Introduction
Rotator cuff injuries represent an extremely common cause of upper extremity disability1, resulting in substantial shoulder pain and dysfunction. Young athletes, middle-aged workers, and a substantial portion of the elderly population can sustain acute or degenerative rotator cuff injuries, which prevent them from working, playing sports, enjoying hobbies, or performing activities of daily living2,3. Active people, including athletes, are highly susceptible to rotator cuff problems, particularly as they advance in age4. It has been estimated that approximately 300,000 rotator cuff surgical procedures are performed each year in the United States.
Traditionally, surgical repair of a rotator cuff consists of reapproximating the tendon edge to an anatomic footprint with the specific type of repair used (open, mini-open, or arthroscopic) dependent on the size, shape, and chronicity of the tear5,6. The disadvantages of the open techniques include a larger dissection and longer operating time, while the arthroscopic repairs have been associated with a limited amount of working area, a longer learning curve, and a higher percentage of recurrent lesions compared with the open or mini-open repair techniques7.
In the past decade, surgical procedures that utilize autografts, cadaveric allografts, or patch grafts made from biological and synthetic materials have been developed to repair massive rotator cuff tears8-11. Several factors limiting the extensive use of these procedures include donor site morbidity, the limited availability of autografts, and the risk of disease transmission with allografts. Recent studies have shown advantages with the use of synthetic augmentation devices to support the healing of a torn rotator cuff, but few of the commercially available matrices truly mimic the biomechanical behavior of a natural rotator cuff tendon12. The integration of biological, chemical, and engineering principles to design a suitable bioresorbable rotator cuff scaffold serves as the basis for the tissue-engineered approach employed in the present investigation.
The objective of this study was to develop a novel therapeutic strategy to support and accelerate the healing of a torn rotator cuff. A tissue-engineering approach with use of a nanostructured resorbable polymeric scaffold was hypothesized to produce a matrix with cellular and biomechanical properties suitable to provide initial strength and enhance the rate of regenerative repair. The components of this investigation included (1) fabrication of a nanofiber polymeric scaffold, (2) in vitro biological characterization, (3) development of an original rodent model, and (4) in vivo biomechanical characterization.
Materials and Methods
Fabrication of a Nanofiber Polymeric Scaffold
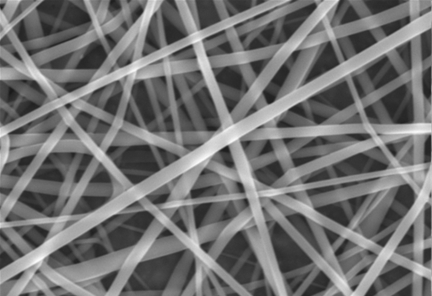
Through the process of electrospinning, a biodegradable polymeric solution was used to create resorbable polymer scaffolds from poly(85 lactic acid-co-15 glycolic acid) (PLAGA; Lakeshore Biomaterials, Birmingham, Alabama). A solution of PLAGA was prepared in a 3:1 solvent mixture of tetrahydrofuran and dimethylformamide and was charged to a high electrical potential of 30 kV. While keeping the controlled variables (voltage, distance, delivery rate, and needle gauge) at previously optimized constants13, ultra-thin fibers with diameters in the 400 to 800-nm range were allowed to collect randomly on a grounded collecting plate (Fig. 1).
Fig. 1.
Randomly oriented nanofiber (400 to 800-nm) mat produced through the process of electrospinning.
In Vitro Biological Characterization of Nanofiber Matrices
Patellar tendon cells were isolated from adult New Zealand White rabbits. The cells were cultured in tissue-culture polystyrene flasks with use of growth medium (Dulbecco modified Eagle medium containing 10% fetal calf serum and 1% penicillin-streptomycin). When the cells reached 80% confluency, they were trypsinized and seeded on the newly developed nanofiber matrices after appropriate passages. Nanofiber matrices measuring 1 × 1 cm were used for this portion of the study. The matrices were irradiated with ultraviolet light for fifteen minutes on each side to reduce bacterial contamination. The cellular density was 50,000 cells per matrix. The cells were cultured for various time periods for analysis, as described below.
