Abstract
Background
As rare myelomas, i.e. the IgD, IgE, IgM and non-secretory forms, constitute only a small proportion of any study, relatively little is known about their prognosis in the era of peripheral stem cell transplantation.
Design and Methods
We used the European Group for Blood and Marrow Transplantation Myeloma Database to compare the outcome following autologous transplantation of over 20,000 patients with common myelomas (IgG, IgA and light chain myeloma) with the outcome of patients with rare myelomas: 379 IgD, 13 IgE, 72 IgM and 976 non-secretory cases.
Results
The study confirms the multiple adverse prognostic factors seen in IgD myeloma. Somewhat surprisingly, patients with IgD and non-secretory myeloma both had higher complete remission rates before and after transplantation than patients with common myelomas. However, while the overall survival of patients with non-secretory myeloma was similar to that of the patients with common myelomas, the survival of patients with IgD myeloma was significantly worse (although better than survival rates reported for non-transplanted patients); this was due to higher transplant-related mortality and relapse/progression rates. The post-transplantation survival of patients with IgE or IgM myeloma appears to be very poor.
Conclusions
This study provides data on the biological features of rare myelomas. The overall survival of patients with IgD, IgE or IgM myeloma is poor following autologous transplantation but substantially better than that reported for patients who were not transplanted.
Keywords: rare myelomas, autologous transplantation, outcome, efficacy
Introduction
More than 90% of myelomas will have IgG, IgA or Bence-Jones protein only ideotypes. IgD and non-secretory myelomas are uncommon while IgM and IgE myelomas are rare or extremely rare.1 Survival of patients with IgD myeloma is generally accepted to be poorer than that of patients with ‘common’ myelomas.2–5 The situation for non-secretory myeloma is less clear; originally thought to confer an adverse prognosis the majority of authorities now suggest that the prognosis of patients with this form of myeloma is similar6–8 to or possibly better9,10 than that of patients with common myelomas. It should be noted that these results are based on only small numbers of cases in the series reported. It would also seem that the survival of patients with IgE or IgM myeloma treated with standard therapy may be somewhat shorter than that of patients with the ‘common’ myelomas.
Little is known about the impact of conventional transplant strategies on the outcome of any of the rare myelomas. The introduction of alternative strategies for allogeneic transplantation with low intensity conditioning, inclusion of new drugs in autologous approaches and new non-transplant therapeutic modalities may improve the outlook for all myeloma patients. It is, therefore, an opportune time to review the outcome of conventional transplantation strategies on the rare myelomas when compared with a group of common myelomas. In this study we used the myeloma database of the European Group for Blood and Marrow Transplantation (EBMT) to study the outcome of autologous transplantation in patients with IgD, IgE, IgM or non-secretory myeloma and compared these patients’ outcome with that of over 20,000 patients with common myelomas.
Design and Methods
A retrospective study was carried out of 22,244 patients with multiple myeloma who underwent autologous transplantation between 1986 and 2007 and for whom complete data on age, sex and type of myeloma were available. Half of the patients were transplanted after the year 2000. The numbers of patients with each type of myeloma are shown in Table 1. Patients with IgG, IgA or Bence-Jones myeloma were collectively described as having ‘common’ myeloma. Patients with plasma cell leukemia were analyzed in a concurrent analysis.11 Cases with solitary plasmacytoma and amyloidosis were also excluded. All patients were reported to the EBMT registry using MED-A (limited data set) or MED-B (more extensive data set) forms. All 22,444 autografted patients were included in the study, regardless of availability of complete MED-A or MED-B data. The number of patients that could be evaluated for each parameter was noted and the proportion of evaluable patients is included in the results. Factors known to affect the transplant outcome from previous EBMT studies12 were also analyzed.
