Abstract
Background
Cytokine-induced killer cells are ex vivo-expanded cells with potent antitumor activity. The infusion of cytokine-induced killer cells in patients with acute myeloid leukemia relapsing after allogeneic hematopoietic stem cell transplant is well tolerated, but limited clinical responses have been observed. To improve their effector functions against acute myeloid leukemia, we genetically modified cytokine-induced killer cells with chimeric receptors specific for the CD33 myeloid antigen.
Design and Methods
SFG-retroviral vectors coding for anti-CD33-ζ and anti-CD33-CD28-OX40-ζ chimeric receptors were used to transduce cytokine-induced killer cells. Transduced cells were characterized in vitro for their ability to lyse leukemic targets (4-hour 51chromium-release and 6-day co-cultures assays on human stromal mesenchymal cells), to proliferate (3H-thymidine-incorporation assay) and to secrete cytokines (flow cytomix assay) after contact with acute myeloid leukemia cells. Their activity against normal CD34+ hematopoietic progenitor cells was evaluated by analyzing the colony-forming unit capacity after co-incubation.
Results
Cytokine-induced killer cells were efficiently transduced with the anti-CD33 chimeric receptors, maintaining their native phenotype and functions and acquiring potent cytotoxicity (up to 80% lysis after 4-hour incubation) against different acute myeloid leukemia targets, as also confirmed in long-term killing experiments. Moreover, introduction of the anti-CD33 chimeric receptors was accompanied by prominent CD33-specific proliferative activity, with the release of high levels of immunostimulatory cytokines. The presence of CD28-OX40 in chimeric receptor endodomain was associated with a significant amelioration of the anti-leukemic activity of cytokine-induced killer cells. Importantly, even though the cytokine-induced killer cells transduced with anti-CD33 chimeric receptors showed toxicity against normal hematopoietic CD34+ progenitor cells, residual clonogenic activity was preserved.
Conclusions
Our results indicate that anti-CD33 chimeric receptors strongly enhance anti-leukemic cytokine-induced killer cell functions, suggesting that cytokine-induced killer cells transduced with these molecules might represent a promising optimized tool for acute myeloid leukemia immunotherapy.
Keywords: chimeric T-cell receptor, acute myeloid leukemia, CD33, cell therapy, gene therapy
Introduction
Acute myeloid leukemia (AML) is the most common form of acute leukemia in adults and it accounts for 30% of leukemia-related deaths in children.1 Current chemotherapy regimens ensure long-term remission only in 30 to 50% of patients and the prognosis of relapsed cases is very poor, with a low survival probability.2 Novel alternative approaches for refractory patients are, therefore, needed. T-cell-mediated immunotherapy using unmanipulated donor lymphocyte infusion for the treatment of leukemia recurrence in hematopoietic stem cell transplant recipients has had some success in AML, but the use of donor lymphocyte infusion carries a significant risk of inducing graft-versus-host disease.3 Cytokine-induced killer (CIK) cells are T lymphocytes enriched in CD3+CD56+ cells,4 which can be easily and rapidly expanded in vitro from human peripheral blood, bone marrow or cord blood mononuclear cells5,6 with the sequential addition of interferon (IFN)-γ, OKT-3 and high doses of interleukin (IL)-2.7,8 It has been demonstrated that CIK cells can lyse a broad array of tumor targets in a non-MHC-restricted manner,4 have the capacity to migrate toward tumor sites9,10 and display anti-tumor activity in vivo.7,9 In contrast, they show negligible alloreactivity and a minimal tendency to induce graft-versus-host disease as compared to allogeneic splenocytes.11,12 The clinical applicability of CIK cells has been proven by various phase I studies performed so far,4,13–15 including our published experience, during which we investigated the safety and toxicity profile of donor-derived CIK cells in patients relapsing after allogeneic hematopoietic stem cell transplantation. Our study clearly indicated that the generation of good manufacturing practice (GMP)-grade allogeneic CIK cells and their subsequent infusion are feasible and well tolerated. However, we registered only limited clinical responses.15 There are several possible reasons for this, but one of the most relevant might be related to the limited basal anti-leukemic activity of CIK cells, which showed, in in vitro testing, only a mean lytic activity of 40% against patients’ leukemic cells with a wide donor-dependent variability.15 Moreover, since CIK cells are terminally differentiated T-effector memory CD45RA+ (EMRA) lymphocytes,16 they might have restricted survival in vivo.
Novel strategies do, therefore, need to be conceived to increase the efficacy and persistence of CIK cells after injection. Chimeric receptors (CAR) represent an innovative technology to redirect T-cell activity against tumors. We previously demonstrated the potency of the CAR approach in redirecting CIK cells against B-lineage acute lymphoblas-tic leukemia and we highlighted, as demonstrated by others,17,18 the crucial role exerted by co-stimulatory molecules in the CAR signaling domain, which significantly improve the anti-tumor effector functions of CAR-expressing CIK cells. Furthermore, a recent study indicated that the inclusion of a tripartite CD28-OX40-ζ cytoplasmic domain into a CAR leads to considerably higher proliferation and cytokine release of in vitro-activated T cells19 than what is observed with the CAR containing only one co-stimulatory domain. The so-called ‘third-generation’ CAR might conceivably represent optimal constructs, as also recently shown in in vivo tumor models.20
With this study we aimed at improving CIK cell activity against AML through the genetic modification of the cells with two different CAR specific for the CD33 myeloid antigen, containing the ζ or the CD28-OX40-ζ signaling domain.
