Abstract
Introduction
High-quality epidemiologic research is essential in reducing chronic diseases. We analyzed the quality of systematic reviews of observational nontherapeutic studies.
Methods
We searched several databases for systematic reviews of observational nontherapeutic studies that examined the prevalence of or risk factors for chronic diseases and were published in core clinical journals from 1966 through June 2008. We analyzed the quality of such reviews by using prespecified criteria and internal quality evaluation of the included studies.
Results
Of the 145 systematic reviews we found, fewer than half met each quality criterion; 49% reported study flow, 27% assessed gray literature, 2% abstracted sponsorship of individual studies, and none abstracted the disclosure of conflict of interest by the authors of individual studies. Planned, formal internal quality evaluation of included studies was reported in 37% of systematic reviews. The journal of publication, topic of review, sponsorship, and conflict of interest were not associated with better quality. Odds of formal internal quality evaluation (odds ratio [OR], 1.10 per year; 95% confidence interval [CI], 1.02-1.19) and either planned, formal internal quality evaluation or abstraction of quality criteria of included studies (OR, 1.17 per year; 95% CI, 1.08-1.26) increased over time, without positive trends in other quality criteria from 1990 through June 2008. Systematic reviews with internal quality evaluation did not meet other quality criteria more often than those that ignored the quality of included studies.
Conclusion
Collaborative efforts from investigators and journal editors are needed to improve the quality of systematic reviews.
Introduction
Valid epidemiologic research is essential in preventing chronic diseases (1-3). Assessing the quality of observational studies is an important part of evidence synthesis (4). Systematic reviews have become key tools in evidence synthesis from a growing number of epidemiologic studies (5). Producing high-quality systematic reviews is essential to developing generalizable and actionable conclusions (6,7). Quality criteria for systematic reviews have been proposed by working groups that developed the Meta-analysis of Observational Studies in Epidemiology (MOOSE), Strengthening the Reporting of Observational Studies in Epidemiology (STROBE), and a measurement tool for assessment of multiple systematic reviews (AMSTAR) (8-12). The working groups and the Cochrane handbook (13) addressed those criteria for systematic reviews that more likely result in biased results, including bias in selection of the studies or the information within studies by the reviewers (14-18) or bias in the publication of positive significant results (6,15,19,20).
Previous research and guidelines (13,21-23) focus on systematic reviews of interventional therapeutic studies. Validity of observational nontherapeutic studies of prevalence of chronic diseases or risk factors for diseases is essential for effective preventive public health actions (24,25). Our aim was to evaluate the quality of systematic reviews of observational nontherapeutic studies that examined the incidence and prevalence of chronic conditions and risk factors for diseases. The criteria we used to determine the reporting and methodologic quality in systematic reviews were from published standards (8-12). We hypothesized that the quality of systematic reviews differs by the time when the study was published, the country in which the study was conducted, the journal of publication, the sponsorship of the study, and whether a conflict of interest was disclosed. We hypothesized also that systematic reviews with internal quality evaluation of the included studies would have better quality, demonstrating commitment to quality of evidence.
Methods
Data sources
We searched MEDLINE via PubMed and via Ovid MEDLINE, the Cochrane Library (26) and working groups, WorldCat (27), and Scirus (28) to find systematic reviews of observational nontherapeutic studies published in English from 1966 through June 2008 in core clinical journals (exact search string is listed in Appendix Table 1). We used the definitions of core clinical journals from the Abridged Index Medicus (119 indexed titles). We defined observational nontherapeutic studies as observations of patient outcomes that did not examine procedures concerned with the remedial treatment or prevention of diseases (29).
Study selection
Three investigators independently decided on the eligibility of the studies according to recommendations from the Cochrane Handbook for Systematic Reviews of Interventions (13). We reviewed abstracts to exclude comments, expert opinions, letters, case reports, systematic reviews of interventional studies, and systematic reviews of studies of diagnostic accuracy of tests.
Data extraction
Evaluations of the studies and data extraction were performed independently by 2 researchers. Predefined categorical responses to the checklist items were abstracted into our spreadsheet. Errors in data extraction were assessed by a comparison of the data charts with the original articles (13,30). Any discrepancies were discussed and resolved. The quality criteria that we abstracted were based on guidelines for determining the reporting and methodologic quality of systematic reviews (8-12).
To evaluate selection bias, we abstracted whether the authors of systematic reviews described the search strategy (yes, no, or partially); yes indicated that the authors reported time periods of searches, searched databases, and exact search string. We abstracted whether the authors of systematic reviews described study flow (yes, no, or partially); yes indicated that the authors reported the list of retrieved citations, the list of excluded studies, and justification for exclusion.
We abstracted as dichotomous variables whether the authors of systematic reviews did any of the following:
Stated the aim of the review and the primary and secondary hypotheses of the review.
Included or justified exclusion of articles published in languages other than English.
Searched for gray literature, including abstracts and unpublished studies, to evaluate publication bias (21).
Described any contact with authors of the included studies.
Analyzed sponsorship of and conflict of interest in the included studies.
We abstracted how the authors of systematic reviews described obtained statistical methods with justification and models for pooling with fixed or random effects models in sufficient detail to be replicated (no pooling, random, or fixed). We abstracted whether the authors of pooling analyses reported statistical tests for heterogeneity and whether heterogeneity was statistically significant (not reported, not significant, or significant).
We used 3 categories to classify whether the authors of systematic reviews had evaluated the quality of included studies by using developed or previously published checklists or scales (31): 1) the authors stated planned, formal internal quality evaluations; 2) the authors abstracted selected criteria of external or internal validity without using a planned, formal, and comprehensive internal quality evaluation; and 3) the authors did not conduct internal quality evaluations. We further categorized the studies that evaluated quality criteria to compare studies with no mention of internal quality evaluation of the included studies. We also compared studies with and without planned formal internal quality evaluation. We abstracted with dichotomous responses blinding and reliability testing (reported or not reported) of internal quality evaluations.
We abstracted several explanatory variables that could be related to the quality of systematic reviews:
The year of publication, defined as a continuous variable. We created categories of 4- or 5-year periods: 1990 to 1994, 1995 to 1999, 2000 to 2004, and 2005 through June 2008.
The journals of publication.
The country where the systematic reviews were performed.
The sponsorship of the reviews. Those that had either governmental or foundational support or were fellowships were defined as having nonprofit support.
The disclosure of conflict of interest by authors of reviews (either not disclosed, disclosed as no conflict of interest, or disclosed conflict of interest).
The number of disclosed relationships with industry, defined as a continuous variable.
The sponsor's participation in data collection, analysis, and interpretation of the results of the review.
The review outcomes as risk factors for prevalence or incidence of chronic conditions or diseases.
Data synthesis
We summarized the results in evidence tables. We used prespecified categories of dependent and independent variables and did not force the data into binary categories for definitive tests of significance. We used univariate logistic regression to examine the association between internal quality evaluation and the year of the publication by using the Wald test. Odds ratios (ORs) were calculated with binary logit models and Fisher's scoring method technique. We computed the fractions of systematic reviews meeting various quality criteria in each of the 4 time periods considered. The proportions of systematic reviews that met different levels of each quality criterion were evaluated by using χ2 tests and Fisher's exact tests in cases of small numbers. All calculations were performed at 95% confidence intervals (CIs) by using 2-sided P values with SAS version 9.1.3 (SAS Institute Inc, Cary, North Carolina).
Results
We found 145 eligible systematic reviews of observational nontherapeutic studies (study flow in the Appendix Figure) (32-176). The number of published systematic reviews increased from 17 during 1990-1994 to 56 during 2005-2008. Most of the studies were conducted in the United States (55 publications) or in the United Kingdom (28 publications) (Appendix Table 2). Half of the systematic reviews (73 publications) were funded by nonprofit organizations; 56 (39%) reviews did not publish their funding sources, 4 reviews received industry support, and 10 were sponsored jointly by industry and nonprofit organizations. Almost three-fourths (106) of the authors of systematic reviews did not disclose conflict of interest; 35 publications stated that the authors do not have any conflict of interest; and 4 studies were conducted by authors who reported conflict of interest. The studies were published in 49 journals. Most systematic reviews (122 studies) assessed risk factors for chronic diseases, 19 summarized estimates of prevalence or incidence, 2 studies reported prevalence and associations with risk factors, and 2 studies examined levels of risk factors. Most studies reported incidence and risk factors for cardiovascular diseases (46 studies) or cancer (26 studies).
Quality of systematic reviews
Less than half of the studies reported study flow (49%), assessed gray literature (27%), or addressed language bias (29%) (Table 1). Only 2% of reviews abstracted sponsorship of individual studies and none abstracted the disclosure of conflict of interest by the authors of individual studies that were eligible for the reviews. Pooling was performed in 137 studies; of these, 62% used a random effects model; 57% reported detecting significant heterogeneity across the studies; and 19% did not provide any information about statistical heterogeneity in pooled estimates. The proportion of systematic reviews that met quality criteria including study flow, assessment of gray literature, or the abstraction of funding sources of included studies did not show significant trends from 1990 through 2008. The proportion of systematic reviews that assessed language bias increased from 8% during 1995-1999 to 41% during 2005-2008. In later years, more studies reported using random effects models (79% during 2005-2008 vs 39% during 1995-1999) and tests for statistical heterogeneity (89% during 2005-2008 vs 65% during 1995-1999).
Table 1.
Quality Criteria of Systematic Reviews of Observational Nontherapeutic Studies Published in Core Clinical Journals, by Year of Publication, 1990 Through June 2008
| Evaluated Criteria | 1990-1994, n (N = 17) | 1995-1999, n (N = 26) | 2000-2004, n (N = 46) | 2005-2008, n (N = 56) | Total, n (N = 145) | P Valuea |
|---|---|---|---|---|---|---|
| Literature search | ||||||
| No information | 0 | 0 | 1 | 0 | 1 | .7 |
| Documented partially | 1 | 1 | 3 | 1 | 6 | |
| Complete documenting of databases used, exact search strings used, and time periods of searches | 16 | 25 | 42 | 55 | 138 | |
| Contact with authors of the included studies | ||||||
| No information | 13 | 17 | 31 | 31 | 92 | .4 |
| The authors of the review attempted to contact the authors of included studies | 4 | 9 | 15 | 25 | 53 | |
| Study flow | ||||||
| Study flow not reported | 10 | 15 | 29 | 18 | 72 | .04 |
| Study flow partially reported | 0 | 0 | 0 | 2 | 2 | |
| Study flow reported with the list of retrieved citations, the list of excluded studies, and justification for exclusion for each study | 7 | 11 | 17 | 36 | 71 | |
| Articles published in languages other than English | ||||||
| Language bias was not addressed | 15 | 24 | 31 | 33 | 103 | .01 |
| Language bias was addressed: the authors included or justified exclusion of the non-English publications | 2 | 2 | 15 | 23 | 42 | |
| Gray literature | ||||||
| Gray literature was not assessed | 15 | 17 | 36 | 38 | 106 | .25 |
| Reporting of the method of handling abstracts and unpublished studies | 2 | 9 | 10 | 18 | 39 | |
| Conflict of interest from included studies | ||||||
| Conflict of interest in included studies was not abstracted | 17 | 26 | 46 | 56 | 145 | NA |
| Sponsorship of the included studies | ||||||
| Sponsorship of included studies was not analyzed | 16 | 25 | 46 | 55 | 142 | .45 |
| Sponsorship of included studies was analyzed | 1 | 1 | 0 | 1 | 3 | |
| Pooled model obtained in the review | ||||||
| Pooling was not obtained | 2 | 0 | 4 | 2 | 8 | <.001 |
| Fixed effects model was obtained for meta-analyses | 10 | 16 | 11 | 10 | 47 | |
| Random effects model was obtained for meta-analyses | 5 | 10 | 31 | 44 | 90 | |
| Heterogeneity across included studies | ||||||
| Heterogeneity across studies was not reported | 6 | 9 | 7 | 6 | 28 | .04 |
| Heterogeneity across studies was not significant | 5 | 6 | 13 | 11 | 35 | |
| Heterogeneity across studies was significant | 6 | 11 | 26 | 39 | 82 | |
| Formal internal quality evaluation of included studies | ||||||
| Planned, formal internal quality evaluation with developed or previously published checklists or scales | 3 | 6 | 20 | 25 | 54 | <.001 |
| Some selected criteria of external or internal quality of included studies were abstracted without planned, formal internal quality evaluation | 2 | 3 | 1 | 20 | 26 | |
| No internal quality evaluation | 12 | 17 | 25 | 11 | 65 | |
| Reliability of internal quality evaluation reported | 2 | 4 | 8 | 18 | 32 | .99 |
| Internal quality evaluation was masked | 1 | 1 | 0 | 1 | 3 | .11 |
Abbreviation: NA, not applicable.
P values for overall χ2 test.
Internal quality evaluation
Planned and detailed quality assessment of included studies was reported in 37% of systematic reviews, and 18% abstracted more than 1 criterion of external or internal quality; significant positive trends were reported during the evaluated time (Table 1). Quality assessment was masked in 3 studies. Development of the appraisals, including references to previously published tools, was reported in 32 studies, but only 6 tested interobserver agreement for quality assessment.
Quality of systematic review by explanatory factors
The quality of systematic reviews did not differ much by study location or by the journal of publication. Systematic reviews of prevalence or incidence or risk factors of the diseases did not differ in their quality measures. Sponsorship was not associated with quality of the reviews. The role of conflict of interest was impossible to establish because the authors of 56 reviews did not disclose funding and authors of 106 reviews did not disclose conflict of interest.
