in 30-day survival between vehicle-injected and DT3-injected groups was statistically significant (P<0.01). (B) Total live cell counts of mouse bone marrow myeloid cells from pooled femur and humerus samples from vehicle control (N=6) and DT3-treated (−24 h or +6 h irradiation) mice (N=6) 1, 8, and 13 days post-irradiation. Total live cell counts of mouse bone marrow myeloid cells 8 days are shown in the upper panel (y axis: 104/mouse). Means±SD. **P<0.01, DT3-treated versus vehicle-treated mice. (C) HE staining of bone marrow from DT3-treated and control mice 8 days post-irradiation (8.75 Gy): longitudinal sections of entire sterna from representative mice in different groups at 20X magnification are shown in the upper panels (panels 1, 2, and 3). Sections in the bottom panels (4, 5, and 6) are higher magnifications of the selected areas of each corresponding upper panel indicated by rectangles (200X). Megakaryocyte foci are identified by arrows.
We further determined the effects of DT3 on γ-irradiated mouse bone marrow hematopoietic progenitor and stem cell survival (Figure 1B). Bone marrow cells were collected from femora and humeri on days 1, 8 and 13 after irradiation and total live bone marrow myeloid cells for pooled samples from each mouse were measured by trypan blue staining. γ-irradiation caused mouse bone marrow cell death within 24 h, resulting in total live cell numbers decreasing from 7.6 ± 2.5×106 cells/mouse before irradiation to 2.3±1.2×106 cells/mouse after irradiation (P<0.01). DT3 administration at either −24 h or +6 h with respect to irradiation did not significantly change the total live cell numbers.
Eight days after irradiation, one of the six mice in the vehicle-treated, TBI group was dead, and the surviving mice exhibited only 10.4±8.5% cell viability (annexin-V−/7AAD−; Online Supplementary Figure S1A) with 0.8±1.2×104 total live cells/mouse. In contrast, by day 8 none of the DT3-treated mice had died. DT3 injection (−24 h) resulted in 33±7.9% total bone marrow cell viability (Online Supplementary Figure S1A) with 19±6×104 and 11±4×104 total live cells/mouse in −24 h and +6 h DT3-treated mice, respectively (P<0.01, Figure 1B upper panel).
Thirteen days after irradiation, DT3 administered at either −24 h or +6 h increased mouse bone marrow myeloid cell numbers to 3.17± 0.7×106 and 2.06±0.9×106, respectively. In the vehicle control group, 40% of mice survived (Figure 1A), with a very low proportion of live bone marrow cells (Figure 1B).
The phenotypes of mouse bone marrow cells after DT3 or vehicle treatment, 24 h post-irradiation, were determined by flow cytometric assays. Interestingly, DT3 treatment resulted in significant increases in Lin−/Sca-1+/ckit+ stem and progenitor cells in marrow regardless of irradiation (Online Supplementary Figure S1B). In addition, clonogenicity was compared between samples collected from individual mice treated with DT3 or vehicle at +6 h. Although DT3 did not enhance total bone marrow live cells 24 h after irradiation, it did increase the total colony number from 27±5×103/mouse (vehicle-treated mice) to 48±6×103/mouse (DT3-treated mice) in non-irradiated samples (P<0.01), and from 12±2×103/mouse (vehicle-treated) to 32±10×103/mouse (DT3-treated) in samples from mice that had been irradiated (8.75 Gy, P<0.01), as shown in Online Supplementary Figure S1C.
Pathological changes in bone marrow from sectioned sternal samples from DT3-treated and control animals at 1 and 8 days post-irradiation were evaluated. Marrow cellularity was measured in microscopic fields for each sectioned sternum. Consistent with total bone marrow cell counts, severe bone marrow cellular failure was observed in vehicle-treated mice 24 h after γ-radiation. Effects of DT3 on bone marrow cell survival were observed but were not statistically significant 24 h post-irradiation (data not shown). We further evaluated the bone marrow 8 days after irradiation (Figure 1C). Cellularity in vehicle control mice decreased from 29% at 24 h to 4% on day 8. Cellularity in DT3-treated mice decreased from 42% at 24 h to 14% on day 8. Compared to the non-irradiated mouse bone marrow (Figure 1C: panels 1 and 4), lethal irradiation reduced marrow cellularity, resulting in no erythroid, rare myeloid, and no megakaryocytic cells (Figure 1C: panels 2 and 5). However, in DT3-treated mice (−24 h), regenerative micro-foci were present (Figure 1C: panels 3 and 6) in the ends of the sternebrae. In DT3-treated mice, regenerative microfoci accounted for up to 50% of cells per low-power (200x) field in ends of sternebrae. Significant megakaryocyte restoration occurred in DT3-treated mice 8 days post-irradiation (Figure 1C: panel 6).