Design and Methods
Cells
Bone marrow and peripheral blood cells were collected from children with AML at diagnosis. Flow cytometry analysis showed that between 80% and 98% of the blasts expressed the CD33 antigen. All leukemia samples were cryopreserved and subsequently thawed for each experiment. The Institutional Review Board approved this study and informed consent was obtained from patients or their guardians. The human B-lineage acute lymphocytic leukemia cell line (SUP-B15) was kindly provided by Dr. Claudia Rossig (University Children’s Hospital, Muenster, Germany), while the human acute myeloid cell lines HL-60 and KG-1 and the human chronic myelogenous leukemia cell line K562 were purchased from the American Type Culture Collection (ATCC). These cell lines were maintained in RPMI-1640 supplemented with 10% fetal calf serum, L-glutamine and antibiotics (complete RPMI medium) (Lonza, Bergamo, Italy). The human telomerase reverse transcriptase (hTERT)+ bone marrow-derived mesenchymal cell line was kindly provided by Prof. Dario Campana (St. Jude Children’s Research Hospital, Memphis, USA) and was maintained in complete RPMI medium with the addition of 10−6 M hydrocortisone (Sigma Aldrich, Milan, Italy). The human renal epithelial cell line 293T was kindly provided by Dr. Martin Pule (University College of London, London, UK) and was maintained in high-glucose Dulbecco’s modified Eagle’s medium (Lonza), supplemented with 10% fetal calf serum, L-glutamine and antibiotics.
Generation of cytokine-induced killer cells
CIK cells were prepared as previously described.10 The method is detailed in the Online Supplementary Appendix.
Flow cytometric analysis
Aliquots of cells were analyzed for the expression of various surface markers using fluorescein isothiocyanate (FITC)-anti-CD8 (Exalpha Biologicals, Shirley, USA), phycoerythrin (PE)-anti-CD4, (Exalpha Biologicals), peridinin-chlorophyll-protein complex (PerCP)-anti-CD3 (Becton Dickinson, BD, San Jose, USA), PE-anti-CD56 (IQ product, Groningen, The Netherlands), FITC-anti-CD45RA (BD), PE-streptavidin-anti-mouse biotin-anti-CCR7 (BD) and anti-CXCR4 (BD), PE-anti-CD33 (BD), allophycocyanin-anti-CD34 (BD) with isotype-matched antibodies (BD), as controls. CAR expression was detected with a cyanine 5 (Cy5) anti-Fc-specific antibody (Jackson ImmunoResearch, Suffolk, UK), as previously described.21 A FACScan (BD) flow cytometer device was used to analyze the samples.
Chemotaxis and trans-Matrigel migration assays
Chemotactic migration assays were performed as previously described10 with a 96-well Transwell insert (5-μm pore size; Corning Costar, Corning, Amsterdam, The Netherlands), adding 300 ng/mL of the chemokine CXCL12 (PeproTech, Rocky Hill, USA). The method is described in the Online Supplementary Appendix.
Plasmids, retrovirus production and retroviral transduction of cytokine-induced killer cells
The high-affinity, humanized rat anti-human CD33, 113, in single chain fragment variable -Fv-(L-(gly4ser)4-H generated using UCB’s Selected Lymphocyte Antibody Method (kindly provided by Dr. Helene Finney, UCB Celltech, Slough, UK), was cloned in frame with CH2CH3-CD28transmembrane-ζ or CH2CH3-CD28transmembrane-CD28-OX40-ζ in the SFG-retroviral construct (kindly provided by Dr. Martin Pule). The retroviral supernatant was produced by FuGENE 6 (Roche Diagnostic S.p.A., Monza, Italy)-mediated co-transfection of 293T cells with the MoMLV gagpol expression plasmid pEQ-PAM3(-E) (kindly provided by Dr. Martin Pule), the RD114 env expression plasmid pRDF (kindly provided by Dr. Yasu Takeuchi, Cancer Research Technology, London, UK) and the SFG-anti-CD33.CAR vectors. Supernatants containing retroviral particles were harvested 48 h and 72 h after transfection, immediately frozen in dry ice, and stored at −80 °C until further use. 293T cells were used to titrate virus concentration. For transduction, 0.5×106 CIK cells at day 5 of culture were resuspended in 2.5 mL of thawed viral supernatant and seeded onto RetroNectin (TaKaRa BioEurope, Gennevilliers, France)-coated 24-well non-tissue culture plates (BD). CIK cells were then spin infected in the presence of IL-2 (600 U/mL) at 1600 rpm for 40 min and incubated for 72 h in a humidified incubator at 37 °C, 5% CO2.
Short-term and long-term cytotoxicity assays
The cytotoxicity of unmanipulated and anti-CD33.CAR-modified CIK cells against leukemic cells was evaluated as previously described.10 The methods are detailed in the Online Supplementary Appendix.
Cell proliferation assay
CD33-specific proliferation was evaluated by 3H-thymidine (Amersham Pharmacia Biotech, Piscataway, USA) incorporation as described in the Online Supplementary Appendix.
Cytokine production detection
For assessment of cytokine production, 2×105 unmanipulated and anti-CD33.CAR-expressing CIK cells were stimulated with γ-irradiated HL-60 cells and primary AML cells at an effector cell:tar-get cell (E:T) ratio of 1:5 ratio for 24 h. Levels of INF-γ, tumor necrosis factor (TNF)-α, TNF-β and IL-2 cytokines in culture supernatants were determined with a Flow Cytomix assay (Multiplex Bender MedSystem, Wien, Austria).
Colony-forming unit assay
CD34+ cells were purified from cord blood by immunomagnetic selection with anti-CD34 beads (Miltenyi Biotech, Bergisch Gladbach, Germany), incubated for 4, 16, 24 and 48 h with unmanipulated or anti-CD33.CAR-transduced CIK cells at an E:T ratio of 10:1. Residual CD34+CD33+ progenitor cells were enumerated by flow cytometry. CD34+ cells were then plated at 5×102 cells/well in a methylcellulose-based medium (MethoCult H4434; StemCell Technologies Inc., Vancouver, Canada). After 14–21 days, colonies were counted as previously described.22
Statistical analysis
The results were compared by using the paired Student’s t test. A P value of 0.05 or less was considered to be statistically significant.