Explanatory factors of internal quality evaluation of included studies
The journal of publication, topic of the review, and continent where the review was conducted were not associated with the likelihood of internal quality evaluation. Systematic reviews of risk factors tended to conduct internal quality evaluation of the included studies more often than reviews of incidence or prevalence or of levels of risk factors. Systematic reviews sponsored by nonprofit organizations conducted internal quality evaluations of individual studies more often than reviews that received corporate funding. Systematic reviews that disclosed conflict of interest conducted internal quality evaluation of individual studies less frequently (10 of 39 studies; 26%) than reviews with no disclosure (44 of 106 studies; 42%). Odds of formal internal quality evaluation (OR, 1.10 per year; 95% CI, 1.02-1.19) and either planned, formal internal quality evaluation or abstraction of quality criteria (OR, 1.17 per year; 95% CI, 1.08-1.26) increased over time. Disclosure of conflict of interest by the authors of systematic reviews was not associated with greater odds of internal quality evaluation.
Quality of systematic reviews by internal quality evaluation
Complete documentation of the literature search including time period, databases searched, and exact literature search strings was less common among reviews with planned, formal internal quality evaluation (48 studies, 35%) than among reviews without it (90 studies, 65%) (Table 2). However, reviews that either abstracted selected quality criteria or planned, formal internal quality evaluation reported partial (6 studies) or complete (74 studies) information about the literature search more often than studies that did not evaluate quality of included studies (64 studies). Reviews that did not justify exclusion of non-English studies ignored quality of individual studies more often (72 studies) than reviews with planned, formal internal quality evaluation (31 studies). The same pattern was present for publication bias: the reviews that did not mention gray literature also ignored the quality of individual studies. The reviews reporting attempts to contact the authors of included studies either performed planned, formal internal quality evaluation or abstracted selected quality criteria more often than reviews without such attempts (OR, 2.3; 95% CI, 1.1-4.7). Reviews with complete reporting of study flow performed planned, formal internal quality evaluation or abstracted quality criteria more often (51 studies) than reviews without study flows (20 studies). More than half of systematic reviews without planned, formal internal quality evaluation (44 studies) also did not report study flow.
Table 2.
Quality of Systematic Reviews, by Internal Quality Evaluation of Included Studies, 1990 Through June 2008
| Quality Criterion | Definition of Formal Internal Quality Evaluation | |||
|---|---|---|---|---|
| Planned, Formal Internal Quality Evaluation or Abstraction of Some Quality Criteria, n | Neither Planned, Formal Internal Quality Evaluation nor Abstraction of Some Quality Criteria, n | Planned, Formal Internal Quality Evaluation, n | No Planned, Formal Internal Quality Evaluation, n | |
| Literature search | P = .04a | P = .004b | ||
| No information | 0 | 1 | 0 | 1 |
| Documented partially | 6 | 0 | 6 | 0 |
| Complete documenting of databases used, exact search strings used, and time periods of searches | 74 | 64 | 48 | 90 |
| Contact with authors of the included studies | P = .02a | P = .25b | ||
| No information | 44 | 48 | 31 | 61 |
| The authors of the review attempted to contact the authors of included studies | 36 | 17 | 23 | 30 |
| Study flow | P < .001a | P = .003b | ||
| Study flow not reported | 28 | 44 | 17 | 55 |
| Study flow partially reported | 1 | 1 | 1 | 1 |
| Study flow reported with the list of retrieved citations, the list of excluded studies, and justification for exclusion for each study | 51 | 20 | 36 | 35 |
| Articles published in languages other than English | P = .001a | P = .01b | ||
| No information | 48 | 55 | 31 | 72 |
| Inclusion of non-English studies or justification for exclusion | 32 | 10 | 23 | 19 |
| Gray literature | P = .09a | P = .04b | ||
| No information | 54 | 52 | 34 | 72 |
| Reporting of the method of handling abstracts and unpublished studies | 26 | 13 | 20 | 19 |
| Conflict of interest from included studies | ||||
| No information | 80 | 65 | 54 | 91 |
| Sponsorship of the included studies | P = .44a | P = .18b | ||
| No information | 79 | 63 | 54 | 88 |
| Sponsorship of included studies was abstracted | 1 | 2 | 0 | 3 |
| Pooled model obtained in the review | P < .001a | P = .06b | ||
| Not applicable (no pooling) | 6 | 2 | 5 | 3 |
| Fixed effects model | 15 | 32 | 12 | 35 |
| Random effects model | 59 | 31 | 37 | 53 |
| Heterogeneity across included studies | P = .27a | P = .67b | ||
| Not reported | 13 | 15 | 9 | 19 |
| Heterogeneity was not significant | 17 | 18 | 15 | 20 |
| Heterogeneity was significant at least for one association | 50 | 32 | 30 | 52 |
P value for overall χ2 test between planned, formal internal quality evaluation or abstraction of some quality criteria versus neither planned, formal internal quality evaluation nor abstraction of some quality criteria.
P value for overall χ2 test between planned, formal internal quality evaluation versus no planned, formal internal quality evaluation.
The association between quality of systematic reviews and sponsor participation in the data collection, analyses, and interpretation was difficult to analyze because this information was either omitted or reported in various ways. Less than 10% of systematic reviews contained a clear statement that the sponsors did not play any role in gathering the studies or analyzing or interpreting the results and did not influence the content of the manuscript. Other reviews omitted mention of the role of the sponsor in approval of the manuscript or provided a general statement that sponsors did not influence the conclusions or the content of the paper. Two reviews included statements of unconditional or unrestricted sponsorship of the meta-analyses.
Discussion
Our analyses showed that less than half of the systematic reviews of nontherapeutic observational studies that were published in core clinical journals met each quality criterion. Quality of systematic reviews did not improve over time. Planned, formal internal quality evaluations of the included studies was reported in less than half of systematic reviews, but the prevalence of internal quality evaluations has increased during the last decade. Our findings are in concordance with previously published methodologic analyses of systematic reviews that also found inconsistent quality and incomplete internal quality evaluation of individual studies (6). Methodologic analyses of systematic reviews that focused on particular diseases or conditions demonstrated that half of the publications had major flaws in design and reporting. For instance, systematic reviews of therapies for renal diseases failed to assess the methodologic quality of included studies (177). Methodologic analyses of systematic reviews of interventions showed that 69% of those randomly selected in MEDLINE meta-analyses did not analyze quality of trials (22). Most (68%) systematic reviews of diagnostic tests for cancer did not provide formal assessments of study quality (178). We also found that the quality of reviews did not differ among types of studies (incidence or risk factors for diseases), types of diseases, or journal of publication.
Journal commitment to high-quality research, however, was associated with improved reporting quality of the publications. For example, adoption by journals of the Consolidated Standards of Reporting Trials (CONSORT) improved the quality of the publications of interventional studies (179,180). An endorsement of the developed standards for observational studies including MOOSE and STROBE checklists may also improve quality of the publications. We did not analyze how many core clinical journals adopted these standards and how quality of the publications changed depending on this adaptation. Peer review of submitted manuscripts should include quality assessment using validated tools (12).
We could not identify the factors that can explain differences in quality of systematic reviews. The role of sponsorship and conflict of interest could not be estimated because of poor reporting of this information. The quality and reliability of quality evaluation of the included studies is unclear because development of the appraisals was described in a small proportion of systematic reviews (32 of 80 studies), and only 6 of 80 studies tested interobserver agreement for quality assessment. We did not evaluate all reviews of observational studies that were published in epidemiologic journals. However, it is unlikely that the quality of reviews published in other journals would be better than those in core clinical journals. Future research should investigate the factors that can explain differences in the quality of systematic reviews.
Peer reviewed publications of high-quality systematic reviews can provide the best available research evidence for evidence-based public health (24). Evidence-based decisions can improve public health practice in preventing incidence and progression of chronic diseases (25). In our analysis, less than half of the systematic reviews of observational nontherapeutic studies met quality criteria established in the MOOSE, STROBE, and AMSTAR statements. Internal quality evaluation of included studies should be an essential part of evidence synthesis, but only half of the reviews reported such evaluation. Collaborative efforts from investigators and journal editors are needed to improve quality of systematic reviews.
Acknowledgments
This article is based on research conducted by the Minnesota Evidence-based Practice Center under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, Maryland (contract no. 290-02-0009).
We thank our reviewers David Atkins, MD, John Hoey, MD, and Christine Laine, MD, for reviewing and commenting on the draft; our collaborating experts, Mohammed Ansari, MBBS, Ethan Balk, MD, Nancy Berkman, PhD, Chantelle Garritty, Mark Grant, MD, Gail Janes, PhD, Margaret Maglione, MPP, David Moher, PhD, Mona Nasser, DDS, Gowri Raman, MD, Karen Robinson, MD, Jodi Segal, MD, and Thomas Trikalinos, PhD, for their scientific input throughout this project; and Carmen Kelly, PharmD, our task order officer, and Stephanie Chang, MD, medical officer, at AHRQ for their guidance throughout the project. We also thank librarian Judith Stanke for her contributions to the literature search; research assistants Emily Zabor, candidate for the master of science degree (MS) in biostatistics, and Akweley Ablorh, candidate for MS in biostatistics, for the data abstraction, quality control, and synthesis of evidence; Zhihua Bian, candidate for MS in biostatistics, for her statistical help; Zhiyuan Xu, candidate for MS in applied economics, for his work creating the ACCESS database; Dean McWilliams for his assistance in database development; Qi Wang, research fellow, for her statistical expertise in reliability testing; Susan Duval, PhD, for her help estimating sample size; Marilyn Eells for editing and formatting the report; and Nancy Russell, MLS, and Rebecca Schultz for their assistance gathering data from the experts and formatting the tables, and Christa Prodzinski for quality control of the data.
Appendix
Table 1.
