In patients with T-cell lymphoblastic leukemia (T-ALL) chromosome rearrangements and gene mutations are used as diagnostic markers for genetic classification and prognostic stratification.1 Gene deregulation in T-ALL is determined by ectopic or over-expressed oncogenes, gain or loss of function mutations, genomic imbalances and, less frequently, gene fusions. In T-ALL rare fusions involving 2 nucleoporins, i.e. NUP98 (NUP98-RAP1GDS1, NUP98-SETBP1, NUP98-C6orf80) and NUP214 (NUP214-ABL1 and SET-NUP214), are believed to predict a poor prognosis.2
We found a SET-NUP214 rearrangement was closely associated with a specific gene profiling signature in adult T-ALL3 and analysis of 69 patients who were enrolled in 3 Italian GIMEMA protocols (LAL0496, LAL2000 and LAL09044) revealed one case had a slightly different profile.
Here we report, for the first time, the case of a 20-year old man, with a chemoresistant pre-T ALL (immunopheno-type: CD3+, CD7+, CD5+, CD34+, CD33+, cKit+) who died 16 months after diagnosis. The karyotype was 46,XY,del(6p)(p21p25) and CI-FISH detected CDKN2A-B/9p21 and NF1/17q11 deletions.5
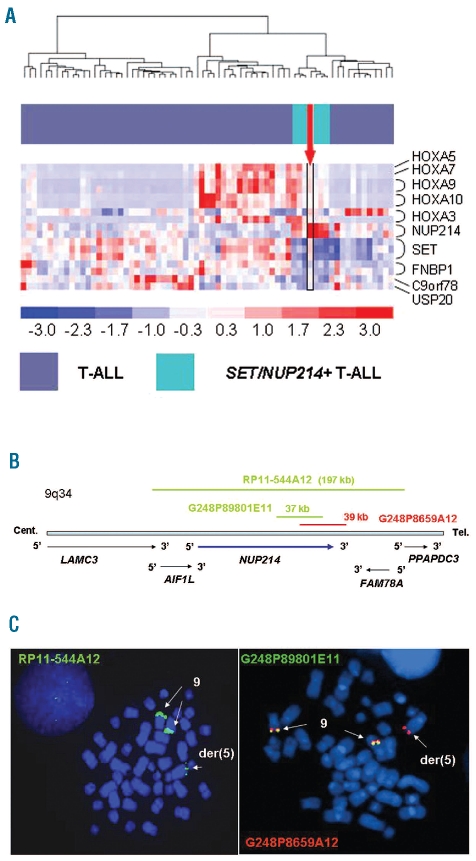
Using the list of genes that were specific for SET/NUP214+ cases,3 this patient clustered tightly with this group and showed high expression levels of HOXA cluster genes (HOXA3, HOXA5, HOXA7, HOXA9, HOXA10) and NUP214 as well as low expression of FNBP1, C9orf78 and USP20 but unlike them, he showed remarkably high SET levels (Figure 1A).
Figure 1.
Gene expression profiles and FISH assays. (A) Identification of additional SET/NUP214+ patients by using the genes specific of SET/NUP214+ signature. Each row represents a probe-set, each column a sample. Color scale: blue and red indicate the lowest and highest expression levels, respectively. The reported case is indicated as an arrow: it clustered with the SET/NUP214+ patients and was characterized by the upregulation of HOXA cluster genes and NUP214 as well as low expression of FNBP1, C9orf78 and USP20 while it showed remarkably high SET levels. (B) Schema of part of chromosome 9 long arm (9q34). DNA clones used for FISH studies are shown with their relative position and size. Centromere to telomere portion of 9q34 indicates gene localization/orientation (arrows). (C) Metaphase FISH results: (left panel) Clone RP11-544A12 (green), hybridized with both chromosomes 9 and one apparently normal chromosome 5 (der (5)). (Right panel) Clone G248P89801E11 (green) (NUP214, exon 25-32) was present on both chromosomes 9. G248P8659A12 (red) (NUP214, exons 31-36) was detected on chromosomes 9 and on der(5).