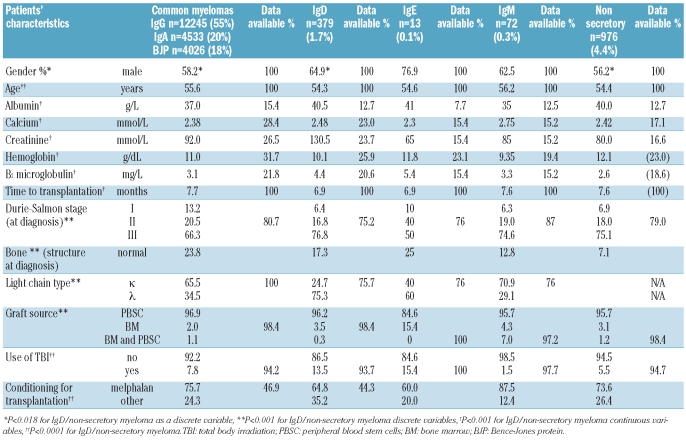
Table 1.
Characteristics of patients with the common types of myeloma and with non-secretory, IgD, IgM or IgE myeloma (n=22244).
Statistical methods
Groups were compared using the χ2 test for frequencies and the Mann-Whitney test for continuous variables. Survival curves were generated according to the Kaplan-Meier method and differences were tested with the log-rank test. Cumulative incidence curves were produced using the proper non-parametric estimator, and Gray’s test was used for comparisons. Adjusted effects on overall survival, progression-free survival, relapse incidence and non-relapse mortality were estimated in terms of hazard ratios by Cox models. Adjusted effects on response were estimated in terms of odds ratios by logistic regression; the constant term represents the baseline odds ratio, that is the ratio between probability of response and probability of no response for a patient with all covariates equal to zero. The adjustment factors considered in the multivariable models were age, gender, disease stage at diagnosis, use of total body irradiation (TBI), interval from diagnosis to transplant and, except for patients with non-secretory myeloma, the light chain type. Overall 16,956 (76%) cases with complete patterns of covariates were included: 15,959 with common myelomas, 215 with IgD myeloma, 47 with IgM myeloma and 735 with non-secretory myeloma. These proportions were similar to those in the whole group. Patients with IgE were excluded on the account of the small number of cases. Relevant selection biases are unlikely as all characteristics, progression-free survival and overall survival appeared very similar in the selected group and in the group for whom some data were missing. For the interpretation of Table 3, if the 95% confidence interval for the hazard ratio or the odds ratio includes the value 1 the effect of the factor is not statistically significant at the 5% level; while intervals all above or all below 1 indicate respectively significant risk/protective factors. The size of the effect is proportional to the deviation from 1. All analyses were carried out using SPSS statistical software (version 12.0.1) except the analyses of cumulative incidence curves, which were carried out using the CMPRSK package in R 2.4.1.
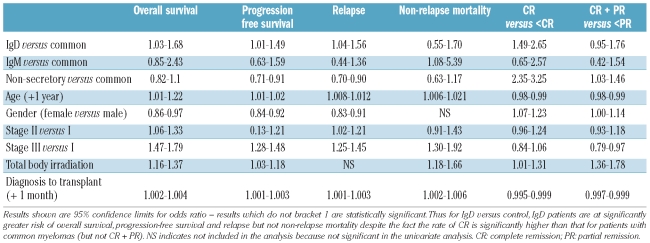
Table 3.
Multivariate analysis of risk factors for outcome.
Results
Characteristics of the myeloma subgroups
The patients’ characteristics at diagnosis are shown in Table 1, with percentage availability of results for each variable shown. In most statistical comparisons the IgE/IgM group was omitted to avoid invalidating the analyses. Comparison with common myelomas showed a statistically significant excess of male patients with IgD, IgE and IgM myeloma, more advanced Salmon Durie stage, and a greater degree of bone involvement. In IgD myeloma the κ:λ ratio was reversed (P=0.001). It can be seen that more patients with IgE myeloma also had an excess of λ light chains while those with IgM myeloma had the usual ratio of κ/λ. Among the patients with non-secretory myeloma there was a higher percentage of patients with stage III myeloma at diagnosis (P<0.001), which may be related at least in part to a significant increase in patients with major bone abnormalities at diagnosis (P<0.001) and the difficulty in diagnosing this form of myeloma due to the absence of a typical ‘spike’ on protein electrophoresis.