δ-tocotrienol protected human CD34+ cells from radiation damage
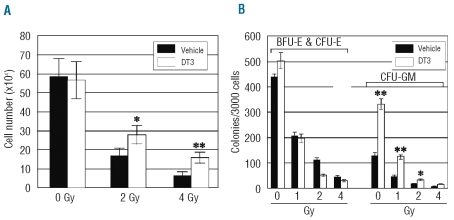
Consistent with the in vivo mouse study, DT3 (2 μM) addition 24 h prior to γ-irradiation of human hematopoietic CD34+ cells (in vitro) increased cell survival 1.6- and 2.4-fold 3 days after 2 or 4 Gy irradiation (P<0.05, Figure 2A). Hematopoietic progenitor granulocyte-macrophage colony-forming units (CFU-GM, Figure 2B) in irradiated human CD34+ cells were markedly enhanced by DT3, but numbers of erythroid lineage colonies (BFU-E and CFU-E) were not changed by DT3. Unlike in the in vivo study, DT3 added 6 h after irradiation was not observed to have effects on the survival of CD34+ cells (data not shown).
Figure 2.
DT3 protected human CD34+ cells (in vitro) from radiation damage. DT3 (2 μM) was added 24 h before irradiation. Following irradiation (0, 2, or 4 Gy), cell expansion (A) and colony formation (B) assays were performed. Total cell numbers from each group (N=3) were counted 3 days after irradiation, and colonies were counted 14 days later. Results are from a total of three experiments and each experiment was performed in triplicate. Means±SD. *P<0.05, **P<0.01, DT3 versus control culture.
δ-tocotrienol induced extracellular signal-related kinase phosphorylation and reversed the inhibition of mammalian target of rapamycin and downstream effector activation by irradiation in human CD34+ cells
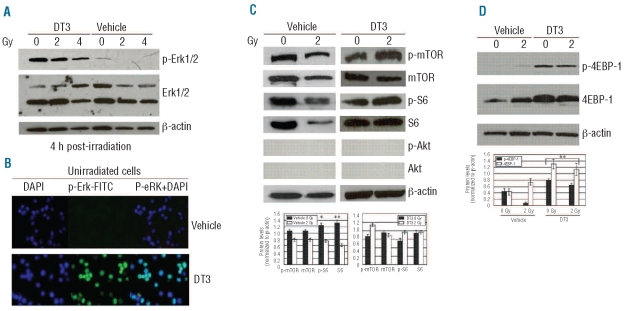
We next evaluated DT3-induced stress-response signal regulation in human CD34+ cells. Immunoblotting data showed that addition of DT3 dramatically induced Erk phosphorylation in CD34+ cells regardless of irradiation (Figure 3A). This result was confirmed by immunofluorescence staining using anti-human phospho-Erk1/2 (p-Erk)-FITC antibody, as shown in Figure 3B. DT3 treatment resulted in Erk phosphorylation in 90% of unirradiated CD34+ cell nuclei. In contrast, untreated human CD34+ cells showed little or no Erk phosphorylation, and γ-radiation did not induce Erk phosphorylation in these cells (Figure 3A).
Figure 3.
Effects of DT3 on Erk, activation of mTOR and S6, and 4EBP-1 phosphorylation in CD34+ cells. (A) Western blot shows total Erk1/2 protein and p-Erk1/2 expression in DT3- or vehicle-treated CD34+ cells 4 h after 2 and 4 Gy-irradiation. β-actin served as the internal loading control. (B) Immunofluorescence staining shows p-Erk1/2 (green) expression in unirradiated CD34+ cells 24 h after DT3 addition. DAPI (blue) defines cell nuclei. (C) m-TOR, S6, and AKT expression and phosphorylation in vehicle control- or DT3-treated CD34+ cells were determined by western blot 4 h after 2 Gy irradiation. (D) Western blot: 4EBP-1 was detectable in all samples, and expression and phosphorylation were significantly increased in DT3-treated samples. Representative immunoblots and statistical data from three experiments are shown. Means ± SD. *P<0.05, **P<0.01. 0 Gy versus 2Gy or DT3-treated versus vehicle-treated.
