Abstract
Background
Donor T lymphocytes are directly responsible for graft-versus-host disease. Molecules important in T-cell function may, therefore, be appropriate targets for graft-versus-host disease therapy and/or prophylaxis. Here we analyzed whether nuclear factor-κ B inducing kinase might have a role in graft-versus-host disease.
Design and Methods
We studied the expression of nuclear factor-κ B inducing kinase in human samples from patients with graft-versus-host disease. We also explored the effect of nuclear factor-κ B inducing kinase in a murine model of graft-versus-host disease using donor cells from aly/aly mice (deficient in nuclear factor-κ B inducing kinase) and C57BL/6 mice (control).
Results
We detected expression of nuclear factor-κ B inducing kinase in T-lymphocytes in the pathological lesions of patients with acute graft-versus-host disease. Mice transplanted with aly/aly T lymphocytes did not develop graft-versus-host disease at all, while mice receiving C57BL/6 cells died of a lethal form of the disease. Deficiency of nuclear factor-κ B inducing kinase did not affect the engrafting ability of donor T cells, but severely impaired their expansion capacity early after transplantation, and aly/aly T cells showed a higher proportion of apoptosis than did C57BL/6 T cells. Effector T lymphocytes were the T-cell subset most affected by nuclear factor-κ B inducing kinase deficiency. We also detected lower amounts of inflammatory cytokines in the serum of mice receiving aly/aly T cells than in the serum of mice receiving C57BL/6 T cells.
Conclusions
Our results show that nuclear factor-κ B inducing kinase has a role in graft-versus-host disease by maintaining the viability of activated alloreactive T lymphocytes.
Keywords: NF-κB, NF-κB inducing kinase, NIK, graft-versus-host disease
Introduction
Hematopoietic stem cell transplantation from an allogeneic donor is the only curative option for many patients with leukemia, primary or acquired marrow failure, primary immunodeficiency syndromes or inborn genetic diseases.1 Over 25,000 patients undergo allogeneic hematopoietic stem cell transplantation each year,2 a number that would greatly increase if clinicians could control one of its major complications: graft-versus-host disease (GVHD). GVHD is the result of the donor’s immune cells attacking the recipient’s organs,3 and is the cause of severe morbidity and mortality. Steroid therapy is the current first-line option for treating patients with GVHD,4 but can fail in up to 30–40% of cases. GVHD refractory to steroids is an unresolved clinical challenge that imposes a costly toll not only on survival but also on the quality of life of patients. Thus, one of the main objectives in transplantation is to identify new therapeutic targets for the development of novel effective drugs for the prophylaxis and treatment of GVHD.
Donor T lymphocytes play a major role in the pathophysiology of GVHD.5 Following infusion into the recipient and alloantigen presentation by antigen-presenting cells, donor T cells undergo activation and then clonally expand. Donor T cells induce damage to target organs either directly through cytolytic attack, or indirectly through the release of inflammatory mediators. Interleukin (IL)-2 and tumor necrosis factor (TNF)-α lead to cellular activation as well as local tissue damage. Alloreactive T-cell responses amplify the systemic inflammation responsible for many of the characteristic causes of transplant-related mortality. Thus, a major line of therapy has been the use of immunosuppressants that can interfere with T-cell activation.2
The nuclear factor-κ B (NF-κB) family of transcription factors plays a role in human immune and inflammatory diseases. NF-κB is found constitutively activated in several inflammatory diseases such as rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis, asthma and psoriasis.6–10 NF-κB inhibition has been proposed as a target for the prevention of GVHD.11 The NF-κB pathway in alloreactive T cells is required for T-cell activation events including IL-2 transcription and may, therefore, be involved in GVHD.12 NF-κB proteins, p50 (NF-κB1), p52 (NF-κB2), p65 (Rel-A), Rel-B and c-Rel, form homodimers and heterodimers in the cytoplasm, which are sequestered by inhibitors of NF-κB (IκB) molecules. IκB kinase phosphorylates IκB molecules, which are then ubiquitinated and degraded by the proteasome, resulting in the translocation of active NF-κB heterodimers to the nucleus and activation of target genes. These activated genes include genes coding for cytokines, chemokines, adhesion molecules, metalloproteinases and HLA molecules, all of which have known roles in immune and inflammatory processes. The canonical NF-κB pathway can be initiated by different stimuli (viruses, inflammatory cytokines, mitogens, T-cell receptor signaling), which activate IκB kinase. A different way for NF-κB activation is known as the alternative or non-canonical pathway.13 Members of the family of TNF receptors (LTβR, CD40, BAFFR, RANK) and some viruses (Epstein-Barr virus, human immunodeficiency virus) trigger the alternative pathway through activation of NF-κB inducing kinase (NIK), which results in the processing of p100 into p52. These two activation pathways modulate NF-κB activity depending upon the cell type and the nature of the stimuli. Besides, it has been reported that NIK may link both pathways of NF-κB activation.14
T lymphocyte proliferation and IL-2 production in response to anti-CD3 stimulation were impaired but not abrogated in NIK-deficient cells, and these effects were related to a diminished NF-κB activity.15 CD28 co-stimulation partly restored this deficiency. We recently found that NIK has a role in T lymphocyte activation, regulating the production of IL-2 through the activation of the CD28 responsive element (CD28RE) of the IL-2 promoter.16 This effect takes place through the phosphorylation of the c-Rel C-terminal transactivation domain by NIK. In the light of the crucial role of IL-2 in GVHD,2 we addressed the role of NIK in GVHD, using primary human samples and a murine model of the disease.