Search Strategy and Exact Search Strings Used to Identify Systematic Reviews of Observational Studies, Scales and Checklists for Internal Quality Evaluation, and Studies About Bias in Observational Research, 1966 Through June 2008
| Search Method | No. of Articles Identified |
|---|---|
| Search strategy for Ovid MEDLINE | |
| 1. exp Research Design/st [Standards] | 4,303 |
| 2. exp Chronic Disease/ep [Epidemiology] | 1,619 |
| 3. exp Urinary Incontinence/ep [Epidemiology] | 1,155 |
| 4. exp Fecal Incontinence/ep [Epidemiology] | 328 |
| 5. exp "Sleep Initiation and Maintenance Disorders"/ep [Epidemiology] | 565 |
| 6. exp Depression/ep [Epidemiology] | 4,700 |
| 7. exp Depressive Disorder/ep [Epidemiology] | 6,816 |
| 8. exp Myocardial Infarction/ | 43,531 |
| 9. 6 or 7 | 11,214 |
| 10. 8 and 9 | 105 |
| 11. 2 or 3 or 4 or 5 or 10 | 3,636 |
| 12. 1 and 11 | 9 |
| 13. exp Data Collection/mt, st [Methods, Standards] | 36,173 |
| 14. exp "Bias (Epidemiology)"/ | 25,369 |
| 15. exp Questionnaires/st [Standards] | 3,879 |
| 16. exp Evidence-Based Medicine/ | 27,487 |
| 17. 13 or 14 or 15 or 16 | 86,857 |
| 18. 11 and 17 | 127 |
| 19. 12 or 18 | 133 |
| 20. limit 19 to english language | 124 |
| 21. exp "Predictive Value of Tests"/ | 62,290 |
| 22. exp "Reproducibility of Results"/ | 126,475 |
| 23. 21 or 22 | 182,941 |
| 24. 11 and 23 | 126 |
| 25. limit 24 to english language | 121 |
| 26. 20 or 25 | 224 |
| 27. exp randomized controlled trial/ | 151,027 |
| 28. 11 and 27 | 74 |
| 29. exp research design/ | 134,468 |
| 30. 28 and 29 | 15 |
| 31. 1 and 16 | 547 |
| 32. ep.fs. | 434,923 |
| 33. exp epidemiology/ | 6,500 |
| 34. 32 or 33 | 437,784 |
| 35. 31 and 34 | 29 |
| 36. exp incidence/ | 81,260 |
| 37. exp prevalence/ | 83,713 |
| 38. 36 or 37 | 157,239 |
| 39. 31 and 38 | 14 |
| 40. 26 or 30 or 35 or 39 | 268 |
| 41. limit 40 to english language | 267 |
| 42. limit 41 to journal article | 251 |
| 43. from 42 keep 1-251 | 251 |
| MEDLINE search via PubMed | |
| ("Biomedical Research/methods"[MeSH] OR "Biomedical Research/organization and administration"[MeSH] OR "Biomedical Research/standards"[MeSH] OR "Biomedical Research/statistics and numerical data"[MeSH] OR "Biomedical Research/trends"[MeSH]) Limits: Humans, Journal Article, English | 3,703 |
| "Epidemiologic Studies"[MeSH] AND "Research Design/standards"[MeSH] AND ("Evaluation Studies as Topic/classification"[MeSH] OR "Evaluation Studies as Topic/methods"[MeSH] OR "Evaluation Studies as Topic/standards"[MeSH]) Limits: Humans, Journal Article, English | 59 |
| "Publishing/standards"[MeSH] AND "Epidemiologic Methods"[MeSH] AND "Research Design/standards"[MeSH] Limits: Humans, Journal Article, English | 65 |
| "STROBE Initiative"[Corporate Author] | 10 |
| "Bias (Epidemiology)"[MeSH] AND "Epidemiologic Studies"[MeSH] AND "Epidemiologic Methods"[MeSH] AND "Research Design/standards"[MeSH] Limits: Humans, Journal Article, English | 97 |
| "Evidence-Based Medicine"[MeSH] AND "Epidemiologic Studies"[MeSH] AND "Epidemiologic Methods"[MeSH] AND "Research Design/standards"[MeSH] Limits: Humans, Journal Article, English | 25 |
| "Research Design/standards"[MeSH] AND "Epidemiologic Studies"[MeSH] AND "Epidemiologic Measurements"[MeSH] AND "Bias (Epidemiology)"[MeSH] Limits: Humans, Journal Article, English AND "Incidence"[MeSH] Limits: Humans, Journal Article, English | 8 |
| "Research Design/standards"[MeSH] AND "Epidemiologic Studies"[MeSH] AND "Epidemiologic Measurements"[MeSH] AND "Bias (Epidemiology)"[MeSH] Limits: Humans, Journal Article, English AND "Prevalence"[MeSH] Limits: Humans, Journal Article, English | 7 |
| ("Prevalence"[MeSH]) AND systematic[sb] "Working group" Limits: English | 15 |
| [CN] Limits: Humans, Meta-Analysis, English, Core clinical journals | 2 |
| ("Prevalence"[MeSH]) AND systematic[sb] Limits: Humans, Meta-Analysis, English, Core clinical journals | 83 |
| Moher D[author] | 198 |
| "Epidemiologic Studies"[MeSH] Limits: Humans, Meta-Analysis, English AND "Incidence"[MeSH] Limits: Humans, Meta-Analysis, English Limits: Humans, Meta-Analysis, English, Core clinical journals | 57 |
| "Epidemiologic Studies"[MeSH] AND "Incidence"[MeSH] Limits: Humans, Meta-Analysis, English | 236 |
| "Epidemiologic Studies"[MeSH] AND "Incidence"[MeSH] AND Evidence Limits: Humans, Meta-Analysis, English | 52 |
| "Incidence"[MeSH] Limits: Humans, Meta-Analysis, English | 635 |
| "Risk"[MeSH] AND "Epidemiologic Studies"[MeSH] Limits: Humans, Meta-Analysis, English, Core clinical journals | 273 |
| "Prevalence"[MeSH] Limits: Humans, Meta-Analysis, English, Core clinical journals | 84 |
| Altman DG[author] | 7 |
| Higgins J[author] | 3 |
| "Review Literature as Topic"[MeSH] AND "Research Design/standards"[MeSH] AND "Epidemiologic Studies"[MeSH] Limits: Humans, English, Core clinical journals | 0 |
| "Review Literature as Topic"[MeSH] AND "Epidemiologic Studies"[MeSH] AND "Quality control"[MeSH] | 1 |
| "Incidence"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Peer Review, Research"[MeSH] AND "Research Design/standards"[MeSH] | 0 |
| "Incidence"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Peer Review, Research"[MeSH] | 0 |
| "Incidence"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Research Design/standards"[MeSH] | 0 |
| "Incidence"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND ("Data Collection/methods"[MeSH] OR "Data Collection/standards"[MeSH]) | 5 |
| "Incidence"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Bias (Epidemiology)"[MeSH] | 1 |
| "Incidence"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND ("Questionnaires/methods"[MeSH] OR "Questionnaires/standards"[MeSH]) | 0 |
| "Incidence"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Evidence-Based Medicine"[MeSH] | 2 |
| "Incidence"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Reproducibility of Results"[MeSH] | 3 |
| "Prevalence"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Peer Review, Research"[MeSH] AND "Research Design/standards"[MeSH] | 0 |
| "Prevalence"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Peer Review, Research"[MeSH] | 0 |
| "Prevalence"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Research Design/standards"[MeSH] | 0 |
| "Prevalence"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND ("Data Collection/methods"[MeSH] OR "Data Collection/standards"[MeSH]) | 16 |
| "Prevalence"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Bias (Epidemiology)"[MeSH] | 6 |
| "Prevalence"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND ("Questionnaires/methods"[MeSH] OR "Questionnaires/standards"[MeSH]) | 1 |
| "Prevalence"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Evidence-Based Medicine"[MeSH] | 0 |
| "Prevalence"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Reproducibility of Results"[MeSH] | 12 |
| "Risk Factors"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Peer Review, Research"[MeSH] AND "Research Design/standards"[MeSH] | 0 |
| "Risk Factors"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Peer Review, Research"[MeSH] | 0 |
| "Risk Factors"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Research Design/standards"[MeSH] | 1 |
| "Risk Factors"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND ("Data Collection/methods"[MeSH] OR "Data Collection/standards"[MeSH]) | 18 |
| "Risk Factors"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Bias (Epidemiology)"[MeSH] | 7 |
| "Risk Factors"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND ("Questionnaires/methods"[MeSH] OR "Questionnaires/standards"[MeSH]) | 1 |
| "Risk Factors"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Evidence-Based Medicine"[MeSH] | 4 |
| "Risk Factors"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Reproducibility of Results"[MeSH] | 10 |
| "Health Care Quality, Access, and Evaluation"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Peer Review, Research"[MeSH] AND "Research Design/standards"[MeSH] | 0 |
| "Health Care Quality, Access, and Evaluation"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Peer Review, Research"[MeSH] | 0 |
| "Health Care Quality, Access, and Evaluation"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Research Design/standards"[MeSH] | 4 |
| "Health Care Quality, Access, and Evaluation"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Evidence-Based Medicine"[MeSH] | 8 |
| "Health Care Quality, Access, and Evaluation"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Bias (Epidemiology)"[MeSH] | 33 |
| "Models, Statistical"[MeSH] AND "Risk Factors"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Research Design/standards"[MeSH] | 0 |
| "Models, Statistical"[MeSH] AND "Incidence"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Research Design/standards"[MeSH] | 0 |
| "Models, Statistical"[MeSH] AND "Prevalence"[MeSH] AND "Chronic Disease/epidemiology"[MeSH] AND "Research Design/standards"[MeSH] | 0 |
| "Epidemiologic Studies"[MeSH] AND "Models, Statistical"[MeSH] AND "Research Design/standards"[MeSH] | 47 |
| "Prevalence"[MeSH] AND "Epidemiologic Studies"[MeSH] AND "Models, Statistical"[MeSH] AND "Bias (Epidemiology)"[MeSH] | 61 |
| "Incidence"[MeSH] AND "Epidemiologic Studies"[MeSH] AND "Models, Statistical"[MeSH] AND "Bias (Epidemiology)"[MeSH] | 66 |
| "Research Design/standards"[MeSH] AND ("Biomedical Research/methods"[MeSH] OR "Biomedical Research/organization and administration"[MeSH] OR "Biomedical Research/standards"[MeSH] OR "Biomedical Research/statistics and numerical data"[MeSH] OR "Biomedical Research/trends"[MeSH]) Limits: Humans, Journal Article, English | 62 |
Abbreviations: MeSH, Medical Subject Heading term; sb, subset; CN, corporate author.
Figure 1.
Study flow to identify systematic reviews of observational studies, scales, and checklists for planned formal internal quality evaluation, and studies about bias in observational research, 1990 through June 2008.
| This flow chart shows 3 boxes. The top box reads |
| Total references — 1,053 |
|
| It is connected by a vertical line to 2 additional boxes. The left box reads |
| Included studies — 397 |
|
| The right box reads |
| Excluded studies — 656 |
|
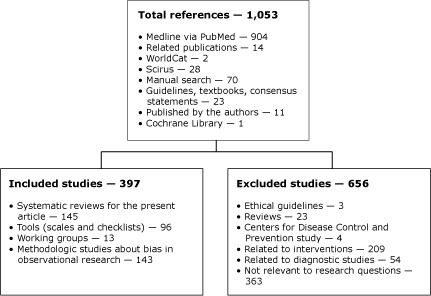
Table 2.
Quality of Systematic Review and Meta-Analyses of Nontherapeutic Observational Studies Published in Core Clinical Journals, 1990 through June 2008
| Publication Characteristics | Outcome | Estimate | Assessment of Quality of Included Studies |
|---|---|---|---|
|
Bracken, 1990 (32)
Country: United States Journal: Obstet Gynecol Sponsorship: Not reported Conflict of interest (COI): Not reported Sponsor participation in data analyses: Not reported |
Congenital malformations in offspring | Risk | No |
|
Romieu et al, 1990 (33)
Country: United States Journal: Cancer Sponsorship: Government COI: Not reported Sponsor participation in data analyses: Not reported |
Breast cancer | Risk | Quality criteria abstracted |
|
Haughey et al, 1992 (34)
Country: United States Journal: Ann Otol Rhinol Laryngol Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Second malignant tumors in head and neck cancer | Risk | No |
|
Lemon et al, 1992 (35)
Country: United States Journal: Cancer Sponsorship: Government COI: Not reported Sponsor participation in data analyses: Not reported |
Nonfamilial breast cancer | Continuous variable | No |
|
McKenna, 1992 (36)
Country: United Kingdom Journal: Am J Med Sponsorship: Nonprofit organization, nursing home COI: Not reported Sponsor participation in data analyses: Not reported |
Differences in vitamin D status | Prevalence | Quality criteria abstracted |
|
Morris et al, 1992 (37)
Country: United States Journal: Am J Public Health Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Cancer | Risk | Yes |
|
Myers and Basinski, 1992 (38)
Country: Canada Journal: Arch Intern Med Sponsorship: Nonprofit organization, award COI: Not reported Sponsor participation in data analyses: Not reported |
Coronary heart disease | Risk | No |
|
Becker et al, 1993 (39)
Country: United States Journal: Ann Emerg Med Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Survival of cardiac arrest | Risk | No |
|
Brownson et al, 1993 (40)
Country: United States Journal: Arch Intern Med Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Adult leukemia | Risk | Yes |
|
Ernst and Resch, 1993 (41)
Country: Austria Journal: Ann Intern Med Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Cardiovascular risk factor | Risk | No |
|
Katerndahl, 1993 (42)
Country: United States Journal: J Nerv Ment Dis Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Panic disorder and mitral valve prolapse | Risk | Yes |
|
Harris and Barraclough, 1994 (43)
Country: United Kingdom Journal: Medicine Sponsorship: Industry COI: Not reported Sponsor participation in data analyses: Not reported |
Suicide | Risk | No |
|
Kawachi et al, 1994 (44)
Country: United States Journal: Br Heart J Sponsorship: Industry, scholarship COI: Not reported Sponsor participation in data analyses: Not reported |
Coronary heart disease | Risk | No |
|
Law et al, 1994 (45)
Country: United Kingdom Journal: BMJ Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Hazards of reducing serum cholesterol | Risk | No |
|
Law et al, 1994 (46)
Country: United Kingdom Journal: BMJ Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Ischemic heart disease | Risk | No |
|
Steffen et al, 1994 (47)
Country: Switzerland Journal: JAMA Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Hepatitis A | Risk | No |
|
Zhang and Begg, 1994 (48)
Country: United States Journal: Int J Epidemiol Sponsorship: Government COI: Not reported Sponsor participation in data analyses: Not reported |
Cervical neoplasia | Risk | No |
|
Everhart and Wright, 1995 (49)
Country: United States Journal: JAMA Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Pancreatic cancer | Risk | No |
|
Feinberg et al, 1995 (50)
Country: United States Journal: Arch Intern Med Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Atrial fibrillation | Prevalence | No |
|
Ritchie and Kildea, 1995 (51)
Country: France Journal: Lancet Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Senile dementia | Prevalence | No |
|
Raman-Wilms et al, 1995 (52)
Country: Canada Journal: Obstet Gynecol Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Fetal genital effects | Risk | No |
|
Hatsukami and Fischman, 1996 (53)
Country: United States Journal: JAMA Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Use of crack cocaine and cocaine hydrochloride | Prevalence | No |
|
Hill and Schoener, 1996 (54)