Reviewing the discrete variables it can be seen that patients with IgD myelomas were marginally younger, had a lower M protein level and higher albumin concentration than those with common myelomas whereas these patients had a significantly lower hemoglobin concentration and higher β2 microglobulin and serum creatinine levels (all comparisons, P<0.0001). While patients with non-secretory myeloma were also significantly younger than those with common myeloma, with significantly higher albumin and hemoglobin levels, their serum creatinine was significantly lower (all comparisons, P=0.0001). The IgM patients had the highest calcium levels and the lowest hemoglobin concentrations, but their levels of β2-microglobulin, albumin and creatinine did not differ greatly from those of patients with common myelomas. The time from diagnosis to transplantation was similar for patients with common, IgE/IgM or non-secretory myeloma while the median time to transplantation of the patients with IgD myeloma was approximately 3 weeks shorter. It should be noted that while the P values for all these differences are highly statistically significant, not all are of biological significance, on account of the number of patients analyzed. Furthermore, the numbers of IgE patients for whom some of the data are reported are too low to allow any conclusions to be drawn. The median time to transplantation suggests that most patients were transplanted as part of their first-line therapy but this was not specified.
Transplant-related variables
Table 2 shows the transplant-related variables including graft type, use of TBI and conditioning regimens. While the differences in the use of stem cells other than peripheral blood stem cells were small, there were small increases in the proportion of bone marrow cells used in the IgE and IgM groups. It can be seen that there was greater use of TBI, either alone or in conjunction with melphalan, among patients with IgD myeloma, whereas patients with IgM or non-secretory myeloma were less likely to receive TBI; there were also some differences in the treatment regimes used between patients with non-secretory myeloma and those with common myeloma.
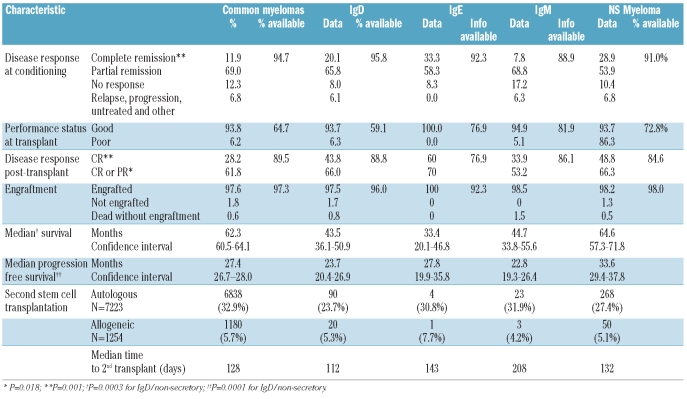
Table 2.
Comparison of disease response to therapy and engraftment between patients with IgD, IgE, IgM and non-secretory myelomas and those with with common types of myeloma.
Transplant outcomes
Comparisons of performance status and disease response to induction therapy in patients undergoing transplantation are shown in Table 2. Again the percentage availability of data for each variable is shown where appropriate. There was no difference in the post-transplant recovery between patients with IgD, IgE/IgM, non-secretory or common myeloma. Disease response in patients with non-secretory myeloma was as defined by the reporting institutions. Kaplan-Meier plots of overall survival, progression-free survival, relapse incidence and non-relapse mortality are shown in Figure 1A–D, respectively.
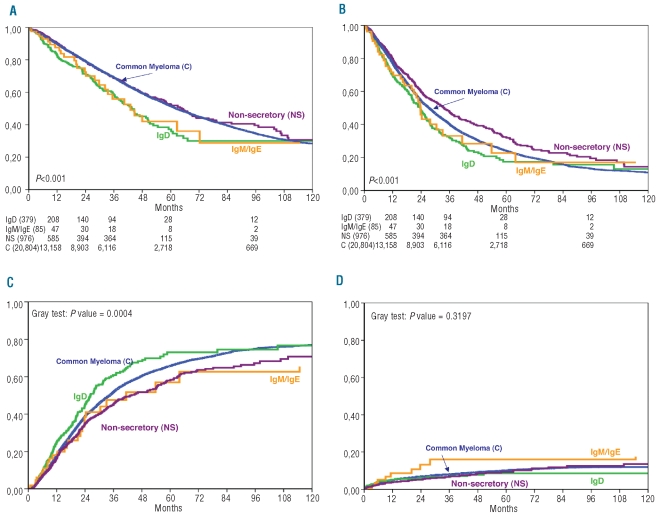
Figure 1.