Results from our immunoblotting data showed mTOR was highly expressed in cultured CD34+ cells. However, levels of mTOR, as well as downstream effector ribosomal protein S6 (S6) expression and phosphorylation, decreased after γ-irradiation (Figure 3C). mTOR plays a central role in regulating protein synthesis, ribosomal protein translation, and cap-dependent translation.12 Major upstream signaling components that control mTOR activity are the PI3K/PTEN/Akt and Ras/Raf/Mek/Erk pathways.14,15 Interestingly, addition of DT3 dramatically induced Erk phosphorylation and reversed the radiation-induced inhibition of mTOR and S6 protein activation in CD34+ cells 4 h after 2 Gy irradiation. In contrast, Akt protein expression and phosphorylation were undetectable (Figure 3C), suggesting DT3 specifically influences Erk signaling. Another direct target of mTOR is 4EBP1, which inhibits the ability of eIF4E to form complexes with eIF4G and mediate cap-initiated mRNA translation in mammalian cells.22,23 mTOR inactivates 4EBP1 through the induction of 4EBP1 phosphorylation, which results in eIF4E release from 4EBP1 binding, and consequently stimulates cap-dependent mRNA translation. In addition, the activation of 4EBP1 can be controlled by the MAPK-Erk pathways.16,24 Both mTOR and Erk inhibit 4EBP1 through phosphorylation of its regulatory sites. To evaluate the consequences of DT3-induced mTOR and Erk activation, we examined 4EBP1 phosphorylation in DT3-treated human CD34+ cells. The western blots in Figure 3D show addition of DT3 dramatically induced 4EBP1 protein phosphorylation and expression regardless of irradiation. In vehicle control-treated cells, 2 Gy γ-irradiation did not significantly up-regulate 4EBP1 phosphorylation although the total 4EBP1 protein level was increased.
δ-tocotrienol up-regulated extracellular signal-related kinase and mammalian target of rapamycin signal pathway activation in mouse marrow cells 4 or 24 hours after γ-irradiation
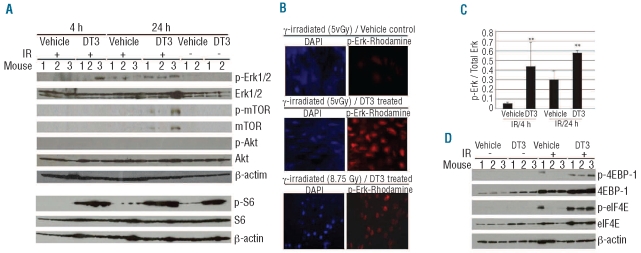
To investigate whether DT3 also induces Erk, mTOR, S6 activation and 4EBP1 phosphorylation in the in vivo mouse model, we evaluated individual bone marrow samples. Since 8.75 Gy irradiation caused massive bone marrow cell death, resulting in insufficient material for immunoblotting, we collected samples from mice irradiated at 5 Gy. Western blots showed phosphorylated Erk, mTOR, and S6 expression in marrow from DT3-treated mice 4 and/or 24 h after 5 Gy irradiation (Figure 4A). p-Erk was expressed in one out of three DT3-treated marrow samples 4 h after irradiation and in all three DT3-treated samples 24 h after irradiation. Comparative immunofluorescence staining showed similar results in mouse sternum marrow slides. In these assays, anti-mouse p-Erk1/2 was labeled with rhodamine. Figure 4B shows that Erk was very weakly phosphorylated in vehicle-treated marrow cells 24 h after 5 Gy γ-irradiation. However, up-regulation of p-Erk was observed in 90% of marrow cells from DT3-treated, 5 or 8.75 Gy-irradiated mice. Figure 4C indicates a significant up-regulation of Erk phosphorylation in DT3-treated, irradiated mice. Unlike in human CD34+ cells, mTOR expression and phosphorylation were observed only in DT3-treated and irradiated mouse bone marrow cells. Marrow contains a mixture of cell types, and levels of mTOR expression may vary in these cells. Akt protein expression was observed in mouse bone marrow cells, but Akt phosphorylation was not detectable. Consistent with findings in human CD34+ cells, DT3 induced S6 phosphorylation in marrow samples with and without irradiation. Furthermore, 4EBP1 protein phosphorylation was observed in all DT3-treated marrow samples 24 h post-irradiation, and correlated with eIF4E activation (Figure 4D). Interestingly, one bone marrow sample from the vehicle control-treated, irradiated group displayed 4EBP-1 phosphorylation and eIF4E activation.
Figure 4.
DT3 stimulated Erk, mTOR, S6, and eIF4E activation, and 4EBP-1 phosphorylation in mouse bone marrow. Mice injected with vehicle or DT3 24 h prior to irradiation (N=6/group). Total bone marrow cells from each femur and humerus were collected 4 or 24 h post-irradiation. (A) Samples were analyzed by immunoblotting. Erk, mTOR, and S6 phosphorylation were up-regulated by DT3 4 and/or 24 h after 5 Gy irradiation. Akt expression but not phosphorylation was observed in all samples. (B) Mouse sterna were fixed with Z-Fix and stained using immuno-fluorescent anti-mouse p-Erk-Rhodamine (red) 24 h after 5 or 8 Gy irradiation. DAPI (blue) defines cell nuclei. Slides were examined with a Zeiss fluorescence microscope using AxioVision MTB2004 configuration. The original magnification was 630X. (C) p-Erk levels were quantified against total Erk protein. The results represent means±SD **P<0.01. (N=6 mice). (D) 4EBP-1 and eIF4E were measured by immunoblotting. Radiation up-regulated 4EBP-1 in marrow cells from both vehicle control- and DT3-injected mice. In 5 Gy irradiated mice, phosphorylation of 4EBP-1 and eIF4E was observed in one vehicle-treated sample and all samples from DT3-injected mice. β-actin served as the internal loading control.