Country: United States Journal: Am J Psychiatry Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Attention deficit hyperactivity disorder | Prevalence | No |
|
Hackshaw et al, 1997 (55)
Country: United Kingdom Journal: BMJ Sponsorship: Government COI: Reported as not a conflict of interest Sponsor participation in data analyses: "The views expressed are those of the authors and not necessarily those of the Department of Health." |
Lung cancer | Risk | No |
|
Kluijtmans et al, 1997 (56)
Country: Netherlands Journal: Circulation Sponsorship: Nonprofit organization, industry COI: Not reported Sponsor participation in data analyses: Not reported |
Coronary artery disease | Risk | No |
|
Law and Hackshaw, 1997 (57)
Country: United Kingdom Journal: BMJ Sponsorship: None COI: Reported as not a conflict of interest Sponsor participation in data analyses: None |
Hip fracture | Risk | No |
|
Law et al, 1997 (58)
Country: United Kingdom Journal: BMJ Sponsorship: Government COI: Reported as not a conflict of interest Sponsor participation in data analyses: "The Department of Health (England) supported this work, although the views are our own." |
Ischemic heart disease | Risk | No |
|
Danesh et al, 1998 (59)
Country: United Kingdom Journal: JAMA Sponsorship: Scholarship, nonprofit organization COI: Not reported Sponsor participation in data analyses: Not reported |
Coronary heart disease | Risk | Yes |
|
French and Brocklehurst, 1998 (60)
Country: United Kingdom Journal: Br J Obstet Gynaecol Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Survival in women infected with human immunodeficiency virus | Risk | Yes |
|
Forgie et al, 1998 (61)
Country: Canada Journal: Arch Intern Med Sponsorship: Industry, government, fellowships, nonprofit organization COI: Not reported Sponsor participation in data analyses: Not reported |
Allogeneic blood transfusion | Risk | No |
|
Huang et al, 1998 (62)
Country: Canada Journal: Gastroenterology Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Gastric cancer | Risk | Yes |
|
Johnston et al, 1998 (63)
Country: United States Journal: Neurology Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Subarachnoid hemorrhage | Risk | No |
|
Lazarou et al, 1998 (64)
Country: Canada Journal: JAMA Sponsorship: Scholarship, nonprofit organization COI: Not reported Sponsor participation in data analyses: Not reported |
Adverse drug reactions in hospitalized patients | Prevalence | Quality criteria abstracted |
|
Ray, 1998 (65)
Country: Canada Journal: Arch Intern Med Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Venous thromboembolic disease | Risk | Quality criteria abstracted |
|
Spencer-Green, 1998 (66)
Country: United States Journal: Arch Intern Med Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Secondary diseases from primary Reynaud phenomenon | Risk | Yes |
|
Stratton et al, 1998 (67)
Country: United Kingdom Journal: Br J Obstet Gynaecol Sponsorship: Research fellowship, nonprofit organization COI: Not reported Sponsor participation in data analyses: Not reported |
Ovarian cancer | Risk | No |
|
Zock and Katan, 1998 (68)
Country: Netherlands Journal: Am J Clin Nutr Sponsorship: Nonprofit organization COI: Not reported Sponsor participation in data analyses: Not reported |
Breast, colorectal, and prostate cancer | Risk | Quality criteria abstracted |
|
Zondervan et al, 1998 (69)
Country: United Kingdom Journal: Br J Obstet Gynaecol Sponsorship: Nonprofit organization COI: Not reported Sponsor participation in data analyses: Not reported |
Chronic pelvic pain in women | Prevalence | No |
|
Angelillo and Villari, 1999 (70)
Country: Italy Journal: Bull World Health Organ Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Childhood leukemia | Risk | Yes |
|
He et al, 1999 (71)
Country: United States Journal: N Engl J Med Sponsorship: Nonprofit organization COI: Not reported Sponsor participation in data analyses: Not reported |
Coronary heart disease | Risk | No |
|
Shaffer et al, 1999 (72)
Country: United States Journal: Am J Public Health Sponsorship: Nonprofit organization COI: Not reported Sponsor participation in data analyses: Not reported |
Disordered gambling behavior | Prevalence | No |
|
Wittrup et al, 1999 (73)
Country: Denmark Journal: Circulation Sponsorship: Government COI: Not reported Sponsor participation in data analyses: Not reported |
Ischemic heart disease | Risk | Yes |
|
Yoder et al, 1999 (74)
Country: United States Journal: Obstet Gynecol Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Fetus with isolated choroid plexus cysts | Risk | No |
|
Christen et al, 2000 (75)
Country: United States Journal: Arch Intern Med Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Cardiovascular disease | Risk | Yes |
|
Cleophas et al, 2000 (76)
Country: Netherlands Journal: Am J Cardiol Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Coronary artery disease | Risk | Yes |
|
DiMatteo et al, 2000 (77)
Country: United States Journal: Arch Intern Med Sponsorship: Industry, scholarship COI: Not reported Sponsor participation in data analyses: Not reported |
Noncompliance with medical treatment | Risk | Quality criteria abstracted |
|
WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality, 2000 (78)
Country: Brazil Journal: Lancet Sponsorship: Nonprofit organization COI: Not reported Sponsor participation in data analyses: Not reported |
Infant and child mortality | Risk | No |
|
Wilson et al, 2000 (79)
Country: Canada Journal: Arch Intern Med Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Mortality after myocardial infarction | Risk | Yes |
|
Zeegers et al, 2000 (80)
Country: Netherlands Journal: Cancer Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Urinary tract cancer | Risk | Yes |
|
Danesh et al, 2001 (81)
Country: United Kingdom Journal: Circulation Sponsorship: Government, scholarship COI: Not reported Sponsor participation in data analyses: Not reported |
Coronary heart disease | Risk | No |
|
Eaden et al, 2001 (82)
Country: United Kingdom Journal: Gut Sponsorship: Nonprofit organization COI: Not reported Sponsor participation in data analyses: Not reported |
Colorectal cancer | Risk | Yes |
|
Faraone et al, 2001 (83)
Country: United States Journal: Am J Psychiatry Sponsorship: Government COI: Not reported Sponsor participation in data analyses: Not reported |
Attention deficit hyperactivity disorder | Risk | Yes |
|
Horta et al, 2001 (84)
Country: Brazil Journal: Am J Public Health Sponsorship: Government COI: Not reported Sponsor participation in data analyses: Not reported |
Early weaning | Risk | Yes |
|
Rebora, 2001 (85)
Country: Italy Journal: Arch Dermatol Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Coronary artery disease | Risk | Yes |
|
Cannon et al, 2002 (86)
Country: United Kingdom Journal: Am J Psychiatry Sponsorship: Research fellowship, nonprofit organization COI: Not reported Sponsor participation in data analyses: Not reported |
Schizophrenia | Risk | No |
|
Hellermann et al, 2002 (87)
Country: United States Journal: Am J Med Sponsorship: Government, nonprofit organization, fellowship COI: Not reported Sponsor participation in data analyses: Not reported |
Heart failure | Risk | No |
|
Huang et al, 2002 (88)
Country: Canada Journal: Lancet Sponsorship: Not reported COI: Reported as not a conflict of interest Sponsor participation in data analyses: Not reported |
Peptic-ulcer disease | Risk | Yes |
|
Huncharek et al, 2002 (89)
Country: United States Journal: Am J Public Health Sponsorship: Nonprofit organization, industry COI: Not reported Sponsor participation in data analyses: Not reported |
Malignant melanoma | Risk | Yes |
|
Juul et al, 2002 (90)
Country: Denmark Journal: Blood Sponsorship: Government, nonprofit organization COI: Not reported Sponsor participation in data analyses: "They had no role in gathering, analyzing, or interpreting the data and had no right to approve or disapprove the submitted paper." |
Factor V Leiden | Risk | Yes |
|
Kelly et al, 2002 (91)
Country: United States Journal: Neurology Sponsorship: Nonprofit organization, industry, fellowship COI: Not reported Sponsor participation in data analyses: Not reported |
Risk of ischemic stroke | Risk | No |
|
Klerk et al, 2002 (92)
Country: Netherlands Journal: JAMA Sponsorship: Government, "public/private partnership" COI: Not reported Sponsor participation in data analyses: Not reported |
Coronary heart disease | Risk | Yes |
|
Kozer et al, 2002 (93)
Country: Canada Journal: Am J Obstet Gynecol Sponsorship: Industry COI: Not reported Sponsor participation in data analyses: Not reported |
Congenital anomalies | Risk | No |
|
Law et al, 2002 (94)
Country: United Kingdom Journal: Arch Intern Med Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Death after myocardial infarction | Risk | No |
|
Wald et al, 2002 (95)
Country: United Kingdom Journal: BMJ Sponsorship: None COI: Reported as not a conflict of interest Sponsor participation in data analyses: None |
Cardiovascular disease | Risk | No |
|
Wald and Link, 2002 (96)
Country: United States Journal: J Infect Dis Sponsorship: Government COI: Not reported Sponsor participation in data analyses: Not reported |
Human immunodeficiency virus infection | Risk | No |
|
Benjamin et al, 2003 (97)
Country: United States Journal: Pediatrics Sponsorship: Government COI: Not reported Sponsor participation in data analyses: Not reported |
End-organ damage | Prevalence | No |
|
Clarfield, 2003 (98)
Country: Israel Journal: Arch Intern Med Sponsorship: Not reported COI: Reported as not a conflict of interest Sponsor participation in data analyses: Not reported |
Reversible dementias | Prevalence | No |
|
Cole and Dendukuri, 2003 (99)
Country: Canada Journal: Am J Psychiatry Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Depression among elderly community subjects | Risk | Yes |
|
Gisbert et al, 2003 (100)
Country: Spain Journal: Gastroenterology Sponsorship: Nonprofit organization COI: Not reported Sponsor participation in data analyses: Not reported |
Hepatitis C virus infection | Risk | Yes |
|
Glatt et al, 2003 (101)
Country: United States Journal: Am J Psychiatry Sponsorship: Government COI: Not reported Sponsor participation in data analyses: Not reported |
Schizophrenia | Risk | No |
|
Halbert et al, 2003 (102)
Country: United States Journal: Chest Sponsorship: Industry COI: Not reported Sponsor participation in data analyses: Not reported |
Prevalence estimates for chronic obstructive pulmonary disease | Prevalence | No |
|
Huang et al, 2003 (103)
Country: Canada Journal: Gastroenterology Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Gastric cancer | Risk | Yes |
|
Rey et al, 2003 (104)
Country: Canada Journal: Lancet Sponsorship: Government COI: Reported as not a conflict of interest Sponsor participation in data analyses: Not reported |
Fetal loss | Risk | Yes |
|
Riboli and Norat, 2003 (105)
Country: France Journal: Am J Clin Nutr Sponsorship: Government COI: Reported as not a conflict of interest Sponsor participation in data analyses: Not reported |
Cancer risk | Risk | No |
|
Scholten-Peeters et al, 2003 (106)
Country: Netherlands Journal: Pain Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Whiplash-associated disorders | Risk | Yes |
|
Thurnham et al, 2003 (107)
Country: United Kingdom Journal: Lancet Sponsorship: Government, fellowship COI: Reported as not a conflict of interest Sponsor participation in data analyses: "The funding source had no role in study design, data collection, data analysis, data interpretation, or in the writing of this report." |
Vitamin A deficiency | Continuous variable | No |
|
Zeegers et al, 2003 (108)
Country: Netherlands Journal: Cancer Sponsorship: Government COI: Not reported Sponsor participation in data analyses: Not reported |
Prostate carcinoma | Risk | No |
|
Burzotta et al, 2004 (109)
Country: Italy Journal: Heart Sponsorship: Fellowship COI: Not reported Sponsor participation in data analyses: Not reported |
Coronary ischemic syndromes | Risk | No |
|
Casas et al, 2004 (110)
Country: United Kingdom Journal: Circulation Sponsorship: Government, 1 author holds a chair of nonprofit organization COI: Not reported Sponsor participation in data analyses: Not reported |
Ischemic heart disease | Risk | No |
|
Casas et al, 2004 (111)
Country: United Kingdom Journal: Arch Neurol Sponsorship: Fellowship COI: Not reported Sponsor participation in data analyses: Not reported |
Ischemic stroke | Risk | No |
|
He et al, 2004 (112)
Country: United States Journal: Circulation Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Coronary heart disease mortality | Risk | No |
|
Huang et al, 2004 (113)
Country: United States Journal: Neurology Sponsorship: Government COI: Not reported Sponsor participation in data analyses: Not reported |
Sporadic Parkinson disease | Risk | No |
|
Klement et al, 2004 (114)
Country: Israel Journal: Am J Clin Nutr Sponsorship: Medical center COI: Reported as not a conflict of interest Sponsor participation in data analyses: Not reported |
Inflammatory bowel disease | Risk | Yes |
|
Kovalevsky et al, 2004 (115)
Country: United States Journal: Arch Intern Med Sponsorship: Not reported COI: Reported as not a conflict of interest Sponsor participation in data analyses: Not reported |
Recurrent pregnancy loss | Risk | No |
|
Levitan et al, 2004 (116)
Country: United States Journal: Arch Intern Med Sponsorship: Not reported COI: Reported as not a conflict of interest Sponsor participation in data analyses: Not reported |
Cardiovascular disease | Risk | No |
|
Lovett et al, 2004 (117)
Country: United Kingdom Journal: Neurology Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Subtype of ischemic stroke | Risk | Yes |
|
Mitsikostas et al, 2004 (118)
Country: Greece Journal: Brain Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Headache | Risk | No |
|
Montanez et al, 2004 (119)
Country: United States Journal: Arch Intern Med Sponsorship: Not reported COI: Reported as not a conflict of interest Sponsor participation in data analyses: Not reported |
Total and cardiovascular mortality and sudden death | Risk | No |
|
Woodbury and Houghton, 2004 (120)
Country: Canada Journal: Ostomy Wound Manage Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Pressure ulcers | Prevalence | Yes |
|
Bolland et al, 2005 (121)
Country: New Zealand Journal: J Clin Endocrinol Metab Sponsorship: Scholarship COI: Not reported Sponsor participation in data analyses: Not reported |
Increased body weight | Risk | Quality criteria abstracted |
|
Contopoulos-Ioannidis et al, 2005 (122)