Cellular Adhesion
Scanning electron microscopy was utilized to evaluate the development of a cellular network and to assist in the qualification of adhesion properties. At three, seven, twenty-one, and twenty-eight-day time points, the cell-seeded matrix scaffolds were washed with phosphate-buffered saline solution and fixed in 3% glutaraldehyde solution. The matrices were then washed and air dried. After gold-coating, imaging was performed with use of scanning electron microscopy to qualify any cellular material that had developed and remained adhered to the scaffold after the washings.
Cellular Proliferation and Viability
The proliferation of cells on the seeded nanofiber matrices was determined quantitatively with use of MTS assays. MTS (a tetrazolium compound) converts to a soluble, colored formazan product in the presence of mitochondrial dehydrogenase of viable cells, and thus serves as an indicator of cell viability. The amount of formazan product is directly proportional to the number of live cells, and its absorbance can be measured spectroscopically. The cell viability on the nanofiber matrices and the increase in cell numbers with time was further evaluated by a standard LIVE/DEAD assay (Invitrogen, Carlsbad, California). In this assay, the live cells display green coloration and any dead cells present with a red color.
Biomechanical and Suture Strength
The Young modulus of the matrices seeded with and without cells was determined under wet conditions with use of a biomechanical testing machine (Instron, Norwood, Massachusetts), with a strain rate of 0.2% per second. For each of the time points, five samples measuring 2 × 2 cm each were tested. The uniform thickness of the scaffold samples was 0.5 mm. The samples were incubated in cell culture media for various time periods (seven, fourteen, and twenty-eight days), at which point the biomechanical properties were determined.
In addition, suture strength was assessed at the same time points to determine both the mode of failure as well as the maximum force that could be withstood at the graft-suture interface. The samples were seeded with cells and incubated in cell culture media. At the designated time point, with use of a template to ensure uniformity, a 5-0 Prolene suture (polypropylene; Ethicon, Somerville, New Jersey) was passed through each scaffold in a horizontal mattress pattern to simulate a clinically relevant configuration. Passes were 5 mm apart and 5 mm from the edge, and suture was brought out to a length of 6 cm from the scaffold. The suture was tied into a knot around an s-shaped hook fixed into the upper grip of the Instron machine. The lower part of the scaffold was fixed to the lower grip. The strain rate used was 12.5 mm per minute. The maximum load to failure was used as a marker of ultimate suture strength, or the point at which the scaffold-suture interface was compromised.
In Vivo Rodent Model
It was hypothesized that the fabricated nanofiber matrices would have the appropriate physical and mechanical properties necessary for use as viable augmentation options for rotator cuff tendon repair. The efficacy for accelerating the rate of healing of a tendon defect was evaluated in an original rodent model. Surgical technique was researched and practiced extensively prior to initiation of the live rodent study in accordance with the Institutional Animal Care and Use Committee regulations. Once the optimal surgical approach was determined, the eight-week in vivo study began.
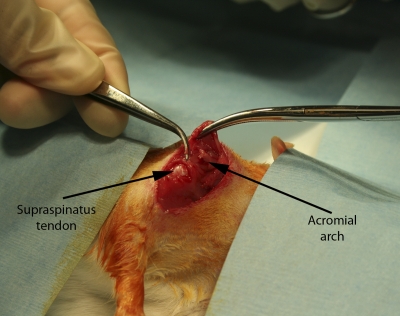
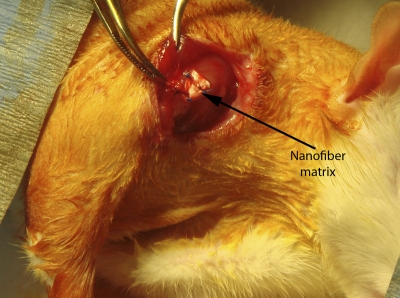
A total of forty-eight adult Sprague-Dawley rats underwent specific operative procedures, according to their predetermined group assignment (Table I). In brief, the supraspinatus was exposed by making a 2-cm skin incision over the scapulohumeral joint, elevating the superficial musculature, and reflecting the coracoacromial arch (Fig. 2-A). In all subjects, the supraspinatus tendon was separated longitudinally from other tissues and detached sharply from the greater tuberosity. To repair the tendon, the supraspinatus was reapproximated primarily, with the cut end secured directly to bone with use of a modified Kessler stitch technique, a common clinical approach for surgical tendon repair. If subsequent augmentation was required, a uniform 3 × 3-mm scaffold sample (thickness, 0.5 mm) was sutured over the primary repair site (Fig. 2-B). The coracoacromial arch, superficial shoulder musculature, and skin were repaired with absorbable suture (Fig. 2-C). After surgery, the animals were allowed to move freely in their cages, without immobilization.