Results
Generation and characterization of anti-CD33.CAR-transduced cytokine-induced killer cells
Healthy donor-derived CIK cells were efficiently generated and transduced with the SFG-anti-CD33-ζ and SFG-anti-CD33-CD28-OX40-ζ retroviral vectors, with a mean CAR expression after 21 days of culture of 64% and 65% (n=20), respectively (Figure 1A and 1B). The transduction process did not alter the CIK cells’ native phenotype and functions, determined at the same time point. In fact, the phenotype of transduced CIK and unmanipulated CIK cells was comparable (Figure 1B), with typical enrichment in the CD3+CD56+ population. Similar expansion rates were registered in anti-CD33.CAR-transduced and unmanipulated CIK cells, with mean fold increases of 31, 25 and 24 (n=20) for anti-CD33-ζ, anti-CD33-CD28-OX40-ζ and unmanipulated cells, respectively (Figure 1C). Moreover, anti-CD33.CAR-transduced CIK cells showed similar or higher killing of the K562 cell line compared to unmanipulated cells (Figure 2A) with a mean lysis, at an E:T ratio of 5:1, of 53% (n=5) for anti-CD33-ζ-expressing CIK cells and of 58% (n=5) for anti-CD33-CD28-OX40-ζexpressing CIK cells, compared to a mean lysis of 31% (n=5; P≤0.05) of unmanipulated cells, which is likely related to the expression of the CD33 antigen on these cells.23 Finally, analogous chemotactic activity and trans-Matrigel migration in response to the CXCL12 chemokine were observed, with a mean migration index (ratio of migrated cells in the presence of CXCL12/migrated cells in the presence of medium alone) of 4.3, 4.4 and 4.4 for anti-CD33-ζ, anti-CD33-CD28-OX40-ζ and unmanipulated CIK cells, respectively (Figure 2B, upper panel), and a mean migration index through Matrigel of 3.9, 3.8 and 3.7 for anti-CD33-ζ, anti-CD33-CD28-OX40-ζ and unmanipulated CIK cells, respectively (Figure 2B, lower panel).
Figure 1.
Transduction with the anti-CD33.CAR does not alter the native phenotype and in vitro expansion capability of CIK cells. (A) The expression of anti-CD33.CAR on the surface of CIK cells was evaluated by flow cytometry with a Cy5-conjugated-anti-human-Fc antibody after 21 days of culture. The results of a representative experiment are shown. (B) The expression of anti-CD33.CAR (Fc+) and of CD3 along with CD56, CD8, CD4 and CXCR4 on the surface of CIK cells and their memory phenotype (evaluated with CCR7/CD45RA staining) was evaluated, by flow cytometry, after 21 days of culture. Data shown are mean ± SD of 20 separate experiments. (C) Expansion of CIK cells was calculated and expressed as the fold increase in cell number at 14 days after transduction versus the day of transduction. Data shown are mean ± SD of 20 separate experiments.
Figure 2.
Transduction with the anti-CD33.CAR does not alter native CIK cell functions. (A) Reactivity of CIK cells against cells of the control K562 cell line was determined after 21 days of culture by a standard 4-h 51chromium-release assay. Data shown are mean ± SD of five separate experiments. Anti-CD33.CAR-transduced CIK cells displayed similar or higher killing of the K562 cell line compared to unmanipulated cells, consistently with the reported expression of CD33 on this cell line (**P≤0.005 and *P≤0.05 versus unmanipulated CIK cells). (B) The migratory activity of CIK cells in response to the CXCL12 chemokine was determined by an in vitro chemotactic assay in the absence (upper panel) and in the presence of reconstituted basement membrane (Matrigel) (lower panel) (n=4). The horizontal line at the migration index 1.0 indicates lack of chemotaxis.
Anti-CD33.CAR-redirected cytokine-induced killer cells acquire potent lytic activity against different acute myeloid leukemia targets
Anti-CD33.CAR-redirected CIK cells showed a strong cytotoxic activity in classical 4-h 51chromium-release assays (short-term cytotoxicity) against different CD33+ targets, including the HL-60 cell line (mean lysis, at an E:T ratio of 5:1, 79%; n=7, for anti-CD33-ζ and mean lysis, 75%; n=7, for anti-CD33-CD28-OX40-ζ CIK cells), the KG-1 cell line (mean lysis, at an E:T ratio of 5:1, 53%; n=4 for anti-CD33-ζ and mean lysis, 50%; n=4, for anti-CD33-CD28-OX40-ζ CIK cells), and primary AML samples (mean lysis, at an E:T ratio of 5:1, 61%; n=8 for anti-CD33-ζ and mean lysis, 65%; n=8, for anti-CD33-CD28-OX40-ζ CIK cells) (Figure 3A). Cytotoxicity of anti-CD33.CAR-transduced CIK cells against AML cells was not dependent on CD33 expression levels or on the subtype of AML, with up to 100% lysis even in the case of CD33-low expressing AML cells (Figure 3B). In contrast, unmanipulated cells were minimally cytotoxic against the same targets (mean lysis, at an E:T ratio of 5:1, of HL-60 cells, 28%; n=7; P≤0.005; mean lysis, at an E:T ratio of 5:1, of the KG-1 cell line, 5%; n=4; P≤0.005 and mean lysis, at an E:T ratio of 5:1, of primary AML, 11%; n=8; P≤0.005) (Figure 3A). The killing activity of transduced cells was specific for CD33-positive targets, as demonstrated by the negligible levels of cytotoxicity of anti-CD33.CAR-transduced CIK cells against the CD19+ CD33− cell line SUP-B15 (Figure 3A).
Figure 3.
CIK cells expressing the anti-CD33.CAR acquire potent cytotoxic activity against AML. (A) Short-term cytotoxicity of CIK cells was evaluated by a standard 4-h 51chromium-release assay after 21 days of culture at E:T ratios of 20:1, 10:1 and 5:1. Anti-CD33.CAR-transduced CIK cells showed a consistent cytolytic activity against the CD33+ targets HL-60, KG-1, and primary AML compared to unmanipulated cells (*P≤0.05;**P≤0.005), while negligible killing, similar to that exerted by unmanipulated cells, was registered against the CD33- cell line SUP-B15. Data shown are mean ± SD of seven, four, eight and four separate experiments, respectively. (B) Correlation of CD33 expression levels on primary AML cells (evaluated as mean fluorescent intensity -MFI-) and cytotoxicity of anti-CD33.CAR-transduced CIK cells at an E:T ratio of 20:1 (upper panel) and lytic efficiency (expressed as % cell lysis at an E:T ratio of 20:1) of anti-CD33.CAR-redirected CIK cells against different AML subtypes (lower panel). (C) CIK cells were co-cultured with HL-60 or primary AML cells at E:T ratios of 1:100 and 1:200 for 6 days on a human stromal mesenchymal cell layer in the absence of IL-2. Leukemic cell recovery was evaluated by flow cytometry (*P≤0.05;**P≤0.005). Data shown are mean ± SD of three and seven independent experiments, respectively.