Design and Methods
Cells
Peripheral blood samples were drawn from children diagnosed with GVHD at Niño Jesús University Hospital (Madrid, Spain), under an Institutional Review Board-approved protocol. Mononuclear cells were obtained after centrifugation over Ficoll (Ficoll-Plaque PLUS, GE Healthcare Bio-Science AB, Uppsala, Sweden). T lymphocytes were purified using an immunomagnetic method (CD3 or CD8 beads, or a pan-T-cell isolation kit, Miltenyi Biotec, Bergisch Gladbach, Germany). Murine T lymphocytes were purified from total splenocytes with the pan-T-cell isolation kit (Miltenyi Biotec). When indicated, samples were depleted of CD44-positive lymphocytes by negative selection with anti-CD44 (IM7) antibody (BD Bioscience). Carboxyl fluorescein succinimidyl ester (CFSE; Molecular Probes, Eugene, Oregon, USA) was added at a final concentration of 2 μM following the manufacturer’s recommendations. Murine marrow cells were flushed from the femora.
Flow cytometry
Anti-mouse monoclonal antibodies were purchased from BD Biosciences: anti-mouse H2Dd (clone 34-2-12), anti-mouse H2Db (clone KH95), anti-mouse B220 (clone RA3-6B2), anti-mouse CD3 (clone 145-2C11), anti-mouse CD4 (clone RM4-5), anti-mouse CD8 (clone 53-6.7), anti-mouse NK1.1 (clone PK-136), anti-mouse CD44 (clone IM7), anti-mouse CD62L (clone MEL-14). Human anti-NIK antibody was from Santa Cruz (H-248 clone, Santa Cruz Biotechnology, Heidelberg, Germany). Annexin-V and 7-amino-actinomycin D were from BD Bioscience. Cells were acquired and analyzed with an EPIC XL (Beckman Coulter, Fullerton, CA, USA) or a FACS Canto II flow cytometer (BD Bioscience). In vivo T lymphocyte cell apoptosis was determined by detection of positivity for annexin V staining.
Immunohistochemistry
Skin and colon biopsies were obtained for diagnostic purposes, and the remaining were used for NIK staining, after informed consent under the Institutional Review Board-approved protocol. Biopsies obtained from patients with histological grade II acute GVHD were stained for NIK (A-12, 1:100, Santa Cruz Biotechnology). An automated staining system (Dako Autostainer, DakoCytomation, Denmark) was used in combination with a two-step peroxidase-labeled polymer system (Envision System, Dako, Denmark). A negative control was generated by substituting the primary antibody with buffer-specific antibody adsorbed with antigen. Human adrenal gland tissue was used as a positive control.
Murine model of graft-versus-host disease
Balb/c (H2Dd) and C57BL/6 (H2Db) breeding pairs, originally obtained from the Jackson Laboratory (Bar Harbor, ME, USA), were bred at the CIEMAT Laboratory Animals Facility (Registration Number 28079-21 A). Aly mice (aly/aly, H2Db) were purchased from Clea Japan. Mice were routinely screened for pathogens, in accordance with recommendations from the Federation of European Laboratory Animal Science Associations. All animals were handled under sterile conditions and maintained in euro-standard type II microisolator cages, with a maximum of five mice in each. All experimental procedures were carried out in accordance with to European and Spanish laws and regulations (European convention ETS 1 2 3, about the use and protection of vertebrate mammals used in experimentation and other scientific purposes and Spanish law 32/2007 and R.D. 1201/2005 about the protection and use of animals in scientific research). Procedures were approved by the CIEMAT Animal Experimentation Ethical Committee according to all external and internal biosafety and bioethics guidelines. Before transplantation, 8- to 12-week old mice underwent total body irradiation with 9 Gy of X-rays (300 kV, 10 mA; Philips MG-324, Hamburg, Germany). For long-term experiments, lethally irradiated Balb/c mice were transplanted with 10×106 marrow cells plus splenocytes (either total or purified T lymphocytes) containing 2×106 CD8 cells from donor mice (C57BL/6 or aly/aly mice). For short-term experiments (5 days), lethally irradiated Balb/c mice were transplanted with purified T lymphocytes containing 2×106 CD8 cells from donor mice (C57BL/6 or aly/aly mice).
Cytokine determinations
Balb/c mice that received either aly/aly or C57BL/6 T lymphocytes were bled at the indicated time-points before being sacrificed. Serum samples were used for quantification of Th1 (IL-2, IL-12, IFN-γ, and TNF-α), Th2 (IL-4), IL-10 and IL-6 cytokine levels. We used cytometric bead array technology (CBA Flex Sets, BD), following the manufacturer’s recommendations. The detection limits for each cytokine were as follows: IL-2, 5 pg/mL; IL-4, 5 pg/mL; IL-6, 1.4 pg/mL; IL-10, 9.6 pg/mL; IL-12, 5 pg/mL; IFN-γ, 2.5 pg/mL; and TNF-α, 6.3 pg/mL.
Statistics
The non-parametric Wilcoxon rank-sum test, also known as the Mann-Whitney two-sample statistic, was used for comparisons of quantitative variables between aly/aly and C57BL/6.
Results
Expression of NIK in human graft-versus-host disease
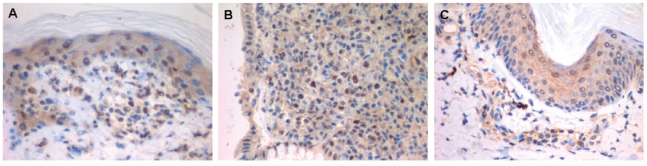
To evaluate whether NIK might play a role in GVHD in humans, we first analyzed the presence of the NIK protein in samples obtained from children who developed acute GVHD after allogeneic hematopoietic stem cell transplantation. All patients were receiving prophylactic cyclosporine at the time of analysis. A majority of circulating T lymphocytes in patients with acute GVHD expressed NIK while T cells from engrafted patients without GVHD did not. These results were observed in both CD4 and CD8 T cells and in all the patients tested (Online Supplementary Table S1). More importantly, we found that the T lymphocytes infiltrating the typical pathological lesions of actue GVHD in the skin and colon expressed NIK (Figure 1). These in vivo results suggest that NIK may play a role in T lymphocyte function during acute GVHD in humans.