TABLE I.
In Vivo Rodent Model Groups and Assigned Surgical Technique
| Group 1 | 4-week evaluation (n = 12) and 8-week evaluation (n = 12) | Supraspinatus tendon acute injury, immediately repaired with suture alone |
| Group 2 | 4-week evaluation (n = 12) and 8-week evaluation (n = 12) | Supraspinatus tendon acute injury, immediately repaired with suture and augmented with nanostructured scaffold |
Fig. 2-A.
Figs. 2-A, 2-B, and 2-C In vivo surgical technique. Fig. 2-A Supraspinatus exposure with overlying acromial arch.
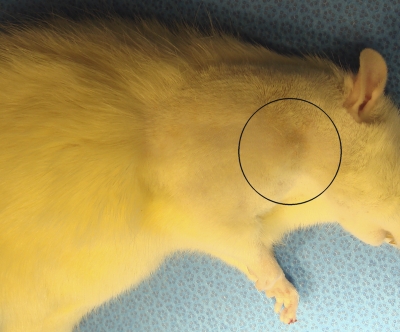
Fig. 2-A Fig. 2-B Fig. 2-C.
Fig. 2-B Repair augmentation with the scaffold. Fig. 2-C A well-healed incision (within the marked region) after eight weeks.
Biomechanical Evaluation
The rodents were killed after surgery, at either the four or eight-week time point, for biomechanical evaluation. The operatively treated limb was removed at the shoulder girdle, and tissue was carefully dissected down to the level of the rotator cuff musculature. For each rodent, the supraspinatus muscle belly, tendon, and humerus were isolated and preserved. The Young modulus of the tendon repair interface was assessed through use of the Instron biomechanical testing machine for continual tensile testing (strain rate, 2% per second) with the supraspinatus portion fixed in the upper grip and the humeral end fixed in the lower grip. Calipers were used to assess the relative thickness measurements of the repair or augmentation regions of tissue over the gauge length. This was important to ensure that the appropriate stress and strain values were obtained during the continuous tensile period of elongation.
Source of Funding
This research investigation was made possible through the National Institutes of Health Musculoskeletal Training Grant: Musculoskeletal Tissue Repair and Regeneration.
Results
In Vitro Biological Characterization of Nanofiber Matrices
Cellular Adhesion
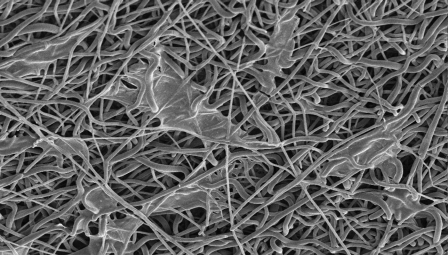
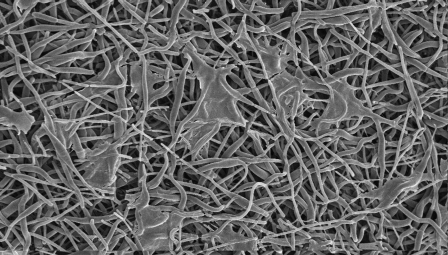
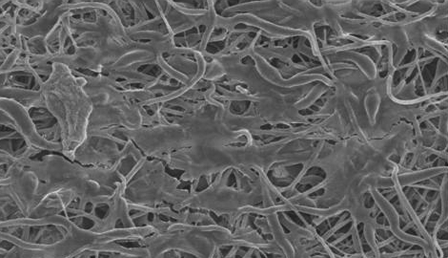
Scanning electron microscopic images of the nanofibers after cellular seeding with isolated tenocytes were evaluated at three, seven, and twenty-eight-day time points as described earlier. As time progressed, increases in the presence of a cellular network on the matrices were observed (Figs. 3-A, 3-B, and 3-C) and used to aid in the qualification of cellular adhesion.
Fig. 3-A Fig. 3-B Fig. 3-C.