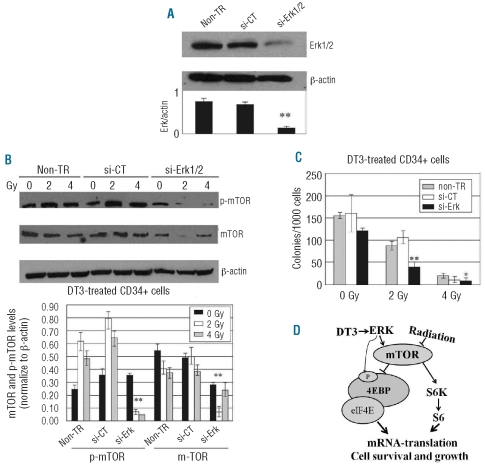
Erk gene knockdown suppressed mammalian target of rapamycin phosphorylation and clonogenicity and increased γ-H2AX foci formation in δ-tocotrienol-treated CD34+ cells after ionizing radiation
Erk is one of the major upstream signaling components controlling mTOR activity.14,15 To verify that DT3 stimulation of the mTOR pathway results from Erk activation, Erk1/2 siRNA was transfected into CD34+ cells before addition of DT3 and γ-irradiation using nucleofector technology.20 Western blots and colony assays were performed 24 h after irradiation, 48 h after Erk siRNA or control siRNA (maxGFP siRNA) transfection or non-gene transfection with DT3 supplementation. Western blots showed that Erk1/2 protein levels markedly decreased after Erk siRNA transfection (Figure 5A). In contrast, control siRNA-transfected cells expressed Erk1/2 at the same level as non-transfected samples. As expected, Erk1/2 knockdown blocked mTOR phosphorylation in DT3-treated CD34+ cells after irradiation (Figure 5B). Furthermore, clonogenicity in DT3-treated CD34+ cells was significantly inhibited by Erk gene knockdown compared with control siRNA-transfected and non-gene-transfected samples after 2 and 4 Gy irradiation (Figure 5C).
Figure 5.
Erk gene knockdown in CD34+ cells after ionizing radiation. (A) SignalSilence Erk1 and Erk2 siRNA from the siGENOME SMARTpool (1.5 μg each) or 1.5 μg of maxGFP siRNA (positive control) were transfected into 3 x 106 human CD34+ cells. Western blots were performed 48 h post-siRNA transfer. (B) Erk1 gene knockdown suppressed m-TOR phosphorylation in DT3-treated CD34+ cells 24 h after 2 or 4 Gy γ-irradiation. Representative immunoblots and statistical data from three experiments are shown. Means ± SD. (C) DT3-induced clonogenicity was significantly reduced by Erk gene knockdown in CD34+ cells after 2 Gy irradiation. Results are from a total of two experiments and each experiment was performed in triplicate. Means ± SD. *P<0.05, **P<0.01. si-CT versus si-Erk. (D) Schematic diagram of the role of DT3 in radioprotection. DT3 reversed irradiation-induced suppression of mTOR activation, resulting in 4EBP1 phosphorylation and S6 and eIF4E activation, which initiates mRNA translation and results in hematopoietic cell survival and growth. The phosphorylation of 4EBP1 can also be controlled directly by the MAPK/Erk pathway.