Figure 1.
Expression of NIK in human graft-versus-host disease (GVHD). (A) Skin and (B) colon biopsies of a patient with acute GVHD. T lymphocytes positive for NIK expression were identified by immunohistochemical staining with an anti-NIK monoclonal antibody. Positive T lymphocytes are recognized by a dark-brown cytoplasmic and nucleic stain. (C) A biospy taken from a patient negative forGVHD showing no positive signal within the T lymphocytes, but only a slight background stain. The darkest spots correspond to melanophagocytes.
Functional NIK deficiency completely prevents the development of graft-versus-host disease in a fully mismatched allogeneic transplant murine model
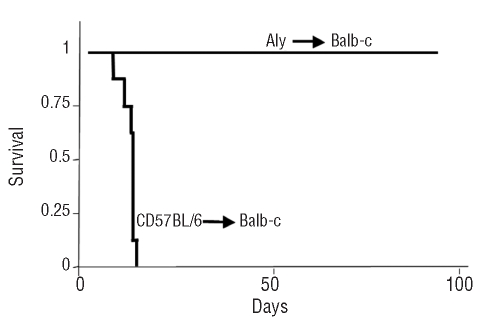
The above data from human samples suggested a role of NIK in the function of alloreactive T cells during acute GVHD. To corroborate this, we explored the effects of suppressing NIK activity in a model of GVHD in mice. In this model, H2Db T lymphocytes transplanted into fully mismatched H2Dd recipients mount an alloreactive reaction that results in a lethal form of the disease. Mice that received major histocompatibility-mismatched lymphocytes with functionally active NIK developed a severe form of acute GVHD with full symptoms (hunched posture, ruffed fur, bloody diarrhea and cachexia) and died in the third week after transplant. In contrast, mice transplanted with T lymphocytes obtained from aly/aly NIK-mutant donors survived (follow-up of 3 months) and did not develop GVHD (Figure 2). Histopathological analysis of skin, gut and liver of these surviving mice showed no sign of GVHD (Online Supplementary Figure S1).
Figure 2.
GVHD across the major histocompatibility complex barrier did not develop with NIK-deficient T lymphocytes. Survival analysis of Balb/c mice transplanted with C57BL/6 or aly/aly cells (107 marrow cells plus splenocytes containing 2×106 CD8 cells). Results shown are from two independent experiments, with a total of 12 mice per group.
Since the proportions of lymphocyte subpopulations differ between aly/aly and C57BL/6 donor mice (Online Supplementary Figure S2A), we adjusted the cell doses so that recipient mice received the same number of T lymphocytes. We determined the proportions of naïve T lymphocytes in the inoculums, since it has been shown that only naïve T cells cause GVHD in this model.17,18 No differences in the proportions of CD62L+CD44− naïve T lymphocytes were detected between aly/aly and C57BL/6 mice (Online Supplementary Figure 2B). We also determined the amount of NKT cells in donor marrow and spleen, since it has been reported that NKT cells play a regulatory role in GVHD19 and aly/aly mice have lower numbers of NKT cells in the marrow and spleen.20 Aly/aly marrow cells contained a lower proportion of NKT cells than did C57BL/6 marrow cells, but the opposite was seen in the spleens (Online Supplementary Figure S2C). The differences observed in GVHD could not, therefore, be ascribed to differences in the number of major cell types infused.
Functional NIK deficiency prevents the initial alloreactive clonal expansion of T lymphocytes in vivo
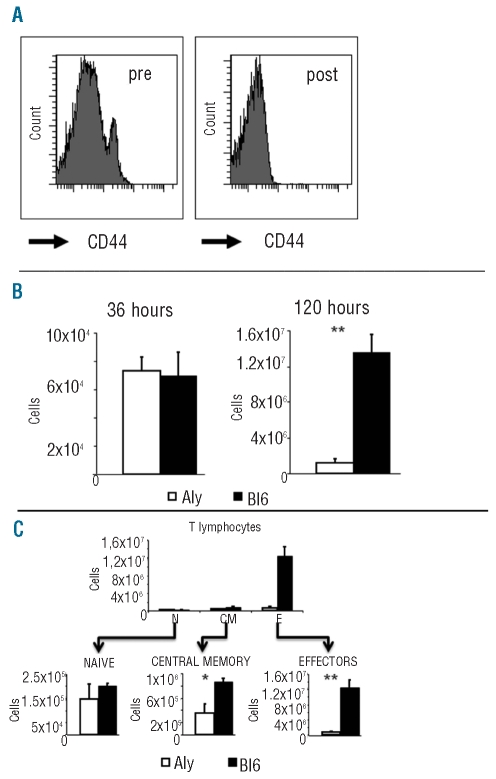
Next, we investigated why aly/aly T cells failed to induce GVHD in vivo, analyzing the fate of the infused T lymphocytes in the early days after transplantation. CD44high effector/memory aly/aly T lymphocytes, but not their wild type NIK T counterparts have been reported to have a suppressive action over the hyperproliferative CD44low naïve T cells.21 To avoid this effect, we transplanted purified CD44-depleted (naïve) T lymphocytes from either C57BL/6 or aly/aly donors into Balb/c mice (Figure 3A for details). By doing this we could assess the direct role of NIK in the cell population responsible for GVHD,17,18 without the interference of suppressor cells.21 Recipient Balb/c mice were sacrificed at 36 h and at 120 h post-transplant. The percentages and absolute numbers of donor T lymphocytes recovered from the spleens were calculated by flow cytometry and cell counts. We found the same numbers of donor T cells independently of the cell source, 36 h after transplant (Figure 3B). This suggested that naïve aly/aly T lymphocytes had the same capacity for engrafting into the spleens as their C57BL/6 counterparts. As expected, C57BL/6 T lymphocyte numbers increased significantly in the following days, as a result of the clonal expansion of alloreactive T cells.22 We found that T lymphocytes from aly/aly mice expanded, but to much lower levels than those seen with C57BL/6 T cells (Figure 3B). We also found the same expansion deficit in two independent experiments started with purified, but not CD44-depleted, T lymphocytes. The expansion defect was found both in CD4 and in CD8 aly/aly T lymphocytes (Online Supplementary Figure S3).