Scanning electron microscopic imaging of the cell-seeded matrix at the three-day (Fig. 3-A), seven-day (Fig. 3-B), and twenty-eight-day time points (Fig. 3-C), showing an increase in the cellular network adhesion.
Cellular Proliferation and Viability
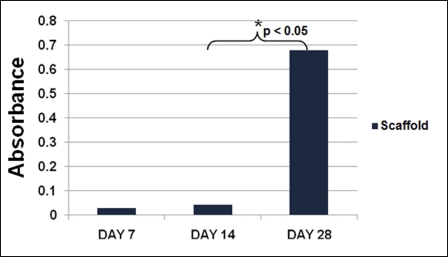
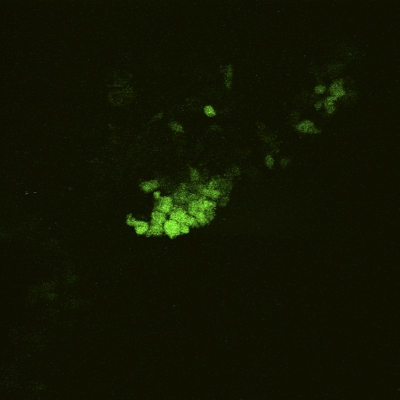
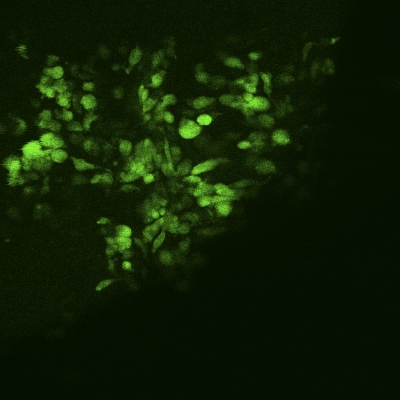
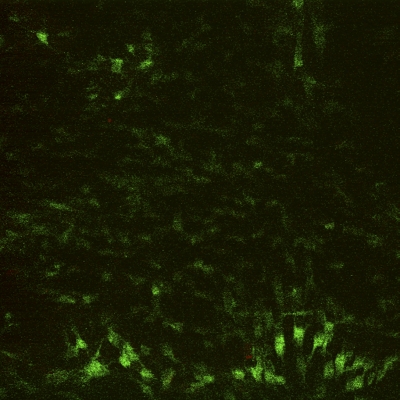
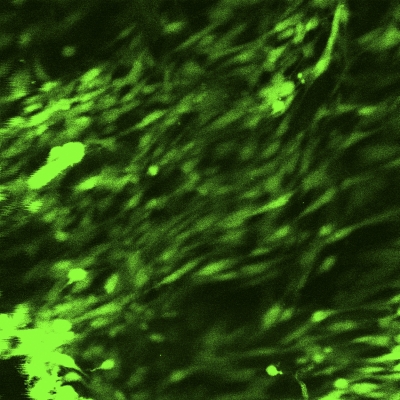
An MTS proliferation assay was performed at seven, fourteen, and twenty-eight-day time points to assess the presence of metabolic activity within the matrix. Between days 7 and 28, there was a significant increase in the measured absorbance from the scaffold, indicating cellular activity and proliferation (p < 0.05; Fig. 4). To determine whether the metabolic activity indeed represented the activity of live cells, a LIVE/DEAD assay was performed on the scaffolds (at three, seven, fourteen, and twenty-eight-day time points), demonstrating qualitative evidence of increases in the surface area of viable cells over time (Figs. 5-A through 5-D).
Fig. 4.
Cellular proliferation MTS assay results. The metabolic activity, represented by absorbance, increased significantly (p < 0.05) between days 14 and 28.
Fig. 5-A Fig. 5-B Fig. 5-C Fig. 5-D.
Figs. 5-A through 5-D LIVE/DEAD assay qualitative images demonstrating persistence of live (green) cellular network proliferation over time. Fig. 5-A Day 3. Fig. 5-B Day 7. Fig. 5-C Day 14. Fig 5-D Day 28.