Outcomes of patients with rare myelomas: (A) overall survival; (B) progression-free survival; (C) relapse incidence; (D) non-relapse mortality.
IgD myeloma
Patients with IgD myeloma appeared to have a better response to therapy than those with common myeloma with a greater proportion of the former patients being transplanted in complete remission; this did not, however, give rise to a difference in performance status. Similarly a greater proportion of IgD myeloma patients achieved complete remission after transplantation. There was no difference in engraftment rates or in the times to neutrophil and platelet recovery post-transplantation.
While the median survival of patients with common myeloma was 62.3 months (Figure 1A) (confidence intervals shown in Table 2), the overall survival of patients with IgD myeloma was significantly shorter, being 43.5 months (P=0.0001), despite the high complete remission rate: this is presumably related to the high relapse rate in patients with IgD myeloma (Figure 1C; P=0.0004). The median progression-free survival was 27.4 and 23.7 months, respectively (P=0.017) (Figure 1B, confidence limits in Table 2). Although the graphs must be interpreted with caution, there appears to be plateau-like flattening of the curve not only for common myeloma but also for IgD myeloma.
IgM myeloma
Although the proportion of patients with IgM myeloma achieving complete remission prior to transplantation was the lowest of all the groups, the transplant appeared to have a beneficial effect with the percentage of patients achieving complete remission rising to 34% with a similar improvement in the proportion of partial remissions. Engraftment appeared to be satisfactory in this group. Overall survival and progression-free survival were markedly similar in these patients to those in patients with IgD myeloma (Figures 1A,B), but the small number of patients results in wider confidence limits, as shown in Table 2.
IgE myeloma
Although the number of patients with IgE myeloma was so small as to make any conclusion tentative, the complete remission rate prior to transplantation was highest in this group. Despite this, the median overall survival of patients in this group was the shortest of all groups even though these patients’ progression-free survival was similar or better than that of patients with IgG or IgM myeloma. There did not appear to be any difficulty with engraftment but the non-relapse mortality rate among IgM/IgE myeloma patients was higher than that of patients with any of the other types of myeloma (Figure 1D), although not statistically significantly so.
Non-secretory myeloma
Table 2 shows that patients with non-secretory myeloma had higher complete and complete + partial remission rates than patients with common myeloma at mobilization and conditioning although this did not result in a difference in performance status at transplantation. The higher complete and complete + partial remission rates are presumed to have contributed to the higher post-transplant response rates, with an observed complete remission rate of 48.8%, the highest of all groups excluding the small IgE group. There was no overall difference in engraftment data. Figure 1A and 1B illustrate the superior overall survival (64.6 months) and progression-free survival (33.6 months) of patients with non-secretory myeloma (confidence interval shown in Table 2); the progression-free survival of the patients with non-secretory myeloma was statistically significantly superior to that of patients with common myeloma (P=0.0002); this was not, however, reflected by a statistically superior overall survival.
Subsequent transplantation
Table 2 also shows the numbers and relative percentages of patients with each type of myeloma proceeding to a further transplant and the median time to the second transplant (irrespective of type). It appears that patients with common myeloma were most likely to receive a second transplant and that IgD myeloma patients were least likely; however, the differences were small and unlikely to have affected the overall survival data. Curiously in IgE/IgM myeloma patients the median time to a second transplant appeared longer. On account of the observational nature of this part of the study, the data are not appropriate for further statistical analyses.
Adjusted analysis of response
Data from this analysis are summarized in Table 3. Patients with ‘light chain type’ myeloma were excluded from the analysis shown in the table but were included in a companion analysis which showed little change in most parameters. The hazard ratio for overall survival, progression-free survival and relapse among patients with IgD myeloma was significantly adverse. When patients with light chain type myeloma were included (data not shown) the hazard ratio was reduced and lost its significance suggesting that the adverse prognosis was related to the much higher incidence of λ light chain in IgD myeloma, demonstrated in Table 2.