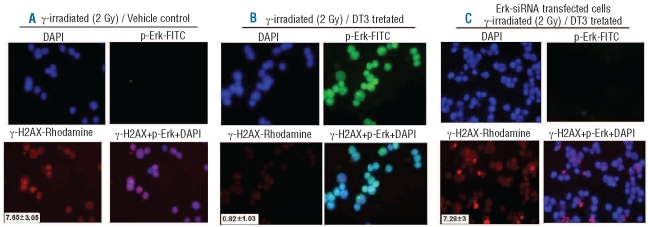
The primary target of radiation-induced cell death in eukaryotes is generally considered to be DNA, although protein oxidation may also trigger signals leading to cell death.25 Double strand breaks are the major lethal lesions.26,27 To verify that DT3-stimulated Erk and mTOR activation is associated with the radiation counteracting function of DT3, we further examined the interaction between phosphorylation of Erk and DNA-damage signal γ-H2AX focus formation28 in human CD34+ cells using immunofluorescence staining with p-Erk1/2 and γ-H2AX antibodies (Figure 6). γ-H2AX foci were formed in vehicle control nuclei: 7.65±3.05 foci per cell, 4 h after 2 Gy irradiation (Figure 6A). p-Erk expression in these nuclei was undetectable. In contrast, addition of DT3 (Figure 6B) up-regulated p-Erk expression in 90% of irradiated cells and decreased γ-H2AX focus formation to 0.82±1.03 per-cell 4 h after 2 Gy irradiation (P<0.001, DT3-treated versus vehicle-treated cells). Finally, γ-H2AX foci were measured in Erk siRNA-transfected and DT3-treated cells to test whether this would reverse protection by DT3 (Figure 6C). Consistent with the clonogenicity results (Figure 5C), Erk knockdown abrogated the effects of DT3 on protection of DNA-double strand breaks in CD34+ cells after γ-irradiation, as reflected by the number of γ-H2AX foci.
Figure 6.
Effects of DT3 on human CD34+ cell DNA-double strand breaks after ionizing radiation are Erk-dependent. Immunofluorescence staining using anti-human- γ-H2AX-Rhodamine (red) and anti-human-phospho-Erk-FITC (green) antibodies in DT3- and control-treated CD34+ cells 4 h after 2 Gy γ-irradiation. Radiation induced γ-H2AX expression (A) in control-treated cells, which expressed p-Erk at undetectable levels. In contrast, DT3 induced p-Erk expression in 90% of cells (B) and decreased γ-H2AX focus formation. Knockdown of Erk1/2 gene (C) resulted in increased γ-H2AX foci in DT3-treated CD34+ cells. Average numbers of γ-H2AX foci per cell were counted in 50–100 cells per sample as shown at the bottom left corners of the γ-H2AX-Rhodamine images (means ± SD. P<0.001, DT3-treated versus vehicle-treated and DT3-treated versus Erk1/2 knockdown/DT3-treated). DAPI (blue) defines cell nuclei. Slides were examined with a Zeiss fluorescence microscope using AxioVision MTB2004 configuration. The original magnification was 630X.
Discussion
We demonstrated that natural DT3 from palm oil or rice bran oil protected mice from a lethal dose of total body γ-irradiation and resulted in 100% 30-day survival compared with 18% survival in vehicle-treated control mice. Exposure to a lethal dose of ionizing radiation decreased viable cells in bone marrow. DT3 administration significantly increased cell viability on day 8 and further improvements were obtained on day 13. Attempts were made to replicate the in vivo studies in human CD34+ cells so that molecular mechanisms could be studied in greater detail. It was found that DT3 added to cell culture 24 h prior to radiation increased cell viability and specifically stimulated CFU-GM colonies (Figure 2). The doses of radiation chosen for CD34+ cells were similar to those used in our previous studies.20
The basic aim of the present study was to determine the mechanism underlying DT3 radioprotection in mice and human CD34+ cells. We found that DT3 administration induced Erk1/2 phosphorylation in mouse bone marrow and human CD34+ cells, and resulted in significant inhibition of the DNA-double strand break marker, γ-H2AX foci formation, in CD34+ cells after γ-irradiation. Erk activity is a component of the pro-survival MAPK pathway, which induces expression of DNA repair and cell growth factors.9,10 However, normal human CD34+ cells have been reported to have little or no Erk phosphorylation,29,30 and our data (Figure 3A,B) confirm this lack of Erk phosphorylation. We, therefore, asked whether DT3-induced Erk phosphorylation is associated with the radioprotective function of DT3 in mice and in human hematopoietic cells. The reason for the preferential stimulus of the granulocyte-macrophage lineage by DT3, as shown in clonogenic assays (Figure 2B), is not known. However, a recent report30 suggested that MEK-ERK signaling plays a critical role in regulation of expansion of myeloid precursor cells through up-regulation of the cyclin D1 gene. Since DT3 induces ERK phosphorylation, this might result in the generation of granulocyte-macrophage progenitor cells.