Biomechanical and Suture Strength
The results of the wet biomechanical testing of the cell-seeded scaffolds are shown in Table II. The Young moduli of these matrices were obtained at days 7, 14, and 28. The cellular network appeared to increase the mechanical properties of the cell-seeded scaffolds, with the calculated moduli continuing to increase over the time period of cellular proliferation. In addition, compared with the properties of scaffolds that were not seeded with cells, the cell-seeded matrices had reached a higher Young modulus than the cell-free scaffold (mean and standard deviation, 78.7 ± 17 MPa vs. 72.3 ± 8 MPa; p > 0.05).
TABLE II.
In Vitro Results of Wet Biomechanical Testing of the Scaffold*
| Scaffold | Young Modulus (MPa) |
| Day 3 (without cells) | 72.3 ± 8 |
| Day 7 (with cells) | 78.7 ± 17 |
| Day 14 (with cells) | 80.5 ± 14 |
| Day 28 (with cells) | 88.7 ± 9 |
The strain rate was 0.2% per second. The values are given as the mean and the standard deviation.
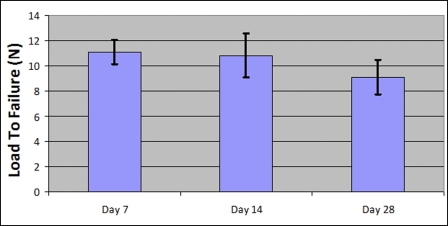
The results of the suture strength testing, performed at the same time points, are displayed in Figure 6. The maximal load to failure, when the scaffold-suture interface was compromised, was used as the marker of suture strength. There was a trend toward decline in the amount of load required to cause construct failure over time, but this was not significant. Furthermore, fourteen of the fifteen samples tested demonstrated suture pullout from the scaffold as the mode of construct breakdown, whereas the remaining sample demonstrated a break in the suture prior to pullout from the scaffold.
Fig. 6.
Suture strength results. The load to failure (mean pullout strength and standard deviation) of the scaffold gradually decreased over time, but the change was not significant (p > 0.05).
In Vivo Rodent Model Biomechanical Results
As described above, forty-eight rodents were killed after surgery at either the four or eight-week time point for biomechanical evaluation, depending on their group assignment (Table I). There were no wound complications (infection or dehiscence) or premature deaths. All subjects regained full use of the operatively treated upper extremity and were able to reach overhead for feeding prior to being killed. The biomechanical testing of the isolated supraspinatus-humerus repair junction was performed, and the results are shown in Table III. The repair junctions of both groups demonstrated similar biomechanical results at the four-week time point. However, between weeks 4 and 8, there was a significant increase in the Young modulus of the group that had received augmentation of their repair with the scaffold compared with the group that had received primary supraspinatus repair alone.
TABLE III.
In Vivo Results of Biomechanical Testing of the Supraspinatus Repair Junction*
| Young Modulus† (MPa) |
||
| 4 Weeks | 8 Weeks | |
| Group 1 (primary repair) | 7.2 ± 3.8 | 3.79 ± 2.1 |
| Group 2 (primary repair and augmentation) | 4.0 ± 0.5 | 48.6 ± 9.5 |
The strain rate was 2% per second. The values are given as the mean and the standard deviation. †A significant increase in biomechanical properties was demonstrated between the four-week and eight-week results for Group 2 and between Group 1 and Group 2 at eight weeks (p < 0.05).
Discussion
Tissue-engineering is an emerging therapeutic technique to accelerate the repair and regeneration of damaged tissue with use of bioresorbable scaffolds, potentially enhanced with cells and bioactive factors. An ideal tissue-engineered construct should mimic the properties of the damaged tissue and perform as a temporary augmentation device, while at the same time functioning as a matrix that allows for the deposition and growth of cells for new tissue formation. Past attempts to mimic the extracellular matrix of cells with synthetic materials have had limited success because of the complexity of these systems.
Most of the biomaterial design and fabrication techniques that have been used to develop three-dimensional scaffolds for tissue-engineered applications have focused on the macrostructure of the tissue without attempting to mimic the nanoscale features of the extracellular matrix. In the case of tendons, the extracellular matrix is composed of specifically oriented collagen fibrils with diameters ranging from 50 to 500 nm. On the basis of this understanding, a logical approach to develop a three-dimensional scaffold for tendon repair is to mimic the structure of collagen fibrils by fabricating a scaffold composed of fibers with diameters and organizations similar to those of the natural matrix (i.e., diameter within the range of 50 to 500 nm).