The last two columns of Table 3 confirm that patients with IgD or non-secretory myeloma had a significantly higher probability of achieving complete remission than that of the control group and that this was not a stage-dependent effect. TBI usage and age were unfavorable factors while female gender was a favorable factor. Somewhat surprisingly, when light chain status was included in the analysis, this was found to be a favorable factor (data not shown). This may be related to the high proportion of patients with λ chain IgD myeloma achieving a good response but having a poor overall survival due to the high relapse rate in this group. However, when complete and complete + partial remission rates were reviewed, there were no differences between patients with IgD, IgM, non-secretory or common myeloma in the rate of achieving at least a partial remission. Stage III became an adverse factor but the effect of chain isotype was lost. There were significant differences in the hazard ratios for age, gender, TBI and time to transplantation but, in general, these retained their impact on the model. In producing this model it was noted that the use of TBI overlapped completely with the year of transplant allowing analysis of only one or other of these variables.
Discussion
Autologous transplantation with stem cells from bone marrow or peripheral blood has been used to treat myeloma for over two decades; the ability of cytokines to mobilize stem cells in the peripheral blood has led to a huge rise in the use of this mode of treatment.13 Initial impressions of benefit shown in a single-center, non-randomized series14 have now been confirmed in multicenter, prospective, randomized clinical trials.15,16 However, the numbers of patients with rare myelomas, such as IgD, IgE and IgM myeloma, in any such trial are usually insufficient to allow any conclusion to be drawn regarding the outcome of these patients, while patients with non-secretory myelomas are often excluded from clinical trials because of the difficulty in assessing response in this condition. We, therefore, used the EBMT myeloma database containing details from more than 22,000 patients to obtain information on the outcome of transplantation in these rare conditions and factors affecting the outcomes.
The overall message of this study is that patients with rare myelomas (IgD, IgE and IgM myeloma) have a worse prognosis than patients with the common myelomas. In contrast, non-secretory myelomas should be considered together with the common myelomas; although patients with the non-secretory form have a better progression-free survival (possibly related to the lack of the usual conventional markers of disease progression in myeloma), overall survival was only marginally better than that of patients with common myelomas. On reviewing our data in more detail, we confirmed that patients with IgD myeloma had a significantly worse prognosis associated with adverse prognostic factors, in keeping with previous series describing this condition.17 This poor prognosis may be related to the high proportion of patients with raised proliferation rates combined with abnormal gene expression profiles demonstrated in a small group (n=12) of patients with IgD myeloma.18 As with common myelomas there was a small tail of long-term survivors confirming the few case reports of prolonged survival in this condition19–21 and also in IgE myeloma.22 Of note, the proportion of patients receiving TBI was higher among subjects with IgD myeloma than among those with common myelomas or all of the other rare myelomas and while this was associated with a poorer outcome,13 it was not sufficient to account for the 19-month difference in overall survival of all patients with IgD myeloma. In fact patients with IgD had higher rates of complete remission both before and after transplantation, but unfortunately this did not translate into a better overall survival due to their very high relapse rate.
In contrast, the non-secretory myelomas have both favorable and adverse prognostic factors.23 The complete remission rates in patients with this type of myeloma appear to be very high, both before and after transplantation, possibly because of the lack of an identifiable para-protein. The progression-free survival of patients with non-secretory myeloma appears to be superior to that of patients with common myeloma (P=0.0003), but there was no difference in overall survival between the two groups. The survival of patients with non-secretory myeloma treated conventionally has been reported to be similar to that of patients with common myeloma.1,24 In a single-institution study of transplanted patients, those with non-secretory myeloma had a significantly better outcome than patients with all other myeloma types although there were only six cases of non-secretory myeloma in the series.25 The current much larger study suggests that non-secretory myeloma behaves similarly to common myelomas and should be treated similarly.