Figure 3.
T-cell expansion early during acute GVHD is impaired in aly/aly mice. (A) Purified T lymphocytes from spleens (Pre) were depleted of CD44-positive cells by immunomagnetic methods. The purity of depleted cells was assessed by flow cytometry (Post). (B) Donor T lymphocyte numbers recovered from the spleens of Balb/c mice transplanted with T cells (as explained in A) from C57BL/6 (solid bars) or aly/aly (open bars) mice. Note that Y labels are different at each time point analyzed. Representative of three experiments. (C) Total numbers of donor naïve (N), central memory (CM) and effector (E) T lymphocytes recovered from the spleens of Balb/c mice transplanted 120 h before with T cells (as explained in A) from aly/aly (open bars) or C57BL/6 (solid squares) mice. Each subpopulation is shown in more detail below. Results show the mean ± standard error, three mice per group. *P<0.05. **P<0.01.
Upon transplantation into mismatched recipients, naïve T lymphocytes generate effector and memory T cells.17 Interestingly, NIK deficiency did not affect different T lymphocyte subsets to the same extent. The population of effector T lymphocytes (CD44highCD62Lneg) was the most affected in terms of cell expansion (Figure 3C), while the expansion of the central memory (CD44highCD62Lhigh) T cells was less severely impaired.
Functional NIK deficiency is dispensable for lymphocyte T proliferation but is required for survival in vivo early after activation
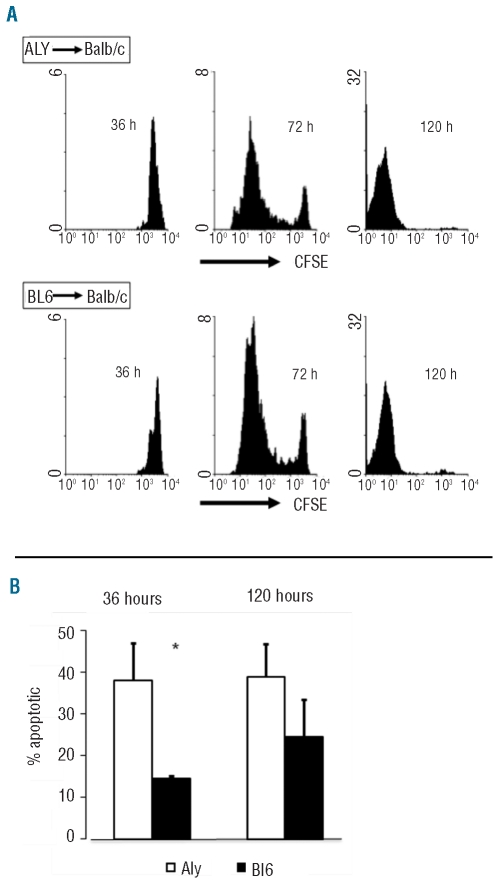
The results in Figure 3 show that T-cell alloreactive expansion was significantly diminished in the absence of fully functional NIK. One possible explanation for this may be that the proliferation of aly/aly T lymphocytes is impaired compared to that of controls. To test this hypothesis, in a different experiment we studied in vivo T-cell divisions as a means for evaluating cell proliferation. CFSE-labeled T lymphocytes from aly/aly mice (and C57BL/6 as controls) were infused into lethally irradiated Balb/c mice, and the recipient mice were sacrificed at 36 h, 72 h or 5 days after transplant. The kinetics of donor T-cell recovery again showed impaired expansion in aly/aly T lymphocytes in the first 5 days after the transplant. Dilution of CFSE was used to monitor T lymphocyte proliferation in vivo. We found that aly/aly and BL6 T lymphocytes displayed a similar relative proliferation efficiency during the first 5 days after transplantation. Most of the T lymphocytes that had engrafted into the spleens at 36 h post-transplant had either not divided or had undergone one cell division (Figure 4A). By 72 h, most of the donor lymphocytes in the spleens had divided, and practically all donor T lymphocytes recovered from the spleens 5 days after transplantation had undergone at least one division. This was detected in both CD4 and CD8 T lymphocytes.
Figure 4.
T-cell proliferation is maintained but apoptosis is enhanced early during acute GVHD in aly/aly mice. (A) Dilution of CFSE in donor T lymphocytes recovered from the spleens of Balb/c mice transplanted with purified T cells from aly/aly (top) or C57BL/6 (bottom) mice. Histograms represent CFSE intensity gated on donor cells. Representative of a single animal per group. (B) Proportions of annexin V-positive donor T lymphocytes recovered from the spleens of Balb/c mice transplanted with T cells from C57BL/6 (solid bars) or aly/aly (open bars) mice. Results shown are from two independent experiments, mean ± standard error, six mice per group per time point. *P<0.05.