Country: Greece Journal: J Allergy Clin Immunol Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Asthma phenotypes | Risk | Quality criteria abstracted |
|
Dauchet et al, 2005 (123)
Country: France Journal: Neurology Sponsorship: Nonprofit organization, educational institute COI: Reported as not a conflict of interest Sponsor participation in data analyses: Not reported |
Stroke | Risk | No |
|
Etminan et al, 2005 (124)
Country: Canada Journal: BMJ Sponsorship: Government, fellowship COI: Reported as not a conflict of interest Sponsor participation in data analyses: Not reported |
Ischemic stroke | Risk | Yes |
|
Fazel et al, 2005 (125)
Country: United Kingdom Journal: Lancet Sponsorship: Nonprofit organization COI: Reported as not a conflict of interest Sponsor participation in data analyses: "The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication." |
Serious mental disorder | Prevalence | Quality criteria abstracted |
|
García-Closas et al, 2005 (126)
Country: United States Journal: Lancet Sponsorship: Nonprofit organization COI: Reported as not a conflict of interest Sponsor participation in data analyses: "The study sponsors had no role in the design of the study; in the collection, analysis, or interpretation of the data; or in the writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit the paper for publication." |
Bladder cancer | Risk | No |
|
Lee et al, 2005 (127)
Country: United States Journal: Arthritis Rheum Sponsorship: Government, industry COI: Not reported Sponsor participation in data analyses: Unrestricted |
Systemic lupus erythematosus | Risk | No |
|
Lin and August, 2005 (128)
Country: United States Journal: Obstet Gynecol Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Preeclampsia | Risk | No |
|
McDonald et al, 2005 (129)
Country: Canada Journal: Am J Obstet Gynecol Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Perinatal outcomes | Risk | Yes |
|
Palmer, 2005 (130)
Country: United States Journal: Arch Gen Psychiatry Sponsorship: Nonprofit organization COI: Not reported Sponsor participation in data analyses: Not reported |
Lifetime risk of suicide in schizophrenia | Prevalence | Quality criteria abstracted |
|
Sin et al, 2005 (131)
Country: Canada Journal: Chest Sponsorship: Nonprofit organization, educational institute COI: Not reported Sponsor participation in data analyses: Not reported |
Cardiovascular mortality | Risk | Yes |
|
Boudville et al, 2006 (132)
Country: Canada Journal: Ann Intern Med Sponsorship: Government, fellowship COI: Reported as not a conflict of interest Sponsor participation in data analyses: "The study sponsors had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication." |
Hypertension | Risk | No |
|
Clark et al, 2006 (133)
Country: United Kingdom Journal: Pediatrics Sponsorship: Fellowship COI: Not reported Sponsor participation in data analyses: Not reported |
Fractures | Risk | Yes |
|
de Boer et al, 2006 (134)
Country: Netherlands Journal: Cancer Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Unemployment | Risk | Yes |
|
Di Castelnuovo et al, 2006 (135)
Country: Italy Journal: Arch Intern Med Sponsorship: Government COI: Not reported Sponsor participation in data analyses: "The sponsor of the study had no involvement in study design; data collection, analysis, or interpretation; writing of the report; or in the decision to submit the paper for publication." |
Total mortality in men and women | Risk | Yes |
|
Flores-Mateo et al, 2006 (136)
Country: United States Journal: Am J Clin Nutr Sponsorship: Nonprofit organization COI: Reported as not a conflict of interest Sponsor participation in data analyses: Not reported |
Coronary heart disease | Risk | Yes |
|
Galassi et al, 2006 (137)
Country: United States Journal: Am J Med Sponsorship: Government COI: Not reported Sponsor participation in data analyses: Not reported |
Cardiovascular disease | Risk | Quality criteria abstracted |
|
Huxley et al, 2006 (138)
Country: Australia Journal: BMJ Sponsorship: Government, fellowship, industry COI: Reported as not a conflict of interest Sponsor participation in data analyses: Unconditional |
Fatal coronary heart disease | Risk | Quality criteria abstracted |
|
Kahlenborn et al, 2006 (139)
Country: United States Journal: Mayo Clin Proc Sponsorship: Government COI: Not reported Sponsor participation in data analyses: Not reported |
Premenopausal breast cancer | Risk | Quality criteria abstracted |
|
Larsson et al, 2006 (140)
Country: Sweden Journal: Gastroenterology Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Esophageal, gastric, and pancreatic cancer | Risk | Quality criteria abstracted |
|
Mahid et al, 2006 (141)
Country: United States Journal: Mayo Clin Proc Sponsorship: Nonprofit organization COI: Not reported Sponsor participation in data analyses: Not reported |
Inflammatory bowel disease | Risk | Yes |
|
Owen et al, 2006 (142)
Country: United Kingdom Journal: Am J Clin Nutr Sponsorship: Nonprofit organization COI: Reported as not a conflict of interest Sponsor participation in data analyses: Not reported |
Type 2 diabetes | Risk | Quality criteria abstracted |
|
Ownby et al, 2006 (143)
Country: United States Journal: Arch Gen Psychiatry Sponsorship: Government COI: Not reported Sponsor participation in data analyses: Not reported |
Alzheimer disease | Risk | Yes |
|
Pavia et al, 2006 (144)
Country: Italy Journal: Am J Clin Nutr Sponsorship: Not reported COI: Reported as not a conflict of interest Sponsor participation in data analyses: Not reported |
Oral cancer | Risk | Yes |
|
Riddle et al, 2006 (145)
Country: United States Journal: Am J Trop Med Hyg Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Diarrhea | Prevalence | Yes |
|
Rutledge et al, 2006 (146)
Country: United States Journal: J Am Coll Cardiol Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Depression | Prevalence/ risk | Quality criteria abstracted |
|
Smith et al, 2006 (147)
Country: United States Journal: J Am Coll Cardiol Sponsorship: Government COI: Not reported Sponsor participation in data analyses: Not reported |
Renal impairment | Risk | Yes |
|
Weis et al, 2006 (148)
Country: United States Journal: Arch Ophthalmol Sponsorship: Government COI: Reported as not a conflict of interest Sponsor participation in data analyses: Not reported |
Uveal melanoma | Risk | Quality criteria abstracted |
|
Williams et al, 2006 (149)
Country: United Kingdom Journal: Arch Dis Child Sponsorship: Nonprofit organization COI: Reported as not a conflict of interest Sponsor participation in data analyses: Not reported |
Autism spectrum disorders | Prevalence/ risk | Quality criteria abstracted |
|
Bahekar et al, 2007 (150)
Country: United States Journal: Am Heart J Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Coronary heart disease | Risk | Yes |
|
Baurecht et al, 2007 (151)
Country: Germany Journal: J Allergy Clin Immunol Sponsorship: Government, university COI: Reported as a conflict of interest Sponsor participation in data analyses: Not reported |
Atopic eczema | Risk | No |
|
Bellamy et al, 2007 (152)
Country: United Kingdom Journal: BMJ Sponsorship: Government, fellowship COI: Reported as not a conflict of interest Sponsor participation in data analyses: Not reported |
Cardiovascular disease | Risk | Quality criteria abstracted |
|
Conde-Agudelo et al, 2007 (153)
Country: Colombia Journal: Am J Obstet Gynecol Sponsorship: Government COI: Not reported Sponsor participation in data analyses: "The content of the paper has not been influenced by the sponsor." |
Maternal health | Risk | Yes |
|
Dehghan et al, 2007 (154)
Country: Netherlands Journal: Diabetes Sponsorship: University, government COI: Not reported Sponsor participation in data analyses: Not reported |
Diabetes | Risk | No |
|
Eichler et al, 2007 (155)
Country: Switzerland Journal: Am Heart J Sponsorship: Nonprofit organization COI: Not reported Sponsor participation in data analyses: "The funding source had no influence on study design; in the collection, analysis, and interpretation of the data; in the writing of the manuscript; and in the decision to submit the manuscript for publication." |
First coronary events | Risk | Yes |
|
Gami et al, 2007 (156)
Country: United States Journal: J Am Coll Cardiol Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Cardiovascular events and death | Risk | Yes |
|
Grulich et al, 2007 (157)
Country: Australia Journal: Lancet Sponsorship: Government, fellowship, scholarship COI: Reported as a conflict of interest Sponsor participation in data analyses: "There was no funding source for this study. All authors had access to all the data. The corresponding author had final responsibility for the decision to submit for publication." |
Cancers | Risk | Yes |
|
Havemann et al, 2007 (158)
Country: United States Journal: Gut Sponsorship: Industry COI: Reported as a conflict of interest Sponsor participation in data analyses: Not reported |
Asthma | Risk | Quality criteria abstracted |
|
Hirtz et al, 2007 (159)
Country: United States Journal: Neurology Sponsorship: Not reported COI: Reported as not a conflict of interest Sponsor participation in data analyses: Not reported |
Common neurologic disorders | Prevalence | Yes |
|
Huxley et al, 2007 (160)
Country: Australia Journal: Am J Clin Nutr Sponsorship: Government, nonprofit organization COI: Reported as not a conflict of interest Sponsor participation in data analyses: "None of the funding sources had any role in the study design, data analysis, data interpretation, writing of the paper, or the decision to submit the paper for publication." |
Ischemic heart disease | Risk | Yes |
|
Krishna and Kim, 2007 (161)
Country: United States Journal: J Neurosurg Sponsorship: Government COI: Not reported Sponsor participation in data analyses: Not reported |
Risk factors for subarachnoid hemorrhage | Risk | Quality criteria abstracted |
|
Langan et al, 2007 (162)
Country: United Kingdom Journal: Arch Dermatol Sponsorship: Nonprofit organization COI: Reported as not a conflict of interest Sponsor participation in data analyses: "The sponsor had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; or in the preparation, review, or approval of the manuscript." |
Eczema | Risk | Quality criteria abstracted |
|
Larsson and Wolk, 2007 (163)
Country: Sweden Journal: Am J Clin Nutr Sponsorship: Nonprofit organization COI: Reported as not a conflict of interest Sponsor participation in data analyses: Not reported |
Colon and rectal cancer risk | Risk | Quality criteria abstracted |
|
Larsson and Wolk, 2007 (164)
Country: Sweden Journal: Gastroenterology Sponsorship: Nonprofit organization COI: Reported as not a conflict of interest Sponsor participation in data analyses: "The sponsor had no role in the study design or in the collection, analysis, and interpretation of the data." |
Liver cancer | Risk | Quality criteria abstracted |
|
Liu et al, 2007 (165)
Country: China Journal: J Am Coll Cardiol Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Recurrence of atrial fibrillation after successful electrical cardioversion | Risk | Yes |
|
Loza and Chang, 2007 (166)
Country: United States Journal: J Allergy Clin Immunol Sponsorship: Government, nonprofit organization COI: Reported as not a conflict of interest Sponsor participation in data analyses: Not reported |
Atopic asthma risk | Risk | Yes |
|
Pittas et al, 2007 (167)
Country: United States Journal: J Clin Endocrinol Metab Sponsorship: Government COI: Reported as not a conflict of interest Sponsor participation in data analyses: Not reported |
Type 2 diabetes | Risk | No |
|
Polanczyk et al, 2007 (168)
Country: Brazil Journal: Am J Psychiatry Sponsorship: Industry, foreign grants COI: Not reported Sponsor participation in data analyses: "There was no involvement of any funding source in the study design, data collection, analysis, interpretation of data, and writing of this article or in the decision to submit the article for publication." |
Attention deficit hyperactivity disorder | Prevalence | No |
|
Rona et al, 2007 (169)
Country: United Kingdom Journal: J Allergy Clin Immunol Sponsorship: Government COI: Reported as a conflict of interest Sponsor participation in data analyses: Not reported |
Food allergy | Prevalence | Quality criteria abstracted |
|
Sarwar et al, 2007 (170)
Country: United Kingdom Journal: Circulation Sponsorship: Government, scholarship, industry COI: Reported as not a conflict of interest Sponsor participation in data analyses: Unrestricted |
Coronary heart disease | Risk | No |
|
Snoep et al, 2007 (171)
Country: Netherlands Journal: Am Heart J Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Clopidogrel nonresponsiveness | Prevalence | Yes |
|
Zintzaras and Kaditis, 2007 (172)
Country: Greece Journal: Arch Pediatr Adolesc Med Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Blood pressure | Risk | Yes |
|
Ageno et al, 2008 (173)
Country: Italy Journal: Circulation Sponsorship: Not reported COI: Not reported Sponsor participation in data analyses: Not reported |
Venous thromboembolism | Risk | Yes |
|
Barclay et al, 2008 (174)
Country: Australia Journal: Am J Clin Nutr Sponsorship: Government COI: Reported as not a conflict of interest Sponsor participation in data analyses: Not reported |
Chronic disease risk | Risk | Quality criteria abstracted |
|
Conde-Agudelo et al, 2008 (175)
Country: United States Journal: Am J Obstet Gynecol Sponsorship: Government COI: Not reported Sponsor participation in data analyses: "The views expressed in this document are solely the responsibility of the authors and do not necessarily represent the views of the World Health Organization." |
Risk of preeclampsia | Risk | Yes |
|
Schunkert et al, 2008 (176)
Country: Germany Journal: Circulation Sponsorship: Government COI: Reported as not a conflict of interest Sponsor participation in data analyses: Not reported |
Coronary artery disease | Risk | No |
Footnotes
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the US Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above. URLs for nonfederal organizations are provided solely as a service to our users. URLs do not constitute an endorsement of any organization by CDC or the federal government, and none should be inferred. CDC is not responsible for the content of Web pages found at these URLs.
Suggested citation for this article: Shamliyan T, Kane RL, Jansen S. Quality of systematic reviews of observational nontherapeutic studies. Prev Chronic Dis 2010;7(6) http://www.cdc.gov/pcd/issues/2010/nov/09_0195.htm. Accessed [date].