This retrospective analysis of ‘rare’ myelomas has resulted in the production of the largest reported database of rare myelomas to date. With the statistical power obtained by large numbers there is the inevitable downside of poor reporting of certain parameters and the fact that while differences may be shown to be statistically significant, their biological significance may be of doubtful importance. Furthermore, while data such as overall survival and progression-free survival are robust, it must be remembered that only the younger and fittest patients will make it through to transplantation. While there is a suggestion from our data that both IgD and non-secretory myelomas tend to affect younger individuals, only 25% of myeloma patients may undergo transplantion, as roughly 50% of all possible cases of myeloma are over the age of 68 years and co-morbid disease may be present in up to 50% of those below that age not undergoing transplantation.26
It has recently been suggested that a very high incidence of the t(11:14) (q13:q32) translocation is a hallmark of non-secretory, IgE and IgM myelomas (but not of the IgD subtype),27 while Feyler et al. suggested that IgM myeloma is characterized by a CD20−CD56−CD117− immunophenotype in addition to the t(11;14).28 This translocation in patients with non-secretory myeloma may be related to the significantly better progression-free survival but was not translated into superior overall survival. In contrast, the survival of patients with IgM and IgE myelomas was very similar to that of patients with IgD myeloma although the small size of these groups means that differences were not statistically significant. Of note there is no information on survival in the report by Avet-Loiseau et al.27
The multivariate study adds support to the idea that non-secretory myeloma is a disease of younger patients, in keeping with other reports of a relatively young median age of patients with this form of myeloma,7,9 which occurs more often in males (male to female ratio, 3:2). Despite their younger age, patients with non-secretory myeloma present with more advanced disease and more bone lesions than do patients with common myeloma (and even IgD myeloma). In contrast, they have significantly better median hemoglobin, albumin and creatinine levels at presentation compared to patients with common myeloma, implying that the major effect of non-secretory myeloma is on the skeletal system rather than on other organs. β2-microglobulin levels were similar to those in patients with common myelomas. The difficulty in defining the level of response in non-secretory myeloma has not been resolved in this study as we used the reporting institutes’ assessments; in years to come serum free light chain analysis29,30 and flow cytometry31 will bring some objectivity to this difficult assessment.
Our study provides robust confirmation of the inversion of the κ:λ light chain ratio in IgD myeloma with three-quarters of patients having λ light chains. The data for IgE myeloma show some similarity while the κ:λ ratio in IgM myelomas is similar to that in common myelomas. For IgD myeloma this would appear to be another adverse prognostic factor to accompany a significantly higher number of bone lesions and a greater proportion of patients with stage III disease. A lower hemoglobin concentration and higher β2 microglobulin and creatinine levels were also associated with poor prognosis. Due to the poorer reporting of these data in the few patients with IgE myeloma, no comments can be made about this group.
Although we found that patients with rare myelomas frequently have more adverse prognostic factors than those with common myelomas and that, despite a higher complete remission rate, this is reflected by a poor median survival following transplantation, it should be noted that the survival rates are significantly better, often more than double, than those in the best historical series, even though these series represent the outcomes of conventional therapy in patients of all ages.17 Two recent publications also suggest a poor prognosis for patients with IgM myeloma.28.32 Figure 1C shows that the relapse incidence in patients with IgD myeloma was significantly higher but associated specifically with the high incidence of λ chain in this isotype (Table 1), while the incidence of non-relapse mortality was much higher among patients with IgE and IgM myelomas than among those with other myelomas (Figure 1D).
In conclusion, this study suggests that patients with IgD, IgE and IgM myelomas have adverse prognostic factors and a worse survival than those with common myelomas and non-secretory myeloma following transplantation. Nevertheless, the survival of transplanted patients with these rare myelomas is much better than anything previously recorded indicating that transplantation should not be abandoned as a therapeutic modality. It remains to be seen what new drugs, such as thalidomide, lenalidomide and bortezomib, can do for these conditions.