An increased rate of apoptosis of proliferating aly/aly T cells could account for the differences in the final total numbers. We, therefore, studied the kinetics of donor lymphocyte apoptosis along the first days after transplant and found that the proportions of apoptotic aly/aly T lymphocytes were higher than those of C57BL/6 lymphocytes (Figure 4B). This was found both in CD4 and in CD8 T lymphocytes (data not shown). These differences disappeared at later time points after transplantation. Therefore, proliferating aly/aly T lymphocytes had a higher rate of apoptosis early after transplant compared to that of C57BL/6 T lymphocytes.
Impaired inflammatory cytokine production in the absence of functional NIK
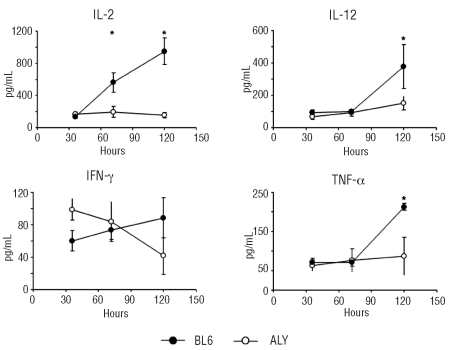
We also quantified the levels of several cytokines in serum samples of Balb/c mice transplanted with aly/aly versus C57BL/6 T lymphocytes. IFN-γ levels at 36 h were higher among mice transplanted with aly/aly T cells than among those transplanted with C57BL/6 cells. The levels of inflammatory circulating cytokines such as TNF-α and IL-12 increased over time after the transplant and, at 5 days after transplantation, were significantly higher in mice that received C57BL/6 T lymphocytes than in those transplanted with aly/aly cells. The differences were not seen at earlier time-points. IL-2 levels also increased in mice that received a C57BL/6 transplant. More interestingly, IL-2 levels did not increase in mice that received aly/aly cells (Figure 6). The production of cytokines associated with GVHD was, therefore, severely impaired when aly/aly T lymphocytes were transplanted into mismatched recipients. We found no differences in the amount of circulating IL-4 (Th2 cytokine), nor in IL-10 or in IL-6, at any time during the first 5 days after transplantation (Online Supplementary Figure S4).
Discussion
There are few reports about the role of NIK in human diseases. Uncontrolled NIK expression has been found in primary samples of human multiple myeloma23,24 and T-cell acute lymphoblastic leukemia and Hodgkin’s disease.25 In the present study we found that T lymphocytes infiltrating the skin and colon of patients with GVHD expressed NIK. The levels of NIK were very low or undetectable in basal conditions13 (Figure 1). NIK expression does not necessarily mean a direct role of NIK in GVHD, so we tested the effect of deleting NIK in an in vivo model of the disease. T lymphocytes with an inactive NIK protein were unable to mount a pathogenic graft-versus-host reaction in a murine model of fully mismatched allogeneic transplantation. Murine models have also suggested a role of NIK in immune diseases. NIK−/− mice were completely resistant to antigen-induced arthritis, suggesting a role of NIK in rheumatoid arthritis.26 NIK was also relevant in a murine model of experimental autoimmune encephalomyelitis.27
Donor T lymphocytes have a major role in the pathophysiology of GVHD.5 After infusion, donor T cells undergo activation upon alloantigen presentation by antigen-presenting cells and then clonally expand. The earlier moments after donor cell infusion are crucial for GVHD development. In vivo studies have shown that donor T lymphocytes first reach the lymphoid organs, then expand, and eventually migrate to the host target tissues of GVHD (skin, liver, gut).22 Thus, the capacity to migrate to the lymphoid organs where antigen-presenting cells reside and the capacity to sustain a clonal expansion after antigen encounter are of vital importance for GVHD to occur. In our experiments, all recipient mice received the same numbers of the major cell populations with a known role in GVHD, such as naïve T lymphocytes17,18 and NKT cells.19 The total numbers of NKT cells transplanted were not different comparing aly/aly and C57BL/6 donors in our long-term experiments. We infused purified T lymphocytes (depleted of NKT cells) in the 5-day experiments. It has been reported that NIK have a different role when considering isolated naïve T cells or a mixture of naïve plus memory T lymphocytes.21 In particular, CD4+CD44high (memory) cells from aly/aly or NIK−/− mice were suppressive both in vivo and in vitro, while CD4+CD44low (naïve) cells had increased expansion and made more IL-2. We transplanted purified CD44-depleted (naïve) T lymphocytes from either C57BL/6 or aly/aly donors to rule out the possibility that memory CD44high CD4 cells from aly/aly mice could interfere with their naïve counterparts. The results were completely similar to those found when unseparated T lymphocytes were infused, confirming that the NIK-deficient naïve T cells were incapable of inducing GVHD upon transplantation in mismatched recipients. Interestingly, NIK seemed to be more important for the expansion of effector T lymphocytes than for that of central memory T cells. Our experiments did not distinguish whether the aly/aly T lymphocytes that survived and expanded moderately in vivo may represent a particular subpopulation, or may indicate that NIK deficiency can be overcome by unknown cellular mechanisms. Some reports have indicated that NIK kinase deficiency resulted in the development of a subset of regulatory T cells with enhanced proliferative capacity upon glucocorticoid-induced TNF receptor family-related gene stimulation.28 This CD4 T lymphocyte subpopulation has regulatory capacity and its proliferation is IL-2-independent. We did not study this particular T-cell subset in our in vivo experiments, but we found that the expansion of both CD4 and CD8 T lymphocytes, particularly those with an effector phenotype, was impaired. Although we cannot definitively discard a contribution of altered cell subpopulations in aly/aly mice, we think that the inability of aly/aly T lymphocytes to induce GVHD in a mismatched model was not due to major differences among the subpopulations infused, but to intrinsic functional defects related to the absence of a functional NIK protein.