We also found mTOR was highly expressed and activated in cultured CD34+ cells (Figure 3C), and that irradiation led to the inhibition of mTOR in these cells. Consistent with our results, Braunstein et al.24 recently reported that in ionizing radiation-sensitive (non-transformed) cells, radiation inhibits cap-dependent protein synthesis through a mechanism that involves inhibition of mTOR and maintained hypophosphorylation of 4EBP1, which binds avidly to eIF4E and inhibits cap-dependent mRNA translation initiation. We found that DT3 reversed ionizing radiation-induced suppression of mTOR activation, resulting in 4EBP1 phosphorylation and S6 and eIF4E activation (Figure 5D). Both 4EBP1 and S6 kinase are mTOR targets, which initiate mRNA translation in mammalian cells.31 The phosphorylation of 4EBP1 can also be directly controlled by the MAPK/Erk pathway.16,24 Translational modulation is more rapid than transcriptional modulation in response to positive and negative stimuli, including radiation stress.22 Regulation of protein synthesis in eukaryotes through mRNA translation plays a critical role in cell cycle, proliferation, differentiation, and apoptosis. In our in vivo studies presented in Figure 4D one bone marrow sample from vehicle control-treated and irradiated mice displayed 4EBP-1 phosphorylation and eIF4E activation. DT3 up-modulated 4EBP-1 phosphorylation and enhanced mouse bone marrow survival. The data suggest that inter-individual variability in baseline activation of mTOR pathways may be related to variations in survival of irradiated, untreated mice.
There are two major upstream regulators of mTOR: Akt and Erk1/2.14,15 In the present study, DT3 upregulated Erk1/2 and mTOR, but not Akt activation, in mouse bone marrow and human hematopoietic progenitor cells. To determine whether DT3-induced mTOR activation is through Erk regulation, we inhibited Erk1/2 gene expression in CD34+ cells using siRNA and found Erk1/2 knockdown significantly blocked the effects of DT3 on mTOR activation and survival of progenitors. Redon et al.28 recently reported a proportional linear response between the initial γ-radiation dose and γ-H2AX foci formation in blood cells and suggested using formation of these foci to monitor DNA damage in blood and skin cells after γ-irradiation. The correlation between Erk phosphorylation and radiation-induced DNA damage was assessed in CD34+ cells using γ-H2AX expression (Figure 6). It is clearly evident from the irradiated, Erk gene knockdown sample that Erk phosphorylation and γ-H2AX foci formation are inversely correlated (Figure 6C).
Previous studies suggest that tocotrienols have anti-cancer properties through apoptotic mechanisms and regulation of PI3K/Akt, NFκB, and MAPK signaling.32,33 Furthermore, many studies revealed that tocotrienols induced apoptosis preferentially in cancer cells but not in normal cells,34 although the mechanisms are not understood. Erk, an anti-apoptotic factor, may participate in the transmission of many mitogenic and oncogenic signals that lead to the accelerated proliferation observed with malignant transformation.35 Although DT3 induces Erk phosphorylation, the induction is transient since Erk and mTOR phosphorylation disappeared in day 13 samples (data not shown). Hence this effect may not be equivalent to the effect of DT3 on cancer cells.
Tumor cells with constitutive activation of NFκB,36 Erk1/2,37 and Akt38 frequently acquire radioresistance. However, the expression and activation of these survival proteins are relatively low in healthy hematopoietic cells, especially in immature progenitor and stem cells. We previously found that ionizing radiation induced degradation of the pro-survival factor NFκB subunit p50 in CD34+ cells, which was associated with a lack of NFκB activation, and may be partly responsible for the radiation sensitivity of CD34+ cells.20 Results from the present study and others29 demonstrate that normal human hematopoietic CD34+ cells have little or no Erk phosphorylation, and our data further show that phosphorylated Erk expression was very low or undetectable after γ-irradiation in mouse bone marrow and human CD34+ cells. Here, studies in mouse bone marrow and human CD34+ cells indicate that Erk/mTOR signaling was necessary for the radioprotective efficacy of DT3, hence this molecular pathway should be explored as a possible target of radiation countermeasure candidates.
In conclusion, our data indicate that DT3, as a pro-proliferative and anti-apoptotic agent, inhibits radiation-induced DNA-double strand breaks in hematopoietic stem and progenitor cells and protects mice and human CD34+ cells from radiation-induced mortality through an increase of bone marrow regeneration capacity and hematopoietic stem and progenitor cell proliferation. The mechanism of DT3-mediated radioprotection may be attributed to this tocotrienol’s stimulation of Erk activation-associated mTOR survival pathways.
Acknowledgments
we thank Dr. Terry C. Pellmar for her helpful suggestions and comments.
Footnotes
Funding: this study was supported by an Armed Forces Radiobiology Research Institute intramural grant (RAB2EI) to MX, a Congressional Grant (G1B2EP) to MX and NIAID-AFRRI-interagency funds (G2B2DB) and a Defense Threat Reduction Agency grant (G1B2BP) to VS.