Contributor Information
Tatyana Shamliyan, Minnesota Evidence-based Practice Center, University of Minnesota School of Public Health; D351 Mayo (MMC 197), 420 Delaware St SE, Minneapolis, MN 55455, Phone: 612-624-1185, Email: shaml005@umn.edu.
Robert L. Kane, University of Minnesota School of Public Health, Minneapolis, Minnesota.
Stacy Jansen, University of Minnesota School of Public Health, Minneapolis, Minnesota.
References
- 1.Bero LA, Jadad AR. How consumers and policymakers can use systematic reviews for decision making. Ann Intern Med. 1997;127(1):37–42. doi: 10.7326/0003-4819-127-1-199707010-00007. [DOI] [PubMed] [Google Scholar]
- 2.Chan KS, Morton SC, Shekelle PG. Am J Manag Care. Vol. 10. 11 Pt 1: 2004. pp. 806–812.Systematic reviews for evidence-based management: how to find them and what to do with them; [PubMed] [Google Scholar]
- 3.Biss PA, Zaza S, Pappaioanou M, Fielding J, Wright-De Agüero, L, Truman BI, et al. Developing an evidence-based Guide to Community Preventive Services — methods. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18(1 Suppl):35–43. doi: 10.1016/s0749-3797(99)00119-1. [DOI] [PubMed] [Google Scholar]
- 4.West S, King V, Carey TS, Lohr KN, McKoy N, Sutton SF, et al. Systems to rate the strength of scientific evidence. Evidence Report/Technology Assessment no. 47 (Prepared by the Research Triangle Institute — University of North Carolina Evidence-based Practice Center under Contract no. 290-97-0011) Rockville (MD): Agency for Healthcare Research and Quality; 2002. AHRQ Publication no. 02-E016. [PMC free article] [PubMed] [Google Scholar]
- 5.US Preventive Services Task Force, Agency for Healthcare Research and Quality . The guide to clinical preventive services, 2008: recommendations of the US Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality; 2008. [Google Scholar]
- 6.Moher D, Tetzlaff J, Tricco AC, Sampson M, Altman DG. Epidemiology and reporting characteristics of systematic reviews. PLoS Med. 2007;4(3):e78. doi: 10.1371/journal.pmed.0040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tricco AC, Tetzlaff J, Sampson M, Fergusson D, Cogo E, Horsley T, et al. Few systematic reviews exist documenting the extent of bias: a systematic review. J Clin Epidemiol. 2008;61(5):422–434. doi: 10.1016/j.jclinepi.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 9.Tooth L, Ware R, Bain C, Purdie DM, Dobson A. Quality of reporting of observational longitudinal research. Am J Epidemiol. 2005;161(3):280–288. doi: 10.1093/aje/kwi042. [DOI] [PubMed] [Google Scholar]
- 10.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology. 2007;18(6):805–835. doi: 10.1097/EDE.0b013e3181577511. [DOI] [PubMed] [Google Scholar]
- 11.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 12.Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Chichester, West Sussex (GB): John Wiley and Sons Ltd; 2008. [Google Scholar]
- 14.Whitehead A. Meta-analysis of controlled clinical trials. Chichester, West Sussex (GB): John Wiley and Sons Ltd; 2002. [Google Scholar]
- 15.Egger M, Smith GD. Bias in location and selection of studies. BMJ. 1998;316(7124):61–66. doi: 10.1136/bmj.316.7124.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickersin K. The existence of publication bias and risk factors for its occurrence. JAMA. 1990;263(10):1385–1389. [PubMed] [Google Scholar]
- 17.Dickersin K. How important is publication bias? A synthesis of available data. AIDS Educ Prev. 1997;9(1 Suppl):15–21. [PubMed] [Google Scholar]
- 18.Dickersin K, Min YI. NIH clinical trials and publication bias. Online J Curr Clin Trials. 1993 Apr 28; Doc No 50:[4,967 words; 53 paragraphs] [PubMed] [Google Scholar]
- 19.Ravnskov U. Cholesterol lowering trials in coronary heart disease: frequency of citation and outcome. BMJ. 1992;305(6844):15–19. doi: 10.1136/bmj.305.6844.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Soeken K, Sampson M, Ben-Porat L, Berman B. Assessing the quality of reports of systematic reviews in pediatric complementary and alternative medicine. BMC Pediatr. 2002;2:3. doi: 10.1186/1471-2431-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Methods guide for comparative effectiveness reviews. Agency for Healthcare Research and Quality. [Accessed June 2010]. http://www.effectivehealthcare.ahrq.gov/repFiles/20090427IdenttifyingTopics.pdf . [PubMed]
- 22.Jadad AR, Cook DJ, Jones A, Klassen TP, Tugwell P, Moher M, et al. Methodology and reports of systematic reviews and meta-analyses: a comparison of Cochrane reviews with articles published in paper-based journals. JAMA. 1998;280(3):278–280. doi: 10.1001/jama.280.3.278. [DOI] [PubMed] [Google Scholar]
- 23.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brownson RC, Fielding JE, Maylahn CM. Evidence-based public health: a fundamental concept for public health practice. Annu Rev Public Health. 2009;30:175–201. doi: 10.1146/annurev.publhealth.031308.100134. [DOI] [PubMed] [Google Scholar]
- 25.Brownson RC, Gurney JG, Land GH. Evidence-based decision making in public health. J Public Health Manag Pract. 1999;5(5):86–97. doi: 10.1097/00124784-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Higgins J, Green S. The Cochrane handbook for systematic reviews of interventions 4.2.6, updated September 2006. [Accessed July 2010]. http://www2.cochrane.org/resources/handbook/handbook.pdf .
- 27.WorldCat. OCLC Online Computer Library Center. [Accessed July 2010]. http://www.oclc.org/worldcat/
- 28.Scirus for scientific information only. Elsevier; [Accessed July 2010]. http://www.scirus.com . [Google Scholar]
- 29.Principles of., epidemiology . Principles of epidemiology in public health practice: an introduction to applied epidemiology and biostatistics. 3rd edition Atlanta (GA): US Department of Health and Human Services, Centers for Disease Control and Prevention, Office of Workforce and Career Development; 2006. [Google Scholar]
- 30.Higgins JPT, Green S, editors. Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions, version 5.0.2 [updated September 2009] London (GB): Cochrane Collaboration; 2009. [Accessed July 2010]. http://www.cochrane-handbook.org/ [Google Scholar]
- 31.Lundh A, Gøtzsche PC. Recommendations by Cochrane Review Groups for assessment of the risk of bias in studies. BMC Med Res Methodol. 2008;8:22. doi: 10.1186/1471-2288-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bracken MB. Obstet Gynecol. Vol. 76. 3 Pt 2: 1990. pp. 552–557.Oral contraception and congenital malformations in offspring: a review and meta-analysis of the prospective studies; [PubMed] [Google Scholar]
- 33.Romieu I, Berlin JA, Colditz G. Oral contraceptives and breast cancer. Review and meta-analysis. Cancer. 1990;66(11):2253–2263. doi: 10.1002/1097-0142(19901201)66:11<2253::aid-cncr2820661102>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 34.Haughey BH, Gates GA, Arfken CL, Harvey J. Ann Otol Rhinol Laryngol. Vol. 101. 2 Pt 1: 1992. pp. 105–112.Meta-analysis of second malignant tumors in head and neck cancer: the case for an endoscopic screening protocol; [DOI] [PubMed] [Google Scholar]
- 35.Lemon HM, Heidel JW, Rodriguez-Sierra JF. Increased catechol estrogen metabolism as a risk factor for nonfamilial breast cancer. Cancer. 1992;69(2):457–465. doi: 10.1002/1097-0142(19920115)69:2<457::aid-cncr2820690231>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 36.McKenna MJ. Differences in vitamin D status between countries in young adults and the elderly. Am J Med. 1992;93(1):69–77. doi: 10.1016/0002-9343(92)90682-2. [DOI] [PubMed] [Google Scholar]
- 37.Morris RD, Audet AM, Angelillo IF, Chalmers TC, Mosteller F. Chlorination, chlorination by-products, and cancer: a meta-analysis. Am J Public Health. 1992;82(7):955–963. doi: 10.2105/ajph.82.7.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myers MG, Basinski A. Coffee and coronary heart disease. Arch Intern Med. 1992;152(9):1767–1772. [PubMed] [Google Scholar]
- 39.Becker LB, Smith DW, Rhodes KV. Incidence of cardiac arrest: a neglected factor in evaluating survival rates. Ann Emerg Med. 1993;22(1):86–91. doi: 10.1016/s0196-0644(05)80257-4. [DOI] [PubMed] [Google Scholar]
- 40.Brownson RC, Novotny TE, Perry MC. Cigarette smoking and adult leukemia. A meta-analysis. Arch Intern Med. 1993;153(4):469–475. [PubMed] [Google Scholar]
- 41.Ernst E, Resch KL. Fibrinogen as a cardiovascular risk factor: a meta-analysis and review of the literature. Ann Intern Med. 1993;118(12):956–963. doi: 10.7326/0003-4819-118-12-199306150-00008. [DOI] [PubMed] [Google Scholar]
- 42.Katerndahl DA. Panic and prolapse. Meta-analysis. J Nerv Ment Dis. 1993;181(9):539–544. doi: 10.1097/00005053-199309000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Harris EC, Barraclough BM. Suicide as an outcome for medical disorders. Medicine (Baltimore) 1994;73(6):281–296. doi: 10.1097/00005792-199411000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Kawachi I, Colditz GA, Stone CB. Does coffee drinking increase the risk of coronary heart disease? Results from a meta-analysis. Br Heart J. 1994;72(3):269–275. doi: 10.1136/hrt.72.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Law MR, Thompson SG, Wald NJ. Assessing possible hazards of reducing serum cholesterol. BMJ. 1994;308(6925):373–379. doi: 10.1136/bmj.308.6925.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Law MR, Wald NJ, Thompson SG. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? BMJ. 1994;308(6925):367–372. doi: 10.1136/bmj.308.6925.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steffen R, Kane MA, Shapiro CN, Billo N, Schoellhorn KJ, van Damme P. Epidemiology and prevention of hepatitis A in travelers. JAMA. 1994;272(11):885–889. [PubMed] [Google Scholar]
- 48.Zhang ZF, Begg CB. Is Trichomonas vaginalis a cause of cervical neoplasia? Results from a combined analysis of 24 studies. Int J Epidemiol. 1994;23(4):682–690. doi: 10.1093/ije/23.4.682. [DOI] [PubMed] [Google Scholar]
- 49.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. JAMA. 1995;273(20):1605–1609. [PubMed] [Google Scholar]
- 50.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155(5):469–473. [PubMed] [Google Scholar]
- 51.Ritchie K, Kildea D. Is senile dementia "age-related" or "ageing-related"? — evidence from meta-analysis of dementia prevalence in the oldest old. Lancet. 1995;346(8980):931–934. doi: 10.1016/s0140-6736(95)91556-7. [DOI] [PubMed] [Google Scholar]
- 52.Raman-Wilms L, Tseng AL, Wighardt S, Einarson TR, Koren G. Fetal genital effects of first-trimester sex hormone exposure: a meta-analysis. Obstet Gynecol. 1995;85(1):141–149. doi: 10.1016/0029-7844(94)00341-a. [DOI] [PubMed] [Google Scholar]
- 53.Hatsukami DK, Fischman MW. Crack cocaine and cocaine hydrochloride. Are the differences myth or reality? JAMA. 1996;276(19):1580–1588. [PubMed] [Google Scholar]
- 54.Hill JC, Schoener EP. Age-dependent decline of attention deficit hyperactivity disorder. Am J Psychiatry. 1996;153(9):1143–1146. doi: 10.1176/ajp.153.9.1143. [DOI] [PubMed] [Google Scholar]
- 55.Hackshaw AK, Law MR, Wald NJ. The accumulated evidence on lung cancer and environmental tobacco smoke. BMJ. 1997;315(7114):980–988. doi: 10.1136/bmj.315.7114.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kluijtmans LA, Kastelein JJ, Lindemans J, Boers GH, Heil SG, Bruschke AV, et al. Thermolabile methylenetetrahydrofolate reductase in coronary artery disease. Circulation. 1997;96(8):2573–2577. doi: 10.1161/01.cir.96.8.2573. [DOI] [PubMed] [Google Scholar]
- 57.Law MR, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ. 1997;315(7112):841–846. doi: 10.1136/bmj.315.7112.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Law MR, Morris JK, Wald NJ. Environmental tobacco smoke exposure and ischaemic heart disease: an evaluation of the evidence. BMJ. 1997;315(7114):973–980. doi: 10.1136/bmj.315.7114.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279(18):1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 60.French R, Brocklehurst P. The effect of pregnancy on survival in women infected with HIV: a systematic review of the literature and meta-analysis. Br J Obstet Gynaecol. 1998;105(8):827–835. doi: 10.1111/j.1471-0528.1998.tb10226.x. [DOI] [PubMed] [Google Scholar]
- 61.Forgie MA, Wells PS, Laupacis A, Fergusson D. Preoperative autologous donation decreases allogeneic transfusion but increases exposure to all red blood cell transfusion: results of a meta-analysis. International Study of Perioperative Transfusion (ISPOT) Investigators. Arch Intern Med. 1998;158(6):610–616. doi: 10.1001/archinte.158.6.610. [DOI] [PubMed] [Google Scholar]