Figure 5.
Th1 cytokine production is severely impaired in mice transplanted with aly/aly T lymphocytes. The levels of circulating IL-2, IL-12, IF-γ and TNF-α in Balb/c mice transplanted with C57BL/6 (black circles) or aly/aly (white circles) purified T lymphocytes. Three mice per time-point per group. Results show the mean ± standard error. *P<0.05.
We studied the fate of donor T lymphocytes in the first days after transplantation to assess the effect of an inactive NIK protein on the capacity of T lymphocytes to migrate to lymphoid organs. It has been reported that aly/aly B lymphocytes have a defect in migration to secondary lymphoid organs (spleen, peritoneum). Chemokine and chemokine-receptor expression seem to be normal, but chemotactic responses are impaired.29 We found the same numbers of donor aly/aly and C57BL/6 T lymphocytes in the spleens of recipient mice at the earliest time evaluated, arguing against a defect in the capacity to engraft into lymphoid organs. We did not evaluate the capacity for secondary migration to peripheral organs, since the primary defect was found in T-cell expansion, and migration studies would have been confounded by the different rates of cell expansion.
Since NIK activity seemed dispensable for T-cell engraftment, we next studied whether it would be needed for T lymphocyte expansion. We found that the number of aly/aly T lymphocytes increased from day +1 to day +5, but to levels significantly lower than those of controls. Cell expansion is the result of cell proliferation and cell apoptosis. We monitored cell proliferation by analysis of CFSE dilution, and found that NIK deficiency was not necessary for T lymphocyte proliferation. Using a NIK knockout mouse model and CFSE dilution to assess cell proliferation, it was also recently found that NIK was dispensable for T lymphocyte proliferation in a murine model of experimental autoimmune encephalomyelitis.27 The apoptosis of aly/aly T lymphocytes was higher than that of controls, at the earliest time after transplantation. Cell numbers increase exponentially during clonal expansion; elimination of cells at initial divisions should, therefore, have the most detrimental effect on total output. We reason that the significantly lower numbers of aly/aly T cells recovered 5 days after infusion were due to the higher numbers of dead cells during the preceding days. This may be related to the lower IL-2 concentration found in aly/aly mice and the poor ability to release IL-2 shown in this study and previously described.16 T-cell apoptosis linked to T-cell activation is a process known as activation-induced cell death.30,31 T lymphocytes are resistant to activation-induced cell death in the earliest period after activation.32 However, we found that aly/aly T lymphocytes had a higher rate of apoptosis than controls immediately after transplantation, suggesting that NIK may be needed to maintain survival of recently activated T lymphocytes. NIK may directly control apoptosis through its ability to modulate NF-κB. Several molecular pathways have been implicated in the resistance or sensitivity of T lymphocytes to activation-induced cell death.33–35 NF-κB has been directly involved, either by activating the expression of anti-apoptotic genes (bcl-xL,36 cFLIP37), or by inhibiting the expression of pro-apoptotic ones (p73).38 It has been suggested that NF-κB has to be inactivated in order for activation-induced cell death to occur.35 Hematopoietic progenitor kinase 1 is a protein that can switch between resistance and sensitivity to activation-induced cell death by inactivating NF-κB.39 Activation of NF-κB through the canonical pathway has been previously implicated in the viability of activated T lymphocytes.40 Our results are the first to show that NIK is involved in the survival of recently activated T lymphocytes.
In addition to the detrimental effect of NIK deficiency in T lymphocyte survival during the first steps of GVHD, we found a low production of inflammatory cytokines (IL-2, IFN-γ, TNF-α, IL-12) in the serum of mice transplanted with aly/aly T lymphocytes. These cytokines have been associated with GVHD in humans.5,41 IL-2 is very important to sustain T lymphocyte clonal expansion and moreover, blocking IL-2 synthesis and/or activity is among therapies for GVHD.2 Several groups have previously shown that NIK has an important role in IL-2 production by T cells.15,16 IL-2 enhances the expansion of T lymphocytes and is crucial for correct CD8 effector function.42 NIK regulates the production of IL-17,27 another cytokine with a role in GVHD.43 We did not measure the levels of IL-17 in our samples. It does not seem likely that a low IL-17 production by aly/aly T lymphocytes could explain the absence of GVHD since a deficit of IL-17 results in exacerbated GVHD in the C57BL/6 into Balb/c model.44 The low in vivo production of the cytokines studied (and presumably of others) may be secondary to a high death rate among activated aly/aly T lymphocytes. Taken together the results on T lymphocyte survival and Th1 cytokine secretion, an inactive NIK profoundly affected the second step in Ferrara’s model of the pathophysiology of GVHD.5 This may be the major reason explaining why NIK-deficient T lymphocytes did not cause GVHD across major histocompatibility class barriers.