In the present study, we attempted to develop a bioresorbable nanostructured scaffold that could serve as an augmentation device for regenerating a torn rotator cuff. The objective was to closely mimic the biomechanical properties of the rotator cuff. In addition, this device needed to present a favorable structure for cell infiltration and matrix deposition for new tissue formation. Poly(85 lactic acid-co-15 glycolic acid) (PLAGA) polymer has been investigated for tissue-engineering applications since it does not elicit a permanent foreign-body reaction and it is gradually resorbed and replaced by natural tissue over time. Furthermore, PLAGA-based devices have been approved by the U.S. Food and Drug Administration (FDA) for many clinical applications. Thus, PLAGA was the material selected for use in this investigation.
We achieved fabrication of nanostructured bioresorbable matrix through the process of electrospinning. Within the in vitro portion of this study, tenocyte cellular adhesion, viability, and proliferation were characterized and found to be supported by the developed scaffold. In addition, preliminary biomechanical properties were evaluated and found to be comparable with the currently available alternative extracellular matrices12. Recent studies have suggested that the addition of specific cells to the repair site could favorably affect the healing process. One relevant investigation demonstrated the feasibility of accelerated rotator cuff regeneration in nude mice with use of genetically modified muscle-derived stem cells14. Another study group has demonstrated the ability to accelerate healing of rotator cuff tissue with use of fibroblast cells transfected with platelet-derived growth factor. The achievability of accelerated regeneration of ligaments using synthetic scaffolds seeded with primary ligament fibroblast cells has also been reported in the literature13.
Accordingly, a major weakness of the in vitro portion of this investigation was the absence of the addition of growth factors or stem cells to evaluate their effect on the cellular properties of our scaffold device. Also, we did not focus on the precollagen gene expression of the tenocyte-seeded nanofibers to investigate potential markers for successful tendon development. Thus, the resultant network of cells that proliferated on the matrices was not entirely characterized in regard to the cellular content. The addition of growth factors, application of marrow-derived mesenchymal stem cells, and exploration of genetic markers that are proven to detect enhanced tenocyte proliferation will be emphasized in subsequent studies that result from this preliminary work.
A major asset for this investigation was the development and validation of an original rodent rotator cuff model. Previous in vivo rotator cuff animal models have demonstrated the utility of the rodent model15-17. The rodent has several advantages that contribute to its popularity for rotator cuff models. For example, similar to human anatomy, there is an identifiable, prominent supraspinatus tendon that inserts onto the humerus and underlies an enclosed coracoacromial arch. In addition, previous studies have identified similarities in mechanical properties during motion. Unlike other animal species, the rodents use their arms for overhead reaching and grasping, adding to their attractiveness for rotator cuff study. Variety in the rodent model across investigators stems from factors such as the manner in which the investigated material is implanted (e.g., as a filler for a tendon defect or as an augmentation on top of a previous repair) and the inclusion or exclusion of postoperative shoulder immobilization. For our original model, we implemented a protocol for augmentation on top of a primary repair in order to maintain consistency with the current U.S. FDA approval for the commercially available matrices to be used only for augmentation of a repair already in place. Also, we chose not to immobilize the rats postoperatively. Our biomechanical studies showed surprisingly substantial increases in repair strength at the eight-week interval when augmentation with the scaffold device was used.
A weakness of our pilot in vivo study is that no substantial histological evaluation that characterized the composition of the repair site in the two major repair groups was performed. Thus, it is not possible to determine the nature of the augmented repair site and deduce any conclusions about the cause of the significant increases in biomechanical properties in this group. Furthermore, without adequate histological results, we cannot determine the presence or extent of a substantial inflammatory tissue response to the matrix, although the shoulder activity of the rodents appeared to recover without any gross complications. Again, subsequent in vivo models implementing our developed scaffold will emphasize histological characterizations of the implanted device.
The proposed nanostructured scaffold has shown promise as a potential replacement option for surgical repair of a torn rotator cuff. Optimized in vitro material characteristics and successful in vivo biological performance for tendon regeneration have been demonstrated. There is no question that a growing clinical need for innovative treatment modalities for rotator cuff repair exists. The use of a novel tissue-engineering nanostructure design approach will be a promising alternative. The current research project will advance construction of an ideal rotator cuff augmentation graft with long-term properties suitable for tendon replacement technology.