The onlive version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6(9):702–13. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 2.Hotz ME, Fliedner TM, Meineke V. Radiation accident preparedness: a European approach to train physicians to manage mass radiation casualties. Health Phys. 2010;98(6):894–7. doi: 10.1097/HP.0b013e3181ab3e71. [DOI] [PubMed] [Google Scholar]
- 3.Xiao M, Whitnall M. Pharmacological countermeasures for the acute radiation syndrome. Curr Mol Pharmacol. 2009;2(1):122–33. doi: 10.2174/1874467210902010122. [DOI] [PubMed] [Google Scholar]
- 4.Coleman CN, Stone HB, Moulder JE, Pellmar TC. Medicine. Modulation of radiation injury. Science. 2004;304(5671):693–4. doi: 10.1126/science.1095956. [DOI] [PubMed] [Google Scholar]
- 5.Sen CK, Khanna S, Roy S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006;78(18):2088–98. doi: 10.1016/j.lfs.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nesaretnam K. Multitargeted therapy of cancer by tocotrienols. Cancer Lett. 2008;269(2):388–95. doi: 10.1016/j.canlet.2008.03.063. [DOI] [PubMed] [Google Scholar]
- 7.Kamat JP, Sarma HD, Devasagayam TP, Nesaretnam K, Basiron Y. Tocotrienols from palm oil as effective inhibitors of protein oxidation and lipid peroxidation in rat liver microsomes. Mol Cell Biochem. 1997;170(1–2):131–7. doi: 10.1023/a:1006853419214. [DOI] [PubMed] [Google Scholar]
- 8.Astsaturov I, Cohen RB, Harari P. Targeting epidermal growth factor receptor signaling in the treatment of head and neck cancer. Expert Rev Anticancer Ther. 2006;6(9):1179–93. doi: 10.1586/14737140.6.9.1179. [DOI] [PubMed] [Google Scholar]
- 9.Krishna M, Narang H. The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cell Mol Life Sci. 2008;65(22):3525–44. doi: 10.1007/s00018-008-8170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambard JC, Lefloch R, Pouyssegur J, Lenormand P. ERK implication in cell cycle regulation. Biochim Biophys Acta. 2007;1773(8):1299–310. doi: 10.1016/j.bbamcr.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Panta GR, Kaur S, Cavin LG, Cortes ML, Mercurio F, Lothstein L, et al. ATM and the catalytic subunit of DNA-dependent protein kinase activate NF-kappaB through a common MEK/extracellular signal-regulated kinase/p90(rsk) signaling pathway in response to distinct forms of DNA damage. Mol Cell Biol. 2004;24(5):1823–35. doi: 10.1128/MCB.24.5.1823-1835.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Proud CG. Signalling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem J. 2007;403(2):217–34. doi: 10.1042/BJ20070024. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, Yang C, Farberman A, Rideout TC, de Lange CF, France J, et al. The mammalian target of rapamycin-signaling pathway in regulating metabolism and growth. J Anim Sci. 2008;86(14 Suppl):E36–50. doi: 10.2527/jas.2007-0567. [DOI] [PubMed] [Google Scholar]
- 14.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441(7092):424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 15.Jiang BH, Liu LZ. Role of mTOR in anti-cancer drug resistance: perspectives for improved drug treatment. Drug Resist Updat. 2008;11(3):63–76. doi: 10.1016/j.drup.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhandari BK, Feliers D, Duraisamy S, Stewart JL, Gingras AC, Abboud HE, et al. Insulin regulation of protein translation repressor 4E-BP1, an eIF4E-binding protein, in renal epithelial cells. Kidney Int. 2001;59(3):866–75. doi: 10.1046/j.1523-1755.2001.059003866.x. [DOI] [PubMed] [Google Scholar]
- 17.Berbee M, Fu Q, Boerma M, Wang J, Kumar KS, Hauer-Jensen M. gamma-Tocotrienol ameliorates intestinal radiation injury and reduces vascular oxidative stress after total-body irradiation by an HMG-CoA reductase-dependent mechanism. Radiat Res. 2009;171(5):596–605. doi: 10.1667/RR1632.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessel JM, Hayflick J, Weyrich AS, Hoffman PA, Gallatin M, McIntyre TM, et al. Coengagement of ICAM-3 and Fc receptors induces chemokine secretion and spreading by myeloid leukocytes. J Immunol. 1998;160(11):5579–87. [PubMed] [Google Scholar]
- 19.Srinivasan V, Doctrow S, Singh VK, Whitnall MH. Evaluation of EUK-189, a synthetic superoxide dismutase/catalase mimetic as a radiation countermeasure. Immunopharmacol Immunotoxicol. 2008;30(2):271–90. doi: 10.1080/08923970801925331. [DOI] [PubMed] [Google Scholar]