The capacity of anti-CD33.CAR-transduced CIK cells to kill leukemic cells over time was then evaluated, in co-culture experiments on human stromal mesenchymal cells, at low E:T ratios (1:200), in the absence of exogenous IL-2. The presence of the CD28-OX40 co-stimulatory moiety in the anti-CD33.CAR potently boosted the cytotoxicity of CIK cells, to significantly higher levels than those observed with anti-CD33-ζ CIK cells, which anyway displayed strong lytic activity in these assays (Figure 3C). In fact, almost all leukemic blasts were eliminated from anti-CD33-CD28-OX40-ζ-transduced CIK cells, with a mean leukemic cell recovery of 4% (n=3) in the case of co-culture with HL-60 cells and of 16% (n=7) in the case of co-culture with primary AML blasts, toward respectively, a mean survival of 87% (n=3; P≤0.005) and 91% (n=7; P≤0.005) in the presence of unmanipulated CIK cells and of 25% (n=3; P≤0.005) and 31% (n=7; P≤0.05) in the presence of anti-CD33-ζ-transduced CIK cells.
Anti-CD33.CAR-redirected cytokine-induced killer cells proliferate and release immunostimulatory cytokines after specific CD33-stimulation, with a more pronounced effect in the presence of the CD28-OX40 domain
The ability of anti-CD33.CAR-transduced CIK cells to expand in vitro after CD33 engagement was measured, by a 3H-thymidine incorporation-assay, after 4 days co-culture with either γ-irradiated HL-60 or primary leukemic cells, in the absence of exogenous IL-2. As outlined in Figure 4, the anti-CD33.CAR containing just the ζ-chain conferred a significant CD33-specific proliferative activity to transduced CIK cells, with a mean proliferation index -calculated as the ratio of stimulated/unstimulated cell proliferation - of 2.2 (n=13) after HL-60-mediated stimulation, and 2.4 (n=11) after primary AML cell-mediated stimulation, compared to 0.9 (n=13; P≤0.005) and 1.4 (n=11; P≤0.05), respectively, for unmanipulated CIK cells. The introduction of the CD28-OX40 domain in the CAR intra-cellular signaling region resulted in an even higher expansion rate, with a mean proliferation index of 4.4 (n=13; P≤0.005) after HL-60-mediated stimulation, and 3.7 (n=11; P≤0.005) after primary AML cell-mediated stimulation. No proliferation was registered when cells were co-cultured with the CD19+ CD33- SUP-B15 cell line (data not shown).
Figure 4.
Anti-CD33.CAR expression on CIK cells confers them a CD33-specific proliferation activity. CIK cells were stimulated with γ-irradiated HL-60 or primary AML cells at an E:T ratio of 1:1 for 72 h and proliferation was estimated by 3H-thymidine incorporation. Data shown are mean ± SD of 13 and 11 independent experiments, respectively (*P≤0.05;**P≤0.005).
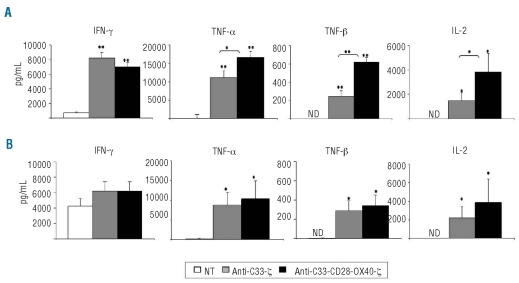
Expression of anti-CD33.CAR on CIK cells resulted in a consistent improvement in the release of a panel of immunostimulatory cytokines, including IFN-γ, TNF-α, TNF-β and IL-2 after 24-h stimulation with γ-irradiated HL-60 and primary AML cells at a 1:5 E:T ratio. HL-60 and primary AML cells alone did not produce TNF-β, and released only low levels of IFN-γ, TNF-α and IL-2 (data not shown), which were subtracted from those measured in CIK-stimulated cultures. After HL-60-mediated stimulation, anti-CD33-ζ and anti-CD33-CD28-OX40-ζ CAR-transduced CIK cells secreted 11-fold and 10-fold more IFN-γ (n=6; P≤0.005 for anti-CD33-ζ and n=6; P≤0.005 for anti-CD33-CD28-OX40-ζ), 120-fold and 180-fold more TNF-α (n=6; P≤0.005 for anti-CD33-ζ and n=6; P≤0.005 for anti-CD33-CD28-OX40-ζ), 250-fold and 600-fold more TNF-β (n=6; P≤0.005 for anti-CD33-ζ and n=6; P≤0.005 for anti-CD33-CD28-OX40-ζ), 1400-fold and 3800-fold more IL-2 (n=6; P≤0.05 for anti-CD33-ζ and n=6; P≤0.05 for anti-CD33-CD28-OX40-ζ), compared to unmanipulated cells (Figure 5A). When primary AML cells were used as stimulators, except for IFN-γ, which was released at high levels also by unmanipulated CIK cells (Figure 5B), as previously described,24 a significant improvement in the production of TNF-α (n=6; P≤0.05 for anti-CD33-ζ and n=6; P≤0.05 for anti-CD33-CD28-OX40-ζ), TNF-β (n=6; P≤0.05 for CD33-ζ and n=6; P≤0.05 for anti-CD33-CD28-OX40-ζ), and IL-2 (n=4; P≤0.05 for anti-CD33-ζ and n=4; P≤0.05 for anti-CD33-CD28-OX40-ζ) was detected in anti-CD33.CAR-expressing CIK cells, compared to unmanipulated CIK cells, without major differences between the two receptors (Figure 5B).
Figure 5.
Anti-CD33.CAR expression on CIK cells results in a consistent release of inflammatory cytokines after specific CD33-stimulation. CIK cells were stimulated with γ-irradiated HL-60 (A) and primary AML cells (B) at an E:T ratio of 1:5 for 24 h. Release of IFN-γ, TNF-α, TNF-β and IL-2 was detected in the culture supernatants by flow cytometry. Data shown are mean ± SD of six independent experiments (*P≤0.05; **P≤0.005).