In summary, we show here that T lymphocytes with an inactive NIK protein were unable to mount a pathogenic graft-versus-host reaction in a murine model of fully mismatched allogeneic transplantation. Our in vivo results fit with those obtained from T-cell activation in vitro and those observed in aly/aly mice15,16 and revealed a new role for NIK. NIK deficiency resulted in a high rate of cell death at a time when activated T lymphocytes are resistant to apoptotic signals. Our results in human and mice experiments support the concept that NIK has a role in GVHD and point out this kinase as an attractive therapeutic target. Aspects of allogeneic transplantation other than GVHD need to be addressed in the light of our findings. In the aly/aly mouse model there is a continuous inhibition of NIK that may present limitations to translating our results into the clinic. For instance, continuous NIK inactivity would negatively affect the graft-versus-leukemia effect and immune reconstitution after transplantation. Further work, ideally with specific NIK inhibitors, should clarify whether NIK may be a molecular target in the prophylaxis and/or treatment of GVHD.
Footnotes
Funding: MF: FIS (PI040993), SAF2005-02220 and SAF2007-61716, RED RICET RD06/0021/0016 from the Spanish Ministry of Science, “Eicosanoids and Nitric Oxide: Mediators of Cardiovascular, Cerebral & Neoplastic Diseases” (LSHM-CT-2004-005033 from the European Union, and S-SAL-0159/2006 from the Comunidad de Madrid MR: PI04/1347 and PI07/0907 from the Instituto de Salud Carlos III, and a grant from the Fundación Mutua Madrileña.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Thomas ED. Bone marrow transplantation: a review. Semin Hematol. 1999;36(4 Suppl 7):95–103. [PubMed] [Google Scholar]
- 2.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–61. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7(5):340–52. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 4.Deeg HJ. How I treat refractory acute GVHD. Blood. 2007;109(10):4119–26. doi: 10.1182/blood-2006-12-041889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara JLM, Cooke KR, Teshima T. The pathophysiology of graft-vs-host disease. In: Ferrara J, Cooke K, Deeg J, editors. Graft-vs-Host Disease. 3rd edition. New York: Marcel Dekker; 2005. pp. 1–34. [Google Scholar]
- 6.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2(10):725–34. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 7.Makarov SS. NF-kappaB as a therapeutic target in chronic inflammation: recent advances. Mol Med Today. 2000;6(11):441–8. doi: 10.1016/s1357-4310(00)01814-1. [DOI] [PubMed] [Google Scholar]
- 8.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107(1):7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107(2):135–42. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liou HC, Hsia CY. Distinctions between c-Rel and other NF-kappaB proteins in immunity and disease. Bioessays. 2003;25(8):767–80. doi: 10.1002/bies.10306. [DOI] [PubMed] [Google Scholar]
- 11.Vodanovic-Jankovic S, Hari P, Jacobs P, Komorowski R, Drobyski WR. NF-kappaB as a target for the prevention of graft-versus-host disease: comparative efficacy of bortezomib and PS-1145. Blood. 2006;107 (2):827–34. doi: 10.1182/blood-2005-05-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain J, Loh C, Rao A. Transcriptional regulation of the IL-2 gene. Curr Opin Immunol. 1995;7(3):333–42. doi: 10.1016/0952-7915(95)80107-3. [DOI] [PubMed] [Google Scholar]
- 13.Dejardin E. The alternative NF-kappaB pathway from biochemistry to biology: pitfalls and promises for future drug development. Biochem Pharmacol. 2006;72(9):1161–79. doi: 10.1016/j.bcp.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Zarnegar B, Yamazaki S, He JQ, Cheng G. Control of canonical NF-kappaB activation through the NIK-IKK complex pathway. Proc Natl Acad Sci. 2008;105(9):3503–8. doi: 10.1073/pnas.0707959105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto M, Yamada T, Yoshinaga SK, Boone T, Horan T, Fujita S, et al. Essential role of NF-kappa B-inducing kinase in T cell activation through the TCR/CD3 pathway. J Immunol. 2002;169(3):1151–8. doi: 10.4049/jimmunol.169.3.1151. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez-Valdepeñas C, Martín AG, Ramakrishnan P, Wallach D, Fresno M. NF-kappaB-inducing kinase is involved in the activation of the CD28 responsive element through phosphorylation of c-Rel and regulation of its transactivating activity. J Immunol. 2006;176(8):4666–74. doi: 10.4049/jimmunol.176.8.4666. [DOI] [PubMed] [Google Scholar]
- 17.Anderson BE, McNiff J, Yan J, Doyle H, Mamula M, Shlomchik MJ, et al. Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest. 2003;112(1):101–8. doi: 10.1172/JCI17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen BJ, Deoliveira D, Cui X, Le NT, Son J, Whitesides JF, et al. Inability of memory T cells to induce graft-versus-host disease is a result of an abortive alloresponse. Blood. 2007;109(7):3115–23. doi: 10.1182/blood-2006-04-016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng D, Lewis D, Dejbakhsh-Jones S, Lan F, García-Ojeda M, Sibley R, et al. Bone marrow NK1.1(−) and NK1.1(+) T cells reciprocally regulate acute graft versus host disease. J Exp Med. 1999;189(7):1073–81. doi: 10.1084/jem.189.7.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elewaut D, Shaikh RB, Hammond KJ, De Winter H, Leishman AJ, Sidobre S, et al. NIK-dependent RelB activation defines a unique signaling pathway for the development of V alpha 14i NKT cells. J Exp Med. 2003;197(12):1623–33. doi: 10.1084/jem.20030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishimaru N, Kishimoto H, Hayashi Y, Sprent J. Regulation of naive T cell function by the NF-kappaB2 pathway. Nat Immunol. 2006;7(7):763–72. doi: 10.1038/ni1351. [DOI] [PubMed] [Google Scholar]