Consequently, we investigated NUP214 and found it was involved in a cryptic unbalanced translocation. FISH with RP11-143H20, flanking NUP214 at the centromeric side, hybridized with both normal chromosomes 9, as expected, while RP11-544A12 spanning NUP214, hybridized with both 9 and an apparently normal chromosome 5. To characterize the rearrangement between chromosome 9 and 5 we used DNA clones for chromosome bands 9q34 and 5q35 and found a trisomic 9q34-qter underwent an unbalanced translocation with chromosome 5, i.e. der(5)t(5;9)(q35;q34) (Figure 1B and C). The 9q34/NUP214 breakpoint was narrowed using two overlapping clones. Clone G248P89801E11 spanning exons 25-32 was detected on two normal chromosomes 9, while clone G248P8659A12 spanning exons 31-36 was present on chromosomes 9 and on der(5) (Figure 1B and C). Thus the breakpoint fell within 3′ region of NUP214.
The 5q35 breakpoint was telomeric to RP11-718N2, a ~1.7Mb region containing 15 candidate genes, with an appropriate centromere-telomere orientation. Since GEP revealed 3/15 genes telomeric to SQSTM1/5q35 were down-modulated, they might have been lost as a result of unbalanced der(5)t(5;9). SQSTM1 was first selected as putative NUP214 fusion partner.
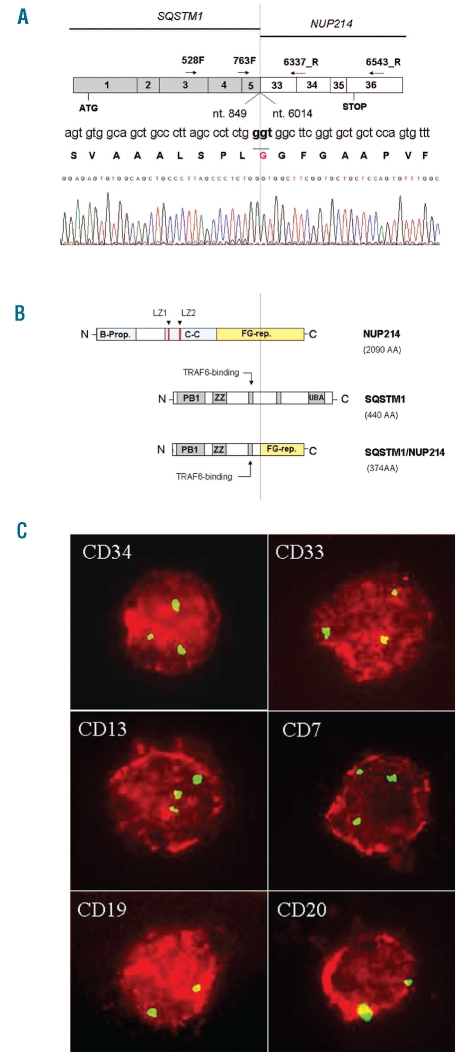
Using primers SQSTM1_ex3_528F (5′-TGCCCAGACTACGACTTGTG-3′) and NUP214_ex36_6543R (5′-AGTAATCATGCGCCTTGTGAGTT-3′), RT-PCR detected an amplification product of 852bp which was sub-cloned into pGEM-T easy vector (Promega, Madison, WI, USA). Sequencing showed an in-frame fusion between nucleotide 849 (exon 5) of SQSTM1 and nucleotide 6014 (exon 33) of NUP214 (Figure 2A and B). To establish the prevalence more accurately we screened another 67 T-ALL patients. We set up a Nested RT-PCR assay, using primers SQSTM1_ex3_528F and NUP214_ex36_6543R in the first amplification round and primers SQSTM1_ex4/5_763F (5′-AATCAGCTTCTGGTCCATCG-3′) and NUP214_ex_33/34_6337R (5′-CAAAGCTGAACCCTCCTGTG-3′) in the second. Nested RT-PCR were also used to validate 15/69 cases analyzed by GEP for which cDNA was available. PCR screening did not identify any other cases with the SQSTM1-NUP214 fusion transcript so the prevalence at present is one in 136 cases.
Figure 2.