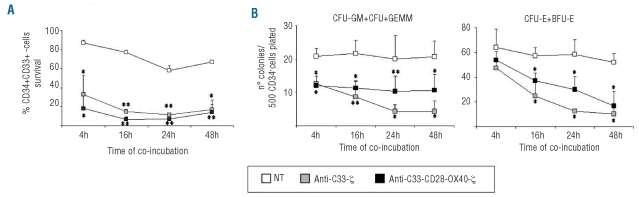
In vitro colony-forming capacity of normal hematopoietic progenitors is preserved after co-culture with anti-CD33.CAR-transduced cytokine-induced killer cells
To assess the toxicity of anti-CD33.CAR-redirected CIK cells against normal CD34+CD33+ progenitors, transduced CIK cells were incubated with cord blood derived-CD34+-immunopurified precursors at an E:T ratio of 10:1 and, at different time-points (4, 16, 24 and 48 h), residual CD34+CD33+ were enumerated and subsequently seeded for colony-forming assays. Anti-CD33.CAR-expressing CIK cells showed significant cytotoxic activity against normal CD34+CD33+ cells, with a mean survival at 48 h of co-culture of 17% (n=3; P≤0.05) for anti-CD33-ζ, and 14% (n=3; P≤0.005) for anti-CD33-CD28-OX40-ζ, compared to 67% (n=3) for unmanipulated cells (Figure 6A). In three different experiments, numbers of colony-forming unit-granulocyte, macrophage (CFU-GM), CFU-granulocyte, erythrocyte, macrophage, megakaryocyte (CFU-GEMM), CFU-erythroid (CFU-E) and burst-forming unit-erythroid (BFU-E) colonies derived from CD34+ cord blood cells (previously incubated for 48 h with anti-CD33-ζ and anti-CD33-CD28-OX40-ζ CAR-expressing CIK cells) were reduced (mean number of CFU-GM + CFU-GEMM, 4; n=3; P≤0.05 for anti-CD33-ζ and 11; n=3; P≤0.05 for anti-CD33-CD28-OX40-ζ and mean number of CFU-E + BFU-E, 10; n=3; P≤0.05 for anti-CD33-ζ and 16; n=3; P≤0.05 for anti-CD33-CD28-OX40-ζ), compared to co-cultures with unmanipulated CIK cells (mean number of CFU-GM + CFU-GEMM, 21; n=3 and mean number of CFU-E + BFU-E, 52; n=3) (Figure 6B). Total numbers of colonies were, however, in the range of normality according to the assay utilized.25
Figure 6.
Cytotoxicity of anti-CD33.CAR-transduced CIK cells against normal myeloid progenitors. (A) CIK cells were incubated with cord blood-purified CD34+ progenitors at an E:T ratio of 10:1 and at different time-points residual CD34+CD33+ cells were enumerated by flow cytometry. Data shown are mean ± SD of three independent experiments (*P≤0.05; **P≤0.005). (B) After co-incubation with CIK cells, CD34+ progenitors were seeded in methylcellulose-based medium and after 14–21 days CFU-GM- CFU-GEMM, CFU-E, and BFU-E colonies were counted. Data shown are mean ± SD of three independent experiments (*P≤0.05; **P≤0.005).
Discussion
In this study we demonstrated that the introduction of anti-CD33-specific CAR into CIK cells is able to significantly improve these cells’ effector functions against AML cells in vitro. In particular, CIK cells acquired a considerably greater lytic activity toward different AML cell lines and primary leukemic samples, including those with a more aggressive subtype. It is noteworthy that the killing efficiency was not dependent on CD33 expression level on target cells, as expected with the high-affinity 113-anti-CD33 scFv,26 thus suggesting that this approach might be very effective also in cases of low antigenic expression. We observed, as well, that anti-CD33.CAR-transduced CIK cells were able to maintain the anti-leukemic cytotoxicity over time in the absence of exogenous cytokines, and this phenomenon might be explained by their acquired CD33-specific proliferative potential. Moreover, the expression of the anti-CD33.CAR on CIK cells resulted in an important release of IFN-γ, TNF-α, TNF-β and IL-2, which have been extensively demonstrated to have a crucial role in tumor eradication.27 The observed potency of the anti-CD33.CAR containing the ζ-chain only, compared to that reported in other studies,17,19,28 might be related to various combined factors, including the high-affinity of the scFv, the choice of the spacer region – human Fc, reported to improve the activity of CAR by limiting the formation of heterodimeric complexes with endogenous T-cell molecules, compared to CD8α spacer – and the activation state induced in CIK cells by the high-doses of IL-2. No direct comparison was performed in our experimental setting between the ζ-chain alone and ‘second-generation’ CAR containing the CD28-ζ and OX40-ζ domains separately, since many data have already been published in the literature on these molecules.18 Instead, we focused on a CAR containing a tripartite endodomain, based on the consideration that inclusion of a primary domain (ζ), an early signaling Ig superfamily member (CD28), with a later signaling TNF superfamily (OX40) member, could be considered an optimal construct to activate T cells fully.18 In line with these considerations, it is interesting to note that the addition of the CD28-OX40 co-stimulatory endodomain to the anti-CD33-ζ.CAR significantly improved CIK cell effector functions, which might be relevant to sustaining the activity of anti-CD33.CAR-transduced CIK cells in vivo upon adoptive transfer, including CD33-specific proliferation and cytokine secretion. Besides, the benefit deriving from the CD28-OX40 endodomain was particularly evident in the long-term cytotoxicity assay, which most likely reflects the potential clinical situation, in which leukemic cells should be in numeric advantage over effector cells and no exogenous IL-2 is administered to the patient. The in vivo efficacy of a CAR-based approach could be limited by its immunogenicity: CAR are artificial proteins that might be abnormally processed and presented, thus generating new epitopes to which the immune system is not tolerant. However, the major cause of immunogenicity is likely to be represented by murine sequences present in the scFv domain of the CAR molecules. Indeed, anti-CAR antibody responses have been seen with some CAR, but this problem could be circumvented by using humanized scFv or scFv derived from human monoclonal antibodies,18,20 as we did in our study.