- 20.Xiao M, Inal CE, Parekh VI, Chang CM, Whitnall MH. 5-Androstenediol promotes survival of gamma-irradiated human hematopoietic progenitors through induction of nuclear factor-kappaB activation and granulocyte colony-stimulating factor expression. Mol Pharmacol. 2007;72(2):370–9. doi: 10.1124/mol.107.035394. [DOI] [PubMed] [Google Scholar]
- 21.Davis TA, Clarke TK, Mog SR, Landauer MR. Subcutaneous administration of genistein prior to lethal irradiation supports multilineage, hematopoietic progenitor cell recovery and survival. Int J Radiat Biol. 2007;83(3):141–51. doi: 10.1080/09553000601132642. [DOI] [PubMed] [Google Scholar]
- 22.Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15(7):807–26. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- 23.Bianchini A, Loiarro M, Bielli P, Busa R, Paronetto MP, Loreni F, et al. Phosphorylation of eIF4E by MNKs supports protein synthesis, cell cycle progression and proliferation in prostate cancer cells. Carcinogenesis. 2008;29(12):2279–88. doi: 10.1093/carcin/bgn221. [DOI] [PubMed] [Google Scholar]
- 24.Braunstein S, Badura ML, Xi Q, Formenti SC, Schneider RJ. Regulation of protein synthesis by ionizing radiation. Mol Cell Biol. 2009;29(21):5645–56. doi: 10.1128/MCB.00711-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daly MJ, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Leapman RD, et al. Protein oxidation implicated as the primary determinant of bacterial radioresistance. PLoS Biol. 2007;5(4):e92. doi: 10.1371/journal.pbio.0050092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burma S, Chen BP, Chen DJ. Role of non-homologous end joining (NHEJ) in maintaining genomic integrity. DNA Repair (Amst) 2006;5(9–10):1042–8. doi: 10.1016/j.dnarep.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 27.Jeggo P, Lobrich M. Radiation-induced DNA damage responses. Radiat Prot Dosimetry. 2006;122(1–4):124–7. doi: 10.1093/rpd/ncl495. [DOI] [PubMed] [Google Scholar]
- 28.Redon CE, Dickey JS, Bonner WM, Sedelnikova OA. gamma-H2AX as a bio-marker of DNA damage induced by ionizing radiation in human peripheral blood lymphocytes and artificial skin. Adv Space Res. 2009;43(8):1171–8. doi: 10.1016/j.asr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ricciardi MR, McQueen T, Chism D, Milella M, Estey E, Kaldjian E, et al. Quantitative single cell determination of ERK phosphorylation and regulation in relapsed and refractory primary acute myeloid leukemia. Leukemia. 2005;19(9):1543–9. doi: 10.1038/sj.leu.2403859. [DOI] [PubMed] [Google Scholar]
- 30.Geest CR, Buitenhuis M, Groot Koerkamp MJ, Holstege FC, Vellenga E, Coffer PJ. Tight control of MEK-ERK activation is essential in regulating proliferation, survival, and cytokine production of CD34+-derived neutrophil progenitors. Blood. 2009;114(16):3402–12. doi: 10.1182/blood-2008-08-175141. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi AA, Proud CG. The rapid activation of protein synthesis by growth hormone requires signaling through mTOR. Am J Physiol Endocrinol Metab. 2007;292 (6):E1647–55. doi: 10.1152/ajpendo.00674.2006. [DOI] [PubMed] [Google Scholar]
- 32.Shibata A, Nakagawa K, Sookwong P, Tsuzuki T, Oikawa S, Miyazawa T. Tumor anti-angiogenic effect and mechanism of action of delta-tocotrienol. Biochem Pharmacol. 2008;76(3):330–9. doi: 10.1016/j.bcp.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 33.Constantinou C, Papas A, Constantinou AI. Vitamin E and cancer: an insight into the anticancer activities of vitamin E isomers and analogs. Int J Cancer. 2008;123(4):739–52. doi: 10.1002/ijc.23689. [DOI] [PubMed] [Google Scholar]
- 34.Miyazawa T, Shibata A, Sookwong P, Kawakami Y, Eitsuka T, Asai A, et al. Antiangiogenic and anticancer potential of unsaturated vitamin E (tocotrienol) J Nutr Biochem. 2009;20(2):79–86. doi: 10.1016/j.jnutbio.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Rubinfeld H, Seger R. The ERK cascade: a prototype of MAPK signaling. Mol Biotechnol. 2005;31(2):151–74. doi: 10.1385/MB:31:2:151. [DOI] [PubMed] [Google Scholar]
- 36.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441(7092):431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 37.Dent P, Yacoub A, Fisher PB, Hagan MP, Grant S. MAPK pathways in radiation responses. Oncogene. 2003;22(37):5885–96. doi: 10.1038/sj.onc.1206701. [DOI] [PubMed] [Google Scholar]
- 38.Valerie K, Yacoub A, Hagan MP, Curiel DT, Fisher PB, Grant S, et al. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6(3):789–801. doi: 10.1158/1535-7163.MCT-06-0596. [DOI] [PubMed] [Google Scholar]