Footnotes
Funding: CM is supported by the Northern Ireland Leukaemia Research Fund. MD was supported by the Belfast City Hospital Haematology Research Fund and by the European Leukemia Net. JA is grateful for support from the NIHR Biomedical Research Centre Funding Scheme.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Kyle RA, Gertz MA, Witzig TE, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 2.Fibbe WE, Jansen J. Prognostic factors in IgD myeloma: a study of 21 cases. Scand J Haematol. 1984;33(5):471–5. doi: 10.1111/j.1600-0609.1984.tb00727.x. [DOI] [PubMed] [Google Scholar]
- 3.Jancelewicz Z, Takatsuki K, Sugai S, Pruzanski W. IgD multiple myeloma. Review of 133 cases. Arch Intern Med. 1975;135(1):87–93. [PubMed] [Google Scholar]
- 4.Shimamoto Y, Anami Y, Yamaguchi M. A new risk grouping for IgD myeloma based on analysis of 165 Japanese patients. Eur J Haematol. 1991;47(4):262–7. doi: 10.1111/j.1600-0609.1991.tb01569.x. [DOI] [PubMed] [Google Scholar]
- 5.Blade J, Lust JA, Kyle RA. Immunoglobulin D multiple myeloma: presenting features, response to therapy, and survival in a series of 53 cases. J Clin Oncol. 1994;12(11):2398–404. doi: 10.1200/JCO.1994.12.11.2398. [DOI] [PubMed] [Google Scholar]
- 6.Cavo M, Galieni P, Gobbi M, Baldrati L, Leardini L, Baccarani M, et al. Nonsecretory multiple myeloma. Presenting findings, clinical course and prognosis. Acta Haematol. 1985;74(1):27–30. doi: 10.1159/000206159. [DOI] [PubMed] [Google Scholar]
- 7.Rubio-Felix D, Giralt M, Giraldo MP, Martinez-Peñuela JM, Oyarzabal F, Sala F, et al. Nonsecretory multiple myeloma. Cancer. 1987;59(10):1847–52. doi: 10.1002/1097-0142(19870515)59:10<1847::aid-cncr2820591027>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 8.Bourantas K. Nonsecretory multiple myeloma. Eur J Haematol. 1996;56(1–2):109–11. doi: 10.1111/j.1600-0609.1996.tb00312.x. [DOI] [PubMed] [Google Scholar]
- 9.Dreicer R, Alexanian R. Nonsecretory multiple myeloma. Am J Hematol. 1982;13(4):313–8. doi: 10.1002/ajh.2830130406. [DOI] [PubMed] [Google Scholar]
- 10.Smith DB, Harris M, Gowland E, Chang J, Scarffe JH. Non-secretory multiple myeloma: a report of 13 cases with a review of the literature. Hematol Oncol. 1986;4(4):307–13. doi: 10.1002/hon.2900040407. [DOI] [PubMed] [Google Scholar]
- 11.Drake MB, Iacobelli S, van Biezen A, Apperley J, Niederwieser D, Bjorkstrand B, et al. Primary plasma cell leukemia and autologous stem cell transplantation. Haematologica. 2010;95(5):804–9. doi: 10.3324/haematol.2009.013334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Björkstrand B, Gahrton G. High-dose treatment with autologous stem cell transplantation in multiple myeloma: past, present, and future. Semin Hematol. 2007;44(4):227–33. doi: 10.1053/j.seminhematol.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Gratwohl A, Baldomero H, Schwendener A, Rocha V, Apperley J, Frauendorfer K, et al. The EBMT activity survey 2007 with focus on allogeneic HSCT for AML and novel cellular therapies. Bone Marrow Transplant. 2009;43(4):275–91. doi: 10.1038/bmt.2009.7. [DOI] [PubMed] [Google Scholar]
- 14.Barlogie B, Jagannath S, Desikan KR, Mattox S, Vesole D, Siegel D, et al. Total therapy with tandem transplants for newly diagnosed multiple myeloma. Blood. 1999;93(1):55–65. [PubMed] [Google Scholar]
- 15.Attal M, Harousseau J L, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335(2):91–7. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 16.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348(19):1875–83. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 17.Blade J, Kyle RA. Nonsecretory myeloma, immunoglobulin D myeloma, and plasma cell leukemia. Hematol Oncol Clin North Am. 1999;13(6):1259–72. doi: 10.1016/s0889-8588(05)70125-8. [DOI] [PubMed] [Google Scholar]