The functional improvements in the activity of CIK cells against AML, obtained through anti-CD33.CAR expression, are not trivial. In the phase I trial previously published by our group,15 in which patients with different hematologic disorders received infusions of CIK cells after allogeneic hematopoietic stem cell transplantation, no evidence of anti-tumor activity of CIK cells in vivo could be found, even in those subjects who received the higher cell doses. Despite these limited results in terms of clinical efficacy, CIK cells still remain an interesting tool to be used for leukemia cell therapy, because they can be generated simply and expanded to high numbers under GMP conditions, and because of their low propensity to cause graft-versus-host disease. Our proposed strategy was, therefore, specifically designed to implement their ability to kill leukemic cells, but more importantly to persist through leukemia-specific proliferation and secretion of IL-2, and to further amplify the anti-leukemic response through the release of IFN-γ, TNF-α and TNF-β. Our results are very promising in this sense and might, at least partially, address the issue related to the limited efficacy of unmanipulated CIK cells observed in vivo in our previously published phase I study.15
The idea of targeting the CD33 molecule was first exploited with the use of gemtuzumab-ozogamicin, an anti-CD33 monoclonal antibody conjugated with calicheamicin, which has shown a certain degree of activity in clinical use, but also a suboptimal safety profile. Even though gemtuzumab-ozogamicin is highly effective as a single treatment for patients with molecular relapse of acute promyelocytic leukemia,29 the complete response rate in AML is around 30%.30 In addition, it has been reported that treatment with gemtuzumab-ozogamicin is associated with myelosuppression, neutropenia and thrombocytopenia in almost all treated patients and severe hepatotoxicity in approximately 20% of patients.30 CAR-mediated cell therapy might have several potential advantages over gemtuzumab-ozogamicin. Anti-CD33.CAR-expressing CIK cells should be able, once infused, to infiltrate the tumor better and to exert a more physiological anti-leukemic response compared to gem-tuzumab-ozogamicin, activating and intensifying a tumor-specific immune response, mediated only by the interaction with the CD33 antigen and subsequent T-cell-mediated lysis, without addition of a chemotherapeutic agent that is toxic per se. Moreover, CIK cells genetically modified with a CD33-specific CAR should avoid the drug-resistance mechanisms that gemtuzumab-ozogam-icin are typically subject to, such as dependence on CD33 expression levels31 and capacity of the cells to internalize the drug or to mediate the efflux from the cells through P-glycoprotein.32 In this study, in fact, we demonstrated that anti-CD33.CAR-redirected CIK cells have a strong capacity to kill cells of the KG-1 cell line, known to be resistant to gemtuzumab-ozogamicin activity.
The use of CD33 as a target antigen is not devoid of problems. This antigen is, in fact, expressed on the surface of normal hematopoietic precursor cells (myeloid, erythroid and megakaryocytic), and on Küppfer cells. This pattern of expression might partially explain the toxicity profile of gemtuzumab-ozogamicin. The CAR-CIK-based immunization strategy might have interesting advantages also with regards to safety. As we demonstrated in our study, even though anti-CD33.CAR-transduced CIK cells killed some normal human hematopoietic progenitor cells, a reduced, but consistent, number of clonogenic progenitors were recovered in in vitro CFU assays. This could be explained by the fact that not all normal CD34+ CD38−human hematopoietic progenitor cells express CD33 at their surface, as reported by Taussig et al.33 It should also be noted that CIK cells could be further manipulated to introduce safety systems that might prevent undesired toxic effects or that might render cells rapidly eliminable from the organisms, in the case of the occurrence of unwanted events. For example, CIK cells might be manipulated to prevent expression of an adhesion molecule or chemokine receptors mediating migration/extravasation to the liver34 or through the introduction of a suicide gene. Many suicide gene systems are available,35 and we are currently comparing their efficacy (unpublished data). Our preliminary data clearly show a potent and rapid clearance capacity of the inducible caspase-9 system, which is at least equally effective as the herpes simplex virus - thymi-dine kinase approach, is clinically applicable,36 and has the important advantage of not being immunogenic. Generally speaking, the addition of a suicide gene could be considered an ideal strategy to optimize the safety of our proposed approach. Other kinds of transduction approaches are also under evaluation, such as self-inactivating vectors and non-viral methods (e.g. transposons). Major technical developments are under constant investigation for a safer clinical translation.37
Finally, in this approach the CD33-independent toxicity caused by free calicheamicin is completely abrogated, while this is the main mechanism of gemtuzumab-ozogamicin toxicity. Indeed, it has been described that this toxin is accumulated in the hepatocytes inducing apoptosis and, therefore, contributes significantly to the hepatic damage38 that is among the limiting side effects observed in patients treated with gemtuzumab-ozogamicin, particularly those receiving a hematopoietic stem cell transplant. This aspect is of great relevance, taking into account that anti-CD33.CAR-expressing cells would likely be used in a post-transplant setting in the presence of molecular relapse of AML.
Besides gemtuzumab-ozogamicin, other anti-CD33 unconjugated-antibody-based approaches are currently under development, such as Micromet's anti-CD33-T-cell receptor engager BiTE and Seattle Genetics' lintuzumab. While no preclinical or clinical data on the former strategy are yet available, the latter has already been tested in clinical studies, in which it showed a moderate success,39,40 and its mechanisms of action are currently under analysis.41 We believe, however, that a T-cell-based, CAR-mediated targeting of AML might be a potentially better approach. T cells, in fact, might have superior homing capabilities, can amplify the anti-tumor immune responses through target-specific cytokine release, and, last but not the least, can proliferate after contact with tumor cells, thus ensuring a boosted and persistent anti-tumor activity and, perhaps, complete eradication of the disease. Obviously, this must be counterbalanced by the possibly greater toxic effects on the normal hematopoietic compartment: only in vivo studies will provide the necessary data on this issue.
Although we are aware that we need to have final proof of the efficacy and safety of our anti-CD33.CAR-transduced CIK cells in a murine xenogenic model, which we are currently setting up, our results indicate that CD33-redirection of CIK cells improves the cells’ effector functions against AML and represents, compared to the unmanipulated counterpart, a step forward in the immunotherapy of resistant and relapsed AML.