- 22.Beilhack A, Schulz S, Baker J, Beilhack GF, Wieland CB, Herman EI, et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106(3):1113–22. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keats JJ, Fonseca R, Chesi M, Schop R, Baker A, Chung WJ, et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12(2):131–44. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Annunziata CM, Davis RE, Demchenko Y, Bellamy W, Gabrea A, Zhan F, et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12(2):115–30. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saitoh Y, Yamamoto N, Dewan MZ, Sugimoto H, Martinez Bruyn VJ, Iwasaki Y, et al. Overexpressed NF-kappaB-inducing kinase contributes to the tumorigenesis of adult T-cell leukemia and Hodgkin Reed-Sternberg cells. Blood. 2008;111(10):5118–29. doi: 10.1182/blood-2007-09-110635. [DOI] [PubMed] [Google Scholar]
- 26.Aya K, Alhawagri M, Hagen-Stapleton A, Kitaura H, Kanagawa O, Novack DV. NF-(kappa)B-inducing kinase controls lymphocyte and osteoclast activities in inflammatory arthritis. J Clin Invest. 2005;115(7):1848–54. doi: 10.1172/JCI23763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin W, Zhou XF, Yu J, Cheng X, Sun SC. Regulation of Th17 cell differentiation and EAE induction by MAP3K NIK. Blood. 2009;113(26):6603–10. doi: 10.1182/blood-2008-12-192914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu LF, Gondek DC, Scott ZA, Noelle RJ. NF kappa B-inducing kinase deficiency results in the development of a subset of regulatory T cells, which shows a hyperproliferative activity upon glucocorticoid-induced TNF receptor family-related gene stimulation. J Immunol. 2005;175(3):1651–7. doi: 10.4049/jimmunol.175.3.1651. [DOI] [PubMed] [Google Scholar]
- 29.Fagarasan S, Shinkura R, Kamata T, Nogaki F, Ikuta K, Tashiro K, et al. Alymphoplasia (aly)-type nuclear factor kappaB-inducing kinase (NIK) causes defects in secondary lymphoid tissue chemokine receptor signaling and homing of peritoneal cells to the gut-associated lymphatic tissue system. J Exp Med. 2000;191(9):1477–86. doi: 10.1084/jem.191.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brenner D, Krammer PH, Arnold R. Concepts of activated T cell death. Crit Rev Oncol Hematol. 2008;66(1):52–64. doi: 10.1016/j.critrevonc.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunol Rev. 2003;193:70–81. doi: 10.1034/j.1600-065x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 32.Schmitz I, Krueger A, Baumann S, Schulze-Bergkamen H, Krammer PH, Kirchhoff S. An IL-2-dependent switch between CD95 signaling pathways sensitizes primary human T cells toward CD95-mediated activation-induced cell death. J Immunol. 2003;171(6):2930–6. doi: 10.4049/jimmunol.171.6.2930. [DOI] [PubMed] [Google Scholar]
- 33.Hildeman DA, Zhu Y, Mitchell TC, Kappler J, Marrack P. Molecular mechanisms of activated T cell death in vivo. Curr Opin Immunol. 2002;14(3):354–9. doi: 10.1016/s0952-7915(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 34.Marrack P, Kappler J. Control of T cell viability. Annu Rev Immunol. 2004;22:765–87. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- 35.Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7(7):532–42. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 36.Khoshnan A, Tindell C, Laux I, Bae D, Bennett B, Nel AE. The NF-kappa B cascade is important in Bcl-xL expression and for the anti-apoptotic effects of the CD28 receptor in primary human CD4+ lymphocytes. J Immunol. 2000;165(4):1743–54. doi: 10.4049/jimmunol.165.4.1743. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz I, Weyd H, Krueger A, Baumann S, Fas SC, Krammer PH, et al. Resistance of short term activated T cells to CD95-mediated apoptosis correlates with de novo protein synthesis of c-FLIPshort. J Immunol. 2004;172(4):2194–200. doi: 10.4049/jimmunol.172.4.2194. [DOI] [PubMed] [Google Scholar]
- 38.Wan YY, DeGregori J. The survival of antigen-stimulated T cells requires NFkappaB-mediated inhibition of p73 expression. Immunity. 2003;18(3):331–42. doi: 10.1016/s1074-7613(03)00053-0. [DOI] [PubMed] [Google Scholar]
- 39.Brenner D, Golks A, Kiefer F, Krammer PH, Arnold R. Activation or suppression of NFkappaB by HPK1 determines sensitivity to activation-induced cell death. EMBO J. 2005;24(24):4279–90. doi: 10.1038/sj.emboj.7600894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mittal A, Papa S, Franzoso G, Sen R. NF-kappaB-dependent regulation of the timing of activation-induced cell death of T lymphocytes. J Immunol. 2006;176(4):2183–9. doi: 10.4049/jimmunol.176.4.2183. [DOI] [PubMed] [Google Scholar]
- 41.Ju XP, Xu B, Xiao ZP, Li JY, Chen L, Lu SQ, et al. Cytokine expression during acute graft-versus-host disease after allogeneic peripheral stem cell transplantation. Bone Marrow Transplant. 2005;35(12):1179–86. doi: 10.1038/sj.bmt.1704972. [DOI] [PubMed] [Google Scholar]
- 42.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441(7095):890–3. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iclozan C, Yu Y, Liu C, Liang Y, Yi T, Anasetti C, et al. T helper17 cells are sufficient but not necessary to induce acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16(2):170–8. doi: 10.1016/j.bbmt.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yi T, Zhao D, Lin CH, Zhang C, Chen Y, Todorov I, et al. Absence of donor Th17 leads to augmented Th1 differentiation and exacerbated acute graft-versus-host disease. Blood. 2008;112 (5):2101–10. doi: 10.1182/blood-2007-12-126987. [DOI] [PMC free article] [PubMed] [Google Scholar]