Acknowledgments
Note: The authors thank the members of the Laurencin Laboratories for their continued assistance and support of this investigation.
Footnotes
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from a National Institutes of Health Musculoskeletal Training Grant. Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity.
References
- 1.Pelinkovic D, Lee JY, Engelhardt M, Rodosky M, Cummins J, Fu FH, Huard J. Muscle cell-mediated gene delivery to the rotator cuff. Tissue Eng. 2003;9:143-51 [DOI] [PubMed] [Google Scholar]
- 2.Cofield RH. Rotator cuff disease of the shoulder. J Bone Joint Surg Am. 1985;67:974-9 [PubMed] [Google Scholar]
- 3.Yamaguchi K, Ditsios K, Middleton WD, Hildebolt CF, Galatz LM, Teefey SA. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am. 2006;88:1699-704 [DOI] [PubMed] [Google Scholar]
- 4.Bigiliani LU, Kimmel J, McCann PD, Wolfe I. Repair of rotator cuff tears in tennis players. Am J Sports Med. 1992;20:112-7 [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin HL. Lesions of the musculotendinous cuff of the shoulder: I. The exposure and treatment of tears with retraction. J Bone Joint Surg Am. 1944;26:31-51 [Google Scholar]
- 6.McLaughlin HL. Rupture of the rotator cuff. J Bone Joint Surg Am. 1962;44:979-83 [Google Scholar]
- 7.Baleani M, Ohman C, Guandalini L, Rotini R, Giavaresi G, Traina F, Viceconti M. Comparative study of different tendon grasping techniques for arthroscopic repair of the rotator cuff. Clin Biomech (Bristol, Avon). 2006;21:799-803 [DOI] [PubMed] [Google Scholar]
- 8.Aoki M, Okamura K, Fukushima S, Takahashi T, Ogino T. Transfer of latissimus dorsi for irreparable rotator-cuff tears. J Bone Joint Surg Br. 1996;78:761-6 [PubMed] [Google Scholar]
- 9.Gerber C. Latissimus dorsi transfer for the treatment of irreparable tears of the rotator cuff. Clin Orthop Relat Res. 1992;275:152-60 [PubMed] [Google Scholar]
- 10.Kimura A, Aoki M, Fukushima S, Ishii S, Yamakoshi K. Reconstruction of a defect of the rotator cuff with polytetrafluoroethylene felt graft. Recovery of tensile strength and histocompatibility in an animal model. J Bone Joint Surg Br. 2003;85:282-7 [DOI] [PubMed] [Google Scholar]
- 11.Bonutti PM, Cremens MJ. Use of direct introduction of allograft anchors for rotator cuff repair. Am J Orthop (Belle Mead NJ). 2005;34:97-9 [PubMed] [Google Scholar]
- 12.Aurora A, McCarron J, Iannotti JP, Derwin K. Commercially available extracellular matrix materials for rotator cuff repairs: state of the art and future trends. J Shoulder Elbow Surg. 2007;16(5 Suppl):S171-8 [DOI] [PubMed] [Google Scholar]
- 13.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613-21 [DOI] [PubMed] [Google Scholar]
- 14.Pelinkovic D, Lee JY, Engelhardt M, Rodosky M, Cummins J, Fu FH, Huard J. Muscle cell-mediated gene delivery to the rotator cuff. Tissue Eng. 2003;9:143-51 [DOI] [PubMed] [Google Scholar]
- 15.Peltz CD, Dourte LM, Kuntz AF, Sarver JJ, Kim SY, Williams GR, Soslowsky LJ. The effect of postoperative passive motion on rotator cuff healing in a rat model. J Bone Joint Surg Am. 2009;91:2421-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gulotta LV, Kovacevic D, Ehteshami JR, Dagher E, Packer JD, Rodeo SA. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009;37:2126-33 [DOI] [PubMed] [Google Scholar]
- 17.Iwata Y, Morihara T, Tachiiri H, Kajikawa Y, Yoshida A, Arai Y, Tokunaga D, Sakamoto H, Matsuda K, Kurokawa M, Kawata M, Kubo T. Behavior of host and graft cells in the early remodeling process of rotator cuff defects in a transgenic animal model. J Shoulder Elbow Surg. 2008;17(1 Suppl):101S-7S [DOI] [PubMed] [Google Scholar]