Acknowledgments
the authors would like to thank Prof. Patrizia Vergani for cord blood collection, Dr. Cristina Bugarin and Dr. Daniela Longoni for helping in setting up the colony-forming assays; Dr. Sarah Tettamanti and Elisabetta Cribioli for their contribution to performing the last experiments and data analysis for the paper revision and Prof. Dominique Bonnet for critically reading the manuscript.
Footnotes
Funding: this work was supported by grants from: STREP 2006 (6th framework; LSHC-CT-2006-037381): “Chimaeric T cells for the treatment of paediatric cancers (Childhope).” See: www.childhope.eu; AIRC 2007 (4069): “The use of chimeric T-cell receptors (ChTCRs) for the therapy of haematological high-risk diseases”; AIRC 2007 (4636): “Childhood ALL: from clinical studies to research questions to understand molecular history and pathogenesis”; the “Progetto Integrato Oncologia 2006”, Ministero della Salute –Direzione Generale della Ricerca Scientifica e Tecnologica; the Fondazione “Matilde Tettamanti”, the “Comitato Stefano Verri” and the “Comitato Maria Letizia Verga”.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Linet MS, Devesa SS. Descriptive epidemiology of childhood leukaemia. Br J Cancer. 1991;63(3):424–9. doi: 10.1038/bjc.1991.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368(9550):1894–907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 3.Takami A, Okumura H, Yamazaki H, Kami M, Kim SW, Asakura H, et al. Prospective trial of high-dose chemotherapy followed by infusions of peripheral blood stem cells and dose-escalated donor lymphocytes for relapsed leukemia after allogeneic stem cell transplantation. Int J Hematol. 2005;82 (5):449–55. doi: 10.1532/IJH97.05086. [DOI] [PubMed] [Google Scholar]
- 4.Verneris MR, Baker J, Edinger M, Negrin RS. Studies of ex vivo activated and expanded CD8+ NK-T cells in humans and mice. J Clin Immunol. 2002;22(3):131–6. doi: 10.1023/a:1015415928521. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med. 1991;174(1):139–49. doi: 10.1084/jem.174.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Introna M, Franceschetti M, Ciocca A, Borleri G, Conti E, Golay J, et al. Rapid and massive expansion of cord blood-derived cytokine-induced killer cells: an innovative proposal for the treatment of leukemia relapse after cord blood transplantation. Bone Marrow Transplant. 2006;38(9):621–7. doi: 10.1038/sj.bmt.1705503. [DOI] [PubMed] [Google Scholar]
- 7.Hoyle C, Bangs CD, Chang P, Kamel O, Mehta B, Negrin RS. Expansion of Philadelphia chromosome-negative CD3(+)CD56(+) cytotoxic cells from chronic myeloid leukemia patients: in vitro and in vivo efficacy in severe combined immunodeficiency disease mice. Blood. 1998;92 (9):3318–27. [PubMed] [Google Scholar]
- 8.Linn YC, Lau LC, Hui KM. Generation of cytokine-induced killer cells from leukaemic samples with in vitro cytotoxicity against autologous and allogeneic leukaemic blasts. Br J Haematol. 2002;116(1):78–86. doi: 10.1046/j.1365-2141.2002.03247.x. [DOI] [PubMed] [Google Scholar]
- 9.Edinger M, Cao YA, Verneris MR, Bachmann MH, Contag CH, Negrin RS. Revealing lymphoma growth and the efficacy of immune cell therapies using in vivo bioluminescence imaging. Blood. 2003;101 (2):640–8. doi: 10.1182/blood-2002-06-1751. [DOI] [PubMed] [Google Scholar]
- 10.Marin V, Dander E, Biagi E, Introna M, Fazio G, Biondi A, et al. Characterization of in vitro migratory properties of anti-CD19 chimeric receptor-redirected CIK cells for their potential use in B-ALL immunotherapy. Exp Hematol. 2006;34(9):1219–29. doi: 10.1016/j.exphem.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Baker J, Verneris MR, Ito M, Shizuru JA, Negrin RS. Expansion of cytolytic CD8(+) natural killer T cells with limited capacity for graft-versus-host disease induction due to interferon gamma production. Blood. 2001;97(10):2923–31. doi: 10.1182/blood.v97.10.2923. [DOI] [PubMed] [Google Scholar]
- 12.Verneris MR, Ito M, Baker J, Arshi A, Negrin RS, Shizuru JA. Engineering hematopoietic grafts: purified allogeneic hematopoietic stem cells plus expanded CD8+ NK-T cells in the treatment of lymphoma. Biol Blood Marrow Transplant. 2001;7(10):532–42. doi: 10.1016/s1083-8791(01)70014-6. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt-Wolf IG, Finke S, Trojaneck B, Denkena A, Lefterova P, Schwella N, et al. Phase I clinical study applying autologous immunological effector cells transfected with the interleukin-2 gene in patients with metastatic renal cancer, colorectal cancer and lymphoma. Br J Cancer. 81(6):1009–16. doi: 10.1038/sj.bjc.6690800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi M, Zhang B, Tang ZR, Lei ZY, Wang HF, Feng YY, et al. Autologous cytokine-induced killer cell therapy in clinical trial phase I is safe in patients with primary hepatocellular carcinoma. World J Gastroenterol. 2004;10(8):1146–51. doi: 10.3748/wjg.v10.i8.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Introna M, Borleri G, Conti E, Franceschetti M, Barbui AM, Broady R, et al. Repeated infusions of donor-derived cytokine-induced killer cells in patients relapsing after allogeneic stem cell transplantation: a phase I study. Haematologica. 2007;92 (7):952–9. doi: 10.3324/haematol.11132. [DOI] [PubMed] [Google Scholar]