Molecular characterization of SQSTM1-NUP214 fusion transcript and FICTION experiments. (A) Schema of SQSTM1-NUP214 fusion transcript. SQSTM1 nucleotide 849 (exon 5) is fused in-frame to NUP214 nucleotide 6014 (exon 33). Arrows indicate the primers used in PCR amplification. Dotted line indicates fusion point. Sequence numbers refer to GenBank accession NM_003900.4 for SQSTM1 and NM_005085.2 for NUP214. (B) Schema of NUP214 (2090 AA) (upper), SQSTM1 (440 AA) (middle) and the predicted SQSTM1/NUP214 fusion protein (374 AA) (bottom) which contains PB1, ZZ and TRAF6 binding domain derived from SQSTM1 and 14/44 FG repeats derived from NUP214. The FG repeat region is indicated in yellow. B-prop: Beta propeller, C-C: Coiled-coil, LZ: Leucine-zipper, FG-rep: Phenylalanine-Glycine repeats, PB1: Phox and Bem1p domain, ZZ: Zinc finger, UBA: ubiquitin associated domain. (C) FICTION with monoclonal antibodies against CD34, CD33, CD13, CD7, CD19, CD20. Red staining detects positive intact cells expressing the specific antigen. Green spots indicate FISH signals in the nuclei using a FITC-labeled genomic probe (RP11-544A12) for NUP214. Two green signals indicate normal cells; three signals hallmark NUP214 rearrangement.
To determine the hematopoietic lineage of this fusion gene we applied FICTION (Fluorescence Immunophenotype and Interphase Cytogenetics as a Tool for Investigations of Neoplasms)6 with RP11-544A12 and found the NUP214 rearrangement in CD34+, CD33+, CD13+, CD14+, CD3+ and in CD7+ bone marrow cells (range of positive cells 50–78%) but not in CD19+ and CD20+ cells (Figure 2C). FICTION results are concordant to the ex vivo immunophenotype, including CD34 positivity. The present case of SQSTM1-NUP214 positive T-ALL shared a few common features with SET-NUP214 positive adult T-ALL:3 immature phenotype and poor disease outcome; additional genomic lesions at karyotype and/or CI-FISH; deregulation of the same set of genes, as shown by GEP.
NUP214 maps on chromosome 9q34.3; with its 36 exons, it covers 108 kb of genomic DNA. The transcript is a 6.6 kb long mRNA which encodes for 214 kDa FXFG nucleoporin of 2090 AA located mainly on the cytoplasmic side of the nuclear pore complex.2
SQSTM1 gene, at chromosome 5q35, encodes a multi-functional adaptor protein that has no intrinsic enzymatic activity but is a critical component of multiple signaling pathways. It is mainly known as an ubiquitin binding protein and a scaffolding/adaptor factor that is involved in NF-κB activation in response to Ras induction.7 SQSTM1 regulates bone remodeling by controlling osteoclastogenesis, inflammatory reaction through T-cell differentiation, NGF receptor internalization and adipocyte differentiation.7 Remarkably, SQSTM1 mutations result in sporadic and familial Paget’s disease of bone8 and its overexpression has been found in several solid human tumors.7 It is involved in tumor growth and bone destruction in patients with multiple myeloma.9
In the predicted SQSTM1-NUP214 fusion protein, as in the SET-NUP214, the fusion partner N-terminal links to the NUP214 C-terminal (Figure 2B). The SET-NUP214 fusion retains 42/44 NUP214 FG repeats with the ability to bind and relocalize the NES receptor hCRM1, thus perturbing nuclear transport.10 SQSTM1/NUP214 contained only 14/44 FG repeats and since it had not maintained the entire hCRM1 binding domain11 other mechanisms might be implicated in the leukemogenic process.
In conclusion, this case illustrates a cryptic unbalanced translocation der(5)t(5;9)(q35;q34) which underlay a new SQSTM1-NUP214 in-frame fusion which joined the SQSTM1 N-terminal with the NUP214 C-terminal. The prevalence at present is one in a total of 136 cases; 69 in the GEP series4 and 67 screened in the present report by RT-PCR.
As NUP214 behaves as a promiscuous gene undergoing different types of rearrangements in T-ALL, we strongly recommend applying a specific FISH assay to detect NUP214 in the diagnostic work up of T-ALL. The incidence and clinical impact of NUP214-rearrangements need to be investigated in large prospective studies.