- 62.Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114(6):1169–1179. doi: 10.1016/s0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- 63.Johnston SC, Colford JM, Jr, Gress DR. Oral contraceptives and the risk of subarachnoid hemorrhage: a meta-analysis. Neurology. 1998;51(2):411–418. doi: 10.1212/wnl.51.2.411. [DOI] [PubMed] [Google Scholar]
- 64.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279(15):1200–1205. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 65.Ray JG. Meta-analysis of hyperhomocysteinemia as a risk factor for venous thromboembolic disease. Arch Intern Med. 1998;158(19):2101–2106. doi: 10.1001/archinte.158.19.2101. [DOI] [PubMed] [Google Scholar]
- 66.Spencer-Green G. Outcomes in primary Raynaud phenomenon: a meta-analysis of the frequency, rates, and predictors of transition to secondary diseases. Arch Intern Med. 1998;158(6):595–600. doi: 10.1001/archinte.158.6.595. [DOI] [PubMed] [Google Scholar]
- 67.Stratton JF, Pharoah P, Smith SK, Easton D, Ponder BA. A systematic review and meta-analysis of family history and risk of ovarian cancer. Br J Obstet Gynaecol. 1998;105(5):493–499. doi: 10.1111/j.1471-0528.1998.tb10148.x. [DOI] [PubMed] [Google Scholar]
- 68.Zock PL, Katan MB. Linoleic acid intake and cancer risk: a review and meta-analysis. Am J Clin Nutr. 1998;68(1):142–153. doi: 10.1093/ajcn/68.1.142. [DOI] [PubMed] [Google Scholar]
- 69.Zondervan KT, Yudkin PL, Vessey MP, Dawes MG, Barlow DH, Kennedy SH. The prevalence of chronic pelvic pain in women in the United Kingdom: a systematic review. Br J Obstet Gynaecol. 1998;105(1):93–99. doi: 10.1111/j.1471-0528.1998.tb09357.x. [DOI] [PubMed] [Google Scholar]
- 70.Angelillo IF, Villari P. Residential exposure to electromagnetic fields and childhood leukaemia: a meta-analysis. Bull World Health Organ. 1999;77(11):906–915. [PMC free article] [PubMed] [Google Scholar]
- 71.He J, Vupputuri S, Allen K, Prerost MR, Hughes J, Whelton PK. Passive smoking and the risk of coronary heart disease — a meta-analysis of epidemiologic studies. N Engl J Med. 1999;340(12):920–926. doi: 10.1056/NEJM199903253401204. [DOI] [PubMed] [Google Scholar]
- 72.Shaffer HJ, Hall MN, Vander Bilt., J Estimating the prevalence of disordered gambling behavior in the United States and Canada: a research synthesis. Am J Public Health. 1999;89(9):1369–1376. doi: 10.2105/ajph.89.9.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wittrup HH, Tybjaerg-Hansen A, Nordestgaard BG. Lipoprotein lipase mutations, plasma lipids and lipoproteins, and risk of ischemic heart disease. A meta-analysis. Circulation. 1999;99(22):2901–2907. doi: 10.1161/01.cir.99.22.2901. [DOI] [PubMed] [Google Scholar]
- 74.Yoder PR, Sabbagha RE, Gross SJ, Zelop CM. The second-trimester fetus with isolated choroid plexus cysts: a meta-analysis of risk of trisomies 18 and 21. Obstet Gynecol. 1999;93(5 Pt 2):869–872. doi: 10.1016/s0029-7844(98)00544-4. [DOI] [PubMed] [Google Scholar]
- 75.Christen WG, Ajani UA, Glynn RJ, Hennekens CH. Blood levels of homocysteine and increased risks of cardiovascular disease: causal or casual? Arch Intern Med. 2000;160(4):422–434. doi: 10.1001/archinte.160.4.422. [DOI] [PubMed] [Google Scholar]
- 76.Cleophas TJ, Hornstra N, van Hoogstraten B, van der Meulen J. Homocysteine, a risk factor for coronary artery disease or not? A meta-analysis. Am J Cardiol. 2000;86(9):1005-9, A8. doi: 10.1016/s0002-9149(00)01137-1. [DOI] [PubMed] [Google Scholar]
- 77.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 78.Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality. Lancet. 2000;355(9202):451–455. [PubMed] [Google Scholar]
- 79.Wilson K, Gibson N, Willan A, Cook D. Effect of smoking cessation on mortality after myocardial infarction: meta-analysis of cohort studies. Arch Intern Med. 2000;160(7):939–944. doi: 10.1001/archinte.160.7.939. [DOI] [PubMed] [Google Scholar]
- 80.Zeegers MP, Tan FE, Dorant E, van Den., Brandt The impact of characteristics of cigarette smoking on urinary tract cancer risk: a meta-analysis of epidemiologic studies. Cancer. 2000;89(3):630–639. doi: 10.1002/1097-0142(20000801)89:3<630::aid-cncr19>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- 81.Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, et al. Fibrin D-dimer and coronary heart disease: prospective study and meta-analysis. Circulation. 2001;103(19):2323–2327. doi: 10.1161/01.cir.103.19.2323. [DOI] [PubMed] [Google Scholar]
- 82.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48(4):526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Faraone SV, Doyle AE, Mick E, Biederman J. Meta-analysis of the association between the 7-repeat allele of the dopamine D(4) receptor gene and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158(7):1052–1057. doi: 10.1176/appi.ajp.158.7.1052. [DOI] [PubMed] [Google Scholar]
- 84.Horta BL, Kramer MS, Platt RW. Maternal smoking and the risk of early weaning: a meta-analysis. Am J Public Health. 2001;91(2):304–307. doi: 10.2105/ajph.91.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rebora A. Baldness and coronary artery disease: the dermatologic point of view of a controversial issue. Arch Dermatol. 2001;137(7):943–947. [PubMed] [Google Scholar]
- 86.Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159(7):1080–1092. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- 87.Hellermann JP, Jacobsen SJ, Gersh BJ, Rodeheffer RJ, Reeder GS, Roger VL. Heart failure after myocardial infarction: a review. Am J Med. 2002;113(4):324–330. doi: 10.1016/s0002-9343(02)01185-3. [DOI] [PubMed] [Google Scholar]
- 88.Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a meta-analysis. Lancet. 2002;359(9300):14–22. doi: 10.1016/S0140-6736(02)07273-2. [DOI] [PubMed] [Google Scholar]
- 89.Huncharek M, Kupelnick B. Use of topical sunscreens and the risk of malignant melanoma: a meta-analysis of 9067 patients from 11 case-control studies. Am J Public Health. 2002;92(7):1173–1177. doi: 10.2105/ajph.92.7.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Juul K, Tybjaerg-Hansen A, Steffensen R, Kofoed S, Jensen G, Nordestgaard BG. Nordestgaard BG. Factor V Leiden: The Copenhagen City Heart Study and 2 meta-analyses. Blood. 2002;100(1):3–10. doi: 10.1182/blood-2002-01-0111. [DOI] [PubMed] [Google Scholar]
- 91.Kelly PJ, Rosand J, Kistler JP, Shih VE, Silveira S, Plomaritoglou A, et al. Homocysteine, MTHFR 677C-->T polymorphism, and risk of ischemic stroke: results of a meta-analysis. Neurology. 2002;59(4):529–536. doi: 10.1212/wnl.59.4.529. [DOI] [PubMed] [Google Scholar]
- 92.Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG. MTHFR 677C-->T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA. 2002;288(16):2023–2031. doi: 10.1001/jama.288.16.2023. [DOI] [PubMed] [Google Scholar]
- 93.Kozer E, Nikfar S, Costei A, Boskovic R, Nulman I, Koren G. Aspirin consumption during the first trimester of pregnancy and congenital anomalies: a meta-analysis. Am J Obstet Gynecol. 2002;187(6):1623–1630. doi: 10.1067/mob.2002.127376. [DOI] [PubMed] [Google Scholar]
- 94.Law MR, Watt HC, Wald NJ. The underlying risk of death after myocardial infarction in the absence of treatment. Arch Intern Med. 2002;162(21):2405–2410. doi: 10.1001/archinte.162.21.2405. [DOI] [PubMed] [Google Scholar]
- 95.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325(7374):1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185(1):45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 97.Benjamin DK, Jr, Poole C, Steinbach WJ, Rowen JL, Walsh TJ. Pediatrics. Vol. 112. 3 Pt 1: 2003. pp. 634–640.Neonatal candidemia and end-organ damage: a critical appraisal of the literature using meta-analytic techniques; [DOI] [PubMed] [Google Scholar]
- 98.Clarfield AM. The decreasing prevalence of reversible dementias: an updated meta-analysis. Arch Intern Med. 2003;163(18):2219–2229. doi: 10.1001/archinte.163.18.2219. [DOI] [PubMed] [Google Scholar]
- 99.Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160(6):1147–1156. doi: 10.1176/appi.ajp.160.6.1147. [DOI] [PubMed] [Google Scholar]
- 100.Gisbert JP, Garcia-Buey L, Pajares JM, Moreno-Otero R. Prevalence of hepatitis C virus infection in B-cell non-Hodgkin's lymphoma: systematic review and meta-analysis. Gastroenterology. 2003;125(6):1723–1732. doi: 10.1053/j.gastro.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 101.Glatt SJ, Faraone SV, Tsuang MT. Association between a functional catechol O-methyltransferase gene polymorphism and schizophrenia: meta-analysis of case-control and family-based studies. Am J Psychiatry. 2003;160(3):469–476. doi: 10.1176/appi.ajp.160.3.469. [DOI] [PubMed] [Google Scholar]
- 102.Halbert RJ, Isonaka S, George D, Iqbal A. Interpreting COPD prevalence estimates: what is the true burden of disease? Chest. 2003;123(5):1684–1692. doi: 10.1378/chest.123.5.1684. [DOI] [PubMed] [Google Scholar]
- 103.Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology. 2003;125(6):1636–1644. doi: 10.1053/j.gastro.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 104.Rey E, Kahn SR, David M, Shrier I. Thrombophilic disorders and fetal loss: a meta-analysis. Lancet. 2003;361(9361):901–908. doi: 10.1016/S0140-6736(03)12771-7. [DOI] [PubMed] [Google Scholar]
- 105.Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr. 2003;78(3 Suppl):559S–569S. doi: 10.1093/ajcn/78.3.559S. [DOI] [PubMed] [Google Scholar]
- 106.Scholten-Peeters GGM, Verhagen AP, Bekkering GE, van der, Windt, Barnsley L, Oostendorp RA, et al. Prognostic factors of whiplash-associated disorders: a systematic review of prospective cohort studies. Pain. 2003;104(1):303–322. doi: 10.1016/s0304-3959(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 107.Thurnham DI, McCabe GP, Northrop-Clewes CA, Nestel P. Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of vitamin A deficiency: meta-analysis. Lancet. 2003;362(9401):2052–2058. doi: 10.1016/s0140-6736(03)15099-4. [DOI] [PubMed] [Google Scholar]
- 108.Zeegers MP, Jellema A, Ostrer H. Empiric risk of prostate carcinoma for relatives of patients with prostate carcinoma: a meta-analysis. Cancer. 2003;97(8):1894–1903. doi: 10.1002/cncr.11262. [DOI] [PubMed] [Google Scholar]
- 109.Burzotta F, Paciaroni K, De Stefano V, Crea F, Maseri A, Leone G, et al. G20210A prothrombin gene polymorphism and coronary ischaemic syndromes: a phenotype-specific meta-analysis of 12,034 subjects. Heart. 2004;90(1):82–86. doi: 10.1136/heart.90.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Casas JP, Bautista LE, Humphries SE, Hingorani AD. Endothelial nitric oxide synthase genotype and ischemic heart disease: meta-analysis of 26 studies involving 23,028 subjects. Circulation. 2004;109(11):1359–1365. doi: 10.1161/01.CIR.0000121357.76910.A3. [DOI] [PubMed] [Google Scholar]
- 111.Casas JP, Hingorani AD, Bautista LE, Sharma P. Meta-analysis of genetic studies in ischemic stroke: thirty-two genes involving approximately 18,000 cases and 58,000 controls. Arch Neurol. 2004;61(11):1652–1661. doi: 10.1001/archneur.61.11.1652. [DOI] [PubMed] [Google Scholar]
- 112.He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, et al. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation. 2004;109(22):2705–2711. doi: 10.1161/01.CIR.0000132503.19410.6B. [DOI] [PubMed] [Google Scholar]
- 113.Huang X, Chen PC, Poole C. APOE-[epsilon]2 allele associated with higher prevalence of sporadic Parkinson disease. Neurology. 2004;62(12):2198–2202. doi: 10.1212/01.wnl.0000130159.28215.6a. [DOI] [PubMed] [Google Scholar]
- 114.Klement E, Cohen RV, Boxman J, Joseph A, Reif S. Breastfeeding and risk of inflammatory bowel disease: a systematic review with meta-analysis. Am J Clin Nutr. 2004;80(5):1342–1352. doi: 10.1093/ajcn/80.5.1342. [DOI] [PubMed] [Google Scholar]
- 115.Kovalevsky G, Gracia CR, Berlin JA, Sammel MD, Barnhart KT. Evaluation of the association between hereditary thrombophilias and recurrent pregnancy loss: a meta-analysis. Arch Intern Med. 2004;164(5):558–563. doi: 10.1001/archinte.164.5.558. [DOI] [PubMed] [Google Scholar]
- 116.Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med. 2004;164(19):2147–2155. doi: 10.1001/archinte.164.19.2147. [DOI] [PubMed] [Google Scholar]
- 117.Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. 2004;62(4):569–573. doi: 10.1212/01.wnl.0000110311.09970.83. [DOI] [PubMed] [Google Scholar]
- 118.Mitsikostas DD, Sfikakis PP, Goadsby PJ. Brain. Vol. 127. Pt 5: 2004. pp. 1200–1209. A meta-analysis for headache in systemic lupus erythematosus: the evidence and the myth; [DOI] [PubMed] [Google Scholar]
- 119.Montanez A, Ruskin JN, Hebert PR, Lamas GA, Hennekens CH. Prolonged QTc interval and risks of total and cardiovascular mortality and sudden death in the general population: a review and qualitative overview of the prospective cohort studies. Arch Intern Med. 2004;164(9):943–948. doi: 10.1001/archinte.164.9.943. [DOI] [PubMed] [Google Scholar]