- 16.Franceschetti M, Pievani A, Borleri G, Vago L, Fleischhauer K, Golay J, et al. Cytokine-induced killer cells are terminally differentiated activated CD8 cytotoxic T-EMRA lymphocytes. Exp Hematol. 2009;37:616–28. e2. doi: 10.1016/j.exphem.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Marin V, Kakuda H, Dander E, Imai C, Campana D, Biondi A, et al. Enhancement of the anti-leukemic activity of cytokine induced killer cells with an anti-CD19 chimeric receptor delivering a 4-1BB-zeta activating signal. Exp Hematol. 2007;35 (9):1388–97. doi: 10.1016/j.exphem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21(2):215–23. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pule MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12(5):933–41. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Berry LJ, Moeller M, Darcy PK. Adoptive immunotherapy for cancer: the next generation of gene-engineered immune cells. Tissue Antigens. 2009;74(4):277–89. doi: 10.1111/j.1399-0039.2009.01336.x. [DOI] [PubMed] [Google Scholar]
- 21.Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108(12):3890–7. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Privitera E, Schiro R, Longoni D, Ronchi A, Rambaldi A, Bernasconi S, et al. Constitutive expression of GATA-1, EPOR, alpha-globin, and gamma-globin genes in myeloid clonogenic cells from juvenile chronic myelocytic leukemia. Blood. 1995;86(1):323–8. [PubMed] [Google Scholar]
- 23.Cornish AL, Freeman S, Forbes G, Ni J, Zhang M, Cepeda M, et al. Characterization of siglec-5, a novel glyco-protein expressed on myeloid cells related to CD33. Blood. 1998;92(6):2123–32. [PubMed] [Google Scholar]
- 24.Linn YC, Wang SM, Hui KM. Comparative gene expression profiling of cytokine-induced killer cells in response to acute myloid leukemic and acute lymphoblastic leukemic stimulators using oligonucleotide arrays. Exp Hematol. 2005;33(6):671–81. doi: 10.1016/j.exphem.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Miller CL, Lai B. Human and mouse hematopoietic colony-forming cell assays. Methods Mol Biol. 2005;290:71–89. doi: 10.1385/1-59259-838-2:071. [DOI] [PubMed] [Google Scholar]
- 26.Chmielewski M, Hombach A, Heuser C, Adams GP, Abken H. T cell activation by antibody-like immunoreceptors: increase in affinity of the single-chain fragment domain above threshold does not increase T cell activation against antigen-positive target cells but decreases selectivity. J Immunol. 2004;173(12):7647–53. doi: 10.4049/jimmunol.173.12.7647. [DOI] [PubMed] [Google Scholar]
- 27.Atkins MB. Cytokine-based therapy and biochemotherapy for advanced melanoma. Clin Cancer Res. 2006;12(7 Pt 2):2353s–8s. doi: 10.1158/1078-0432.CCR-05-2503. [DOI] [PubMed] [Google Scholar]
- 28.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106(1):376–83. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo-Coco F, Cimino G, Breccia M, Noguera NI, Diverio D, Finolezzi E, et al. Gemtuzumab ozogamicin (Mylotarg) as a single agent for molecularly relapsed acute promyelocytic leukemia. Blood. 2004;104 (7):1995–9. doi: 10.1182/blood-2004-04-1550. [DOI] [PubMed] [Google Scholar]
- 30.Stasi R, Evangelista ML, Buccisano F, Venditti A, Amadori S. Gemtuzumab ozogamicin in the treatment of acute myeloid leukemia. Cancer Treat Rev. 2008;34(1):49–60. doi: 10.1016/j.ctrv.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Walter RB, Raden BW, Kamikura DM, Cooper JA, Bernstein ID. Influence of CD33 expression levels and ITIM-dependent internalization on gemtuzumab ozogamicin-induced cytotoxicity. Blood. 2005;105(3):1295–302. doi: 10.1182/blood-2004-07-2784. [DOI] [PubMed] [Google Scholar]
- 32.Walter RB, Gooley TA, van der Velden VH, Loken MR, van Dongen JJ, Flowers DA, et al. CD33 expression and P-glycoprotein-mediated drug efflux inversely correlate and predict clinical outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin monotherapy. Blood. 2007;109(10):4168–70. doi: 10.1182/blood-2006-09-047399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taussig DC, Pearce DJ, Simpson C, Rohatiner AZ, Lister TA, Kelly G, et al. Hematopoietic stem cells express multiple myeloid markers: implications for the origin and targeted therapy of acute myeloid leukemia. Blood. 2005;106(13):4086–92. doi: 10.1182/blood-2005-03-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14(5):409–26. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 35.Bonini C, Bondanza A, Perna SK, Kaneko S, Traversari C, Ciceri F, et al. The suicide gene therapy challenge: how to improve a successful gene therapy approach. Mol Ther. 2007;15(7):1248–52. doi: 10.1038/sj.mt.6300190. [DOI] [PubMed] [Google Scholar]
- 36.Di Stasi A, Tey SK, Fujita Y, Cruz R, Martinez C, Kennedy-Nasser A, et al. CASPALLO: phase I clinical trial of allodepleted T cells transduced with inducible caspase 9 suicide gene after haploidentical stem cell transplantation. Mol Ther. 2010;18:S110. [Google Scholar]
- 37.Brenner MK, Okur FV. Overview of gene therapy clinical progress including cancer treatment with gene-modified T cells. Hematology Am Soc Hematol Educ Program. 2009:675–81. doi: 10.1182/asheducation-2009.1.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDonald GB. Management of hepatic sinusoidal obstruction syndrome following treatment with gemtuzumab ozogamicin (Mylotarg) Clin Lymphoma. 2002;2 (Suppl 1):S35–9. doi: 10.3816/clm.2002.s.007. [DOI] [PubMed] [Google Scholar]
- 39.Castillo J, Winer E, Quesenberry P. Newer monoclonal antibodies for hematological malignancies. Exp Hematol. 2008;36(7):755–68. doi: 10.1016/j.exphem.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 40.Raza A, Jurcic JG, Roboz GJ, Maris M, Stephenson JJ, Wood BL, et al. Complete remissions observed in acute myeloid leukemia following prolonged exposure to lintuzumab: a phase 1 trial. Leuk Lymphoma. 2009;50(8):1336–44. doi: 10.1080/10428190903050013. [DOI] [PubMed] [Google Scholar]
- 41.Sutherlan MK, Yu C, Lewis TS, Miyamoto JB, Morris-Tilden CA, Jonas M, et al. Anti-leukemic activity of lintuzumab (SGN-33) in preclinical models of acute myeloid leukemia. MAbs. 2009;1(5):481–90. doi: 10.4161/mabs.1.5.9288. [DOI] [PMC free article] [PubMed] [Google Scholar]