- 18.Nair B, Waheed S, Szymonifka J, Shaughnessy JD, Jr, Crowley J, Barlogie B. Immunoglobulin isotypes in multiple myeloma: laboratory correlates and prognostic implications in total therapy protocols. Br J Haematol. 2009;145(1):134–7. doi: 10.1111/j.1365-2141.2008.07547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kettle P, Morris TC. Late relapse in IgD myeloma: value of immunohistochemistry in trephine biopsy specimens. J Clin Pathol. 1994;47(8):773–4. doi: 10.1136/jcp.47.8.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zitouni M, Barbouche MR, Ayed K, Makni S. Clinical features, autoantibody activity, and survival in nine Tunisian patients with IgD myeloma. Rev Rhum Eng Ed. 1999;66 (2):122. [PubMed] [Google Scholar]
- 21.Bemelmans RH, van Toorn DW, van Leeuwen L, Schaar CG. Long-term complete remission in IgD-myeloma. Eur J Haematol. 2006;76(4):339–41. doi: 10.1111/j.1600-0609.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 22.Hayes MJ, Carey JL, Krauss JC, Hedstrom DL, Gulbranson RL, Keren DF. Low IgE monoclonal gammopathy level in serum highlights 20-yr survival in a case of IgE muliple myeloma. Eur J Haematol. 2007;78 (4):353–7. doi: 10.1111/j.1600-0609.2007.00825.x. [DOI] [PubMed] [Google Scholar]
- 23.Morris TCM, Iacobelli S, Brand R, Bjorkstrand B, Drake M, Niederwieser D, et al. Benefit and timing of second transplantations in multiple myeloma; clinical findings and methodological limitations in an EBMT registry study. J Clin Oncol. 2004;22(9):1674–81. doi: 10.1200/JCO.2004.06.144. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S, Pérez WS, Zhang MJ, Ballen K, Bashey A, To LB, et al. Comparable outcomes in nonsecretory and secretory multiple myeloma after autologous stem cell transplantation. Biol Blood Marrow Transplant. 2008;14(10):1134–40. doi: 10.1016/j.bbmt.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terpos E, Apperley JF, Samson D, Giles C, Crawley C, Kanfer E, et al. Autologous stem cell transplantation in multiple myeloma: improved survival in nonsecretory multiple myeloma but lack of influence of age, status at transplant, previous treatment and conditioning regimen. A single-centre experience in 127 patients. Bone Marrow Transplant. 2003;31(3):163–70. doi: 10.1038/sj.bmt.1703818. [DOI] [PubMed] [Google Scholar]
- 26.Morris TCM, Velangi M, Jackson G, Marks DI, et al. Less than half of patients aged 65 years or under with myeloma proceed to transplantation: results of a two region population based survey. Br J Haematol. 2005;128(4):510–2. doi: 10.1111/j.1365-2141.2004.05340.x. [DOI] [PubMed] [Google Scholar]
- 27.Avet-Loiseau H, Garand R, Lode L, Harousseau JL, Bataille R Intergroupe Francophone du Myélome. Translocation t(11;14)(q13;q32) is the hallmark of IgM, IgE, and nonsecretory multiple myeloma variants. Blood. 2003;101(4):1570–1. doi: 10.1182/blood-2002-08-2436. [DOI] [PubMed] [Google Scholar]
- 28.Feyler S, O’Connor SJ, Rawstron AC, Subash C, Ross FM, Pratt G, et al. IgM myeloma: a rare entity characterized by a CD20-CD56-CD117- immunophenotype and the t(11;14) Br J Haematol. 2008;140 (5):547–51. doi: 10.1111/j.1365-2141.2007.06969.x. [DOI] [PubMed] [Google Scholar]
- 29.Bradwell AR, Carr-Smith HD, Mead GP, Tang LX, Showell PJ, Drayson MT, et al. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem. 2001;47(4):673–80. [PubMed] [Google Scholar]
- 30.Dispenzieri A, Kyle R, Merlini G, Miguel JS, Ludwig H, Hajek R, et al. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23(2):215–24. doi: 10.1038/leu.2008.307. [DOI] [PubMed] [Google Scholar]
- 31.Rawstron AC, Orfao A, Beksac M, Bezdickova L, Brooimans RA, Bumbea H, et al. Report of the European Myeloma Network on multiparametric flow cytometry in multiple myeloma and related disorders. Haematologica. 2008;93(3):431–8. doi: 10.3324/haematol.11080. [DOI] [PubMed] [Google Scholar]
- 32.Annibali O, Petrucci MT, Del Bianco P, Gallucci C, Levi A, Foà R, et al. IgM multiple myeloma: report of four cases and review of the literature. Leuk Lymphoma. 2006;47(8):1565–9. doi: 10.1080/10428190600604450. [DOI] [PubMed] [Google Scholar]