- 120.Woodbury MG, Houghton PE. Prevalence of pressure ulcers in Canadian healthcare settings. Ostomy Wound Manage. 2004;50(10):22-4, 26, 28 passim. [PubMed] [Google Scholar]
- 121.Bolland MJ, Grey AB, Gamble GD, Reid IR. Association between primary hyperparathyroidism and increased body weight: a meta-analysis. J Clin Endocrinol Metab. 2005;90(3):1525–1530. doi: 10.1210/jc.2004-1891. [DOI] [PubMed] [Google Scholar]
- 122.Contopoulos-Ioannidis DG, Manoli EN, Ioannidis JP. Meta-analysis of the association of beta2-adrenergic receptor polymorphisms with asthma phenotypes. J Allergy Clin Immunol. 2005;115(5):963–972. doi: 10.1016/j.jaci.2004.12.1119. [DOI] [PubMed] [Google Scholar]
- 123.Dauchet L, Amouyel P, Dallongeville J. Fruit and vegetable consumption and risk of stroke: a meta-analysis of cohort studies. Neurology. 2005;65(8):1193–1197. doi: 10.1212/01.wnl.0000180600.09719.53. [DOI] [PubMed] [Google Scholar]
- 124.Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischaemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330(7482):63. doi: 10.1136/bmj.38302.504063.8F. [Errata published in: BMJ 2005;330(7491):596. BMJ 2005;330(7487):345.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fazel M, Wheeler J, Danesh J. Prevalence of serious mental disorder in 7000 refugees resettled in western countries: a systematic review. Lancet. 2005;365(9467):1309–1314. doi: 10.1016/S0140-6736(05)61027-6. [DOI] [PubMed] [Google Scholar]
- 126.García-Closas M, Malats N, Silverman D, Dosemeci M, Kogevinas M, Hein DW, et al. NAT2 slow acetylation, GSTM1 null genotype, and risk of bladder cancer: results from the Spanish Bladder Cancer Study and meta-analyses. Lancet. 2005;366(9486):649–659. doi: 10.1016/S0140-6736(05)67137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee YH, Witte T, Momot T, Schmidt RE, Kaufman KM, Harley JB, et al. The mannose-binding lectin gene polymorphisms and systemic lupus erythematosus: two case-control studies and a meta-analysis. Arthritis Rheum. 2005;52(12):3966–3974. doi: 10.1002/art.21484. [DOI] [PubMed] [Google Scholar]
- 128.Lin J, August P. Genetic thrombophilias and preeclampsia: a meta-analysis. Obstet Gynecol. 2005;105(1):182–192. doi: 10.1097/01.AOG.0000146250.85561.e9. [DOI] [PubMed] [Google Scholar]
- 129.McDonald S, Murphy K, Beyene J, Ohlsson A. Perinatal outcomes of in vitro fertilization twins: a systematic review and meta-analyses. Am J Obstet Gynecol. 2005;193(1):141–152. doi: 10.1016/j.ajog.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 130.Palmer BA, Pankratz VS, Bostwick JM. The lifetime risk of suicide in schizophrenia: a reexamination. Arch Gen Psychiatry. 2005;62(3):247–253. doi: 10.1001/archpsyc.62.3.247. [DOI] [PubMed] [Google Scholar]
- 131.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127(6):1952–1959. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 132.Boudville N, Prasad GV, Knoll G, Muirhead N, Thiessen-Philbrook H, Yang RC, et al. Meta-analysis: risk for hypertension in living kidney donors. Ann Intern Med. 2006;145(3):185–196. doi: 10.7326/0003-4819-145-3-200608010-00006. [DOI] [PubMed] [Google Scholar]
- 133.Clark EM, Tobias JH, Ness AR. Association between bone density and fractures in children: a systematic review and meta-analysis. Pediatrics. 2006;117(2):e291–e297. doi: 10.1542/peds.2005-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.de Boer AG, Verbeek JH, van Dijk FJ. Adult survivors of childhood cancer and unemployment: a metaanalysis. Cancer. 2006;107(1):1–11. doi: 10.1002/cncr.21974. [DOI] [PubMed] [Google Scholar]
- 135.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166(22):2437–2445. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- 136.Flores-Mateo G, Navas-Acien A, Pastor-Barriuso R, Guallar E. Selenium and coronary heart disease: a meta-analysis. Am J Clin Nutr. 2006;84(4):762–773. doi: 10.1093/ajcn/84.4.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119(10):812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 138.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332(7533):73–78. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kahlenborn C, Modugno F, Potter DM, Severs WB. Oral contraceptive use as a risk factor for premenopausal breast cancer: a meta-analysis. Mayo Clin Proc. 2006;81(10):1290–1302. doi: 10.4065/81.10.1290. [DOI] [PubMed] [Google Scholar]
- 140.Larsson SC, Giovannucci E, Wolk A. Folate intake, MTHFR polymorphisms, and risk of esophageal, gastric, and pancreatic cancer: a meta-analysis. Gastroenterology. 2006;131(4):1271–1283. doi: 10.1053/j.gastro.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 141.Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc. 2006;81(11):1462–1471. doi: 10.4065/81.11.1462. [DOI] [PubMed] [Google Scholar]
- 142.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am J Clin Nutr. 2006;84(5):1043–1054. doi: 10.1093/ajcn/84.5.1043. [DOI] [PubMed] [Google Scholar]
- 143.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63(5):530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pavia M, Pileggi C, Nobile CG, Angelillo IF. Association between fruit and vegetable consumption and oral cancer: a meta-analysis of observational studies. Am J Clin Nutr. 2006;83(5):1126–1134. doi: 10.1093/ajcn/83.5.1126. [DOI] [PubMed] [Google Scholar]
- 145.Riddle MS, Sanders JW, Putnam SD, Tribble DR. Incidence, etiology, and impact of diarrhea among long-term travelers (US military and similar populations): a systematic review. Am J Trop Med Hyg. 2006;74(5):891–900. [PubMed] [Google Scholar]
- 146.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure: a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48(8):1527–1537. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 147.Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, et al. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47(10):1987–1996. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 148.Weis E, Shah CP, Lajous M, Shields JA, Shields CL. The association between host susceptibility factors and uveal melanoma: a meta-analysis. Arch Ophthalmol. 2006;124(1):54–60. doi: 10.1001/archopht.124.1.54. [DOI] [PubMed] [Google Scholar]
- 149.Williams JG, Higgins JP, Brayne CE. Systematic review of prevalence studies of autism spectrum disorders. Arch Dis Child. 2006;91(1):8–15. doi: 10.1136/adc.2004.062083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bahekar AA, Singh S, Saha S, Molnar J, Arora R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: a meta-analysis. Am Heart J. 2007;154(5):830–837. doi: 10.1016/j.ahj.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 151.Baurecht H, Irvine AD, Novak N, Illig T, Bühler B, Ring J, et al. Toward a major risk factor for atopic eczema: meta-analysis of filaggrin polymorphism data. J Allergy Clin Immunol. 2007;120(6):1406–1412. doi: 10.1016/j.jaci.2007.08.067. [DOI] [PubMed] [Google Scholar]
- 152.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Conde-Agudelo A, Rosas-Bermudez A, Kafury-Goeta AC. Effects of birth spacing on maternal health: a systematic review. Am J Obstet Gynecol. 2007;196(4):297–308. doi: 10.1016/j.ajog.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 154.Dehghan A, Kardys I, de Maat MP, Uitterlinden AG, Sijbrands EJ, Bootsma AH, et al. Genetic variation, C-reactive protein levels, and incidence of diabetes. Diabetes. 2007;56(3):872–878. doi: 10.2337/db06-0922. [DOI] [PubMed] [Google Scholar]
- 155.Eichler K, Puhan MA, Steurer J, Bachmann LM. Prediction of first coronary events with the Framingham score: a systematic review. Am Heart J. 2007;153(5):722-31, 731.e1-8. doi: 10.1016/j.ahj.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 156.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49(4):403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 157.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 158.Havemann BD, Henderson CA, El-Serag HB. The association between gastro-oesophageal reflux disease and asthma: a systematic review. Gut. 2007;56(12):1654–1664. doi: 10.1136/gut.2007.122465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the "common" neurologic disorders? Neurology. 2007;68(5):326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- 160.Huxley R, Owen CG, Whincup PH, Cook DG, Rich-Edwards J, Smith GD, et al. Is birth weight a risk factor for ischemic heart disease in later life? Am J Clin Nutr. 2007;85(5):1244–1250. doi: 10.1093/ajcn/85.5.1244. [DOI] [PubMed] [Google Scholar]
- 161.Krishna V, Kim DH. Ethnic differences in risk factors for subarachnoid hemorrhage. J Neurosurg. 2007;107(3):522–529. doi: 10.3171/JNS-07/09/0522. [DOI] [PubMed] [Google Scholar]
- 162.Langan SM, Flohr C, Williams HC. The role of furry pets in eczema: a systematic review. Arch Dermatol. 2007;143(12):1570–1577. doi: 10.1001/archderm.143.12.1570. [DOI] [PubMed] [Google Scholar]
- 163.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86(3):556–565. doi: 10.1093/ajcn/86.3.556. [DOI] [PubMed] [Google Scholar]
- 164.Larsson SC, Wolk A. Coffee consumption and risk of liver cancer: a meta-analysis. Gastroenterology. 2007;132(5):1740–1745. doi: 10.1053/j.gastro.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 165.Liu T, Li G, Li L, Korantzopoulos P. Association between C-reactive protein and recurrence of atrial fibrillation after successful electrical cardioversion: a meta-analysis. J Am Coll Cardiol. 2007;49(15):1642–1648. doi: 10.1016/j.jacc.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 166.Loza MJ, Chang BL. Association between Q551R IL4R genetic variants and atopic asthma risk demonstrated by meta-analysis. J Allergy Clin Immunol. 2007;120(3):578–585. doi: 10.1016/j.jaci.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 167.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92(6):2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 169.Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120(3):638–646. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 170.Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115(4):450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 171.Snoep JD, Hovens MM, Eikenboom JC, van der Bom JG, Jukema JW, Huisman MV. Clopidogrel nonresponsiveness in patients undergoing percutaneous coronary intervention with stenting: a systematic review and meta-analysis. Am Heart J. 2007;154(2):221–231. doi: 10.1016/j.ahj.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 172.Zintzaras E, Kaditis AG. Sleep-disordered breathing and blood pressure in children: a meta-analysis. Arch Pediatr Adolesc Med. 2007;161(2):172–178. doi: 10.1001/archpedi.161.2.172. [DOI] [PubMed] [Google Scholar]
- 173.Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117(1):93–102. doi: 10.1161/CIRCULATIONAHA.107.709204. [DOI] [PubMed] [Google Scholar]
- 174.Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, et al. Glycemic index, glycemic load, and chronic disease risk — a meta-analysis of observational studies. Am J Clin Nutr. 2008;87(3):627–637. doi: 10.1093/ajcn/87.3.627. [DOI] [PubMed] [Google Scholar]
- 175.Conde-Agudelo A, Villar J, Lindheimer M. Maternal infection and risk of preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 2008;198(1):7–22. doi: 10.1016/j.ajog.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 176.Schunkert H, Gotz A, Braund P, McGinnis R, Tregouet DA, Mangino M, et al. Repeated replication and a prospective meta-analysis of the association between chromosome 9p21.3 and coronary artery disease. Circulation. 2008;117(13):1675–1684. doi: 10.1161/CIRCULATIONAHA.107.730614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mrkobrada M, Thiessen-Philbrook H, Haynes RB, Iansavichus AV, Rehman F, Garg AX. Need for quality improvement in renal systematic reviews. Clin J Am Soc Nephrol. 2008;3(4):1102–1114. doi: 10.2215/CJN.04401007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mallett S, Deeks JJ, Halligan S, Hopewell S, Cornelius V, Altman DG. Systematic reviews of diagnostic tests in cancer: review of methods and reporting. BMJ. 2006;333(7565):413. doi: 10.1136/bmj.38895.467130.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Needleman I, Worthington H, Moher D, Schulz K, Altman DG. Improving the completeness and transparency of reports of randomized trials in oral health: the CONSORT statement. Am J Dent. 2008;21(1):7–12. [PubMed] [Google Scholar]
- 180.Plint AC, Moher D, Morrison A, Schulz K, Altman DG, Hill C, et al. Does the CONSORT checklist improve the quality of reports of randomised controlled trials? A systematic review. Med J Aust. 2006;185(5):263–267. doi: 10.5694/j.1326-5377.2006.tb00557.x. [DOI] [PubMed] [Google Scholar]