Acknowledgments
the authors would like to thank GIMEMA (Gruppo Italiano Malattie Ematologiche dell’Adulto), and Dr G A Boyd for assistance in the preparation of the manuscript.
Footnotes
Funding: this work was supported by a grant from IAP (Interuniversity Attraction Poles, University of Leuven, Belgium), AIRC (Associazione Italiana Ricerca sul Cancro), PRIN-MIUR (Programmi di Ricerca Cofinanziati-Ministero per l’Istruzione, l’Università e la Ricerca Scientifica, Italy), Fondazione Cassa di Risparmio di Perugia, Associazione “Sergio Luciani”, Fabriano, Italy; Compagnia di San Paolo, Turin, Italy and European Community, Ricerca finalizzata Regione Umbria 2008.
References
- 1.De Keersmaecker K, Marynen P, Cools J. Genetic insights in the pathogenesis of T-cell acute lymphoblastic leukemia. Haematologica. 2005;90(8):1116–27. [PubMed] [Google Scholar]
- 2.Xu S, Powers MA. Nuclear pore proteins and cancer. Semin Cell Dev Biol. 2009;20(5):620–30. doi: 10.1016/j.semcdb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorello P, La Starza R, Varasano E, Chiaretti S, Elia L, Pierini V, et al. Combined interphase fluorescence in situ hybridization elucidates the genetic heterogeneity of T-cell acute lymphoblastic leukemia in adults. Haematologica. 2010;95(1):79–86. doi: 10.3324/haematol.2009.010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiaretti S, Messina M, Tavolaro S, Zardo G, Elia L, Vitale A, et al. Gene expression profiling identifies a subset of adult T-cell acute lymphoblastic leukemia (T-ALL) with myeloid-like gene features and overexpression of miR-223. Haematologica. 2010;95 (7):1114–21. doi: 10.3324/haematol.2009.015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matteucci C, Barba G, Varasano E, Vitale A, Mancini M, Testoni N, et al. Rescue of genomic information in adult acute lymphoblastic leukaemia (ALL) with normal/failed cytogenetics: a GIMEMA centralized biological study. Br J Haematol. 2010;149(1):70–8. doi: 10.1111/j.1365-2141.2009.08056.x. [DOI] [PubMed] [Google Scholar]
- 6.Crescenzi B, La Starza R, Beacci D, Rosti V, Galli A, Specchia G, et al. FIP1L1-PDGFRA in CEL and BCR-ABL1 in CML affect different leukemic cells. Leukemia. 2007;21(3):397–402. doi: 10.1038/sj.leu.2404510. [DOI] [PubMed] [Google Scholar]
- 7.Pursiheimo JP, Rantanen K, Heikkinen PT, Johansen T, Jaakkola PM. Hypoxia-activated autophagy accelerates degradation of SQSTM1/p62. Oncogene. 2009;28(3):334–44. doi: 10.1038/onc.2008.392. [DOI] [PubMed] [Google Scholar]
- 8.Laurin N, Brown JP, Morissette J, Raymond V. Recurrent mutation of the gene encoding sequestosome 1 (SQSTM1/p62) in Paget disease of bone. Am J Hum Genet. 2002;70(6):1582–8. doi: 10.1086/340731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiruma Y, Honjo T, Jelinek DF, Windle JJ, Shin J, Roodman GD, et al. Increased signaling through p62 in the marrow microenvironment increases myeloma cell growth and osteoclast formation. Blood. 2009;113(20):4894–902. doi: 10.1182/blood-2008-08-173948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saito S, Miyaji-Yamaguchi M, Nagata K. Aberrant Intracellular localization of SET-CAN fusion protein, associated with a leukemia, disorganizes nuclear export. Int J Cancer. 2004;111 (4):501–7. doi: 10.1002/ijc.20296. [DOI] [PubMed] [Google Scholar]
- 11.Fornerod M, Boer J, van Baal S, Morreau H, Grosveld G. Interaction of cellular proteins with the leukemia specific fusion proteins DEK-CAN and SET-CAN and their normal counterpart, the nucleoporin CAN. Oncogene. 1996;13(8):1801–8. [PubMed] [Google Scholar]