Abstract
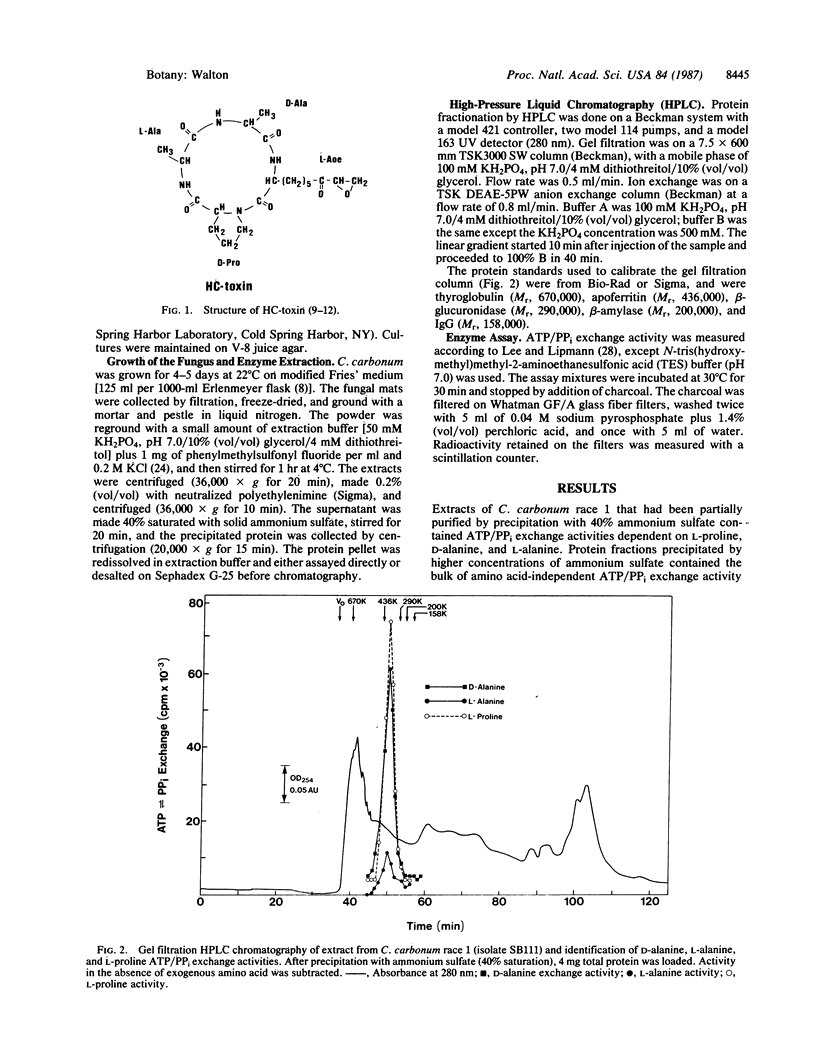
Cochliobolus carbonum race 1 produces a cyclic tetrapeptide HC-toxin, which is necessary for its exceptional virulence on certain varieties of maize. Previous genetic analysis of HC-toxin production by the fungus has indicated that a single genetic locus controls HC-toxin production. Enzymes involved in the biosynthesis of HC-toxin have been sought by following the precedents established for the biosynthetic enzymes of cyclic peptide antibiotics. Two enzymatic activities from C. carbonum race 1 were found, a D-alanine- and an L-proline-dependent ATP/PPi exchange, which by biochemical and genetic criteria were shown to be involved in the biosynthesis of HC-toxin. These two activities were present in all tested race 1 isolates of C. carbonum, which produce HC-toxin, and in none of the tested race 2 and race 3 isolates, which do not produce the toxin. In a genetic cross between two isolates of C. carbonum differing at the tox locus, all tox+ progeny had both activities, and all tox- progeny lacked both activities.
Keywords: cyclic peptide biosynthesis, maize, plant disease
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Closse A., Huguenin R. Isolierung und Strukturaufklärung von Chlamydocin. Helv Chim Acta. 1974 Apr 27;57(3):533–545. doi: 10.1002/hlca.19740570306. [DOI] [PubMed] [Google Scholar]
- Dimroth P., Walter H., Lynen F. Biosynthese von 6-Methylsalicylsäure. Eur J Biochem. 1970 Mar 1;13(1):98–110. doi: 10.1111/j.1432-1033.1970.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Froyshov O., Laland S. G. On the biosynthesis of bacitracin by a soluble enzyme complex from Bacillus licheniformis. Eur J Biochem. 1974 Jul 15;46(2):235–242. doi: 10.1111/j.1432-1033.1974.tb03616.x. [DOI] [PubMed] [Google Scholar]
- Gevers W., Kleinkauf H., Lipmann F. Peptidyl transfers in gramicidin S bisoynthesis from enzyme-bound thioester intermediates. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1335–1342. doi: 10.1073/pnas.63.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilhuus-Moe C. C., Kristensen T., Bredesen J. E., Zimmer T. -L., Laland S. G. The presence and possible role of phosphopantothenic acid in gramicidin S synthetase. FEBS Lett. 1970 Apr 16;7(3):287–290. doi: 10.1016/0014-5793(70)80183-1. [DOI] [PubMed] [Google Scholar]
- Hirota A., Suzuki A., Aizawa K., Tamura S. Mass spectrometric determination of amino acid sequence in Cyl-2, a novel cyclotetrapeptide from Cylindrocladium scoparium. Biomed Mass Spectrom. 1974 Feb;1(1):15–19. doi: 10.1002/bms.1200010106. [DOI] [PubMed] [Google Scholar]
- Kawai M., Pottorf R. S., Rich D. H. Structure and solution conformation of the cytostatic cyclic tetrapeptide WF-3161, cyclo[L-leucyl-L-pipecolyl-L-(2-amino-8-oxo-9, 10-epoxydecanoyl)-D-phenylalanyl]. J Med Chem. 1986 Nov;29(11):2409–2411. doi: 10.1021/jm00161a046. [DOI] [PubMed] [Google Scholar]
- Kawai M., Rich D. H., Walton J. D. The structure and conformation of HC-toxin. Biochem Biophys Res Commun. 1983 Mar 16;111(2):398–403. doi: 10.1016/0006-291x(83)90319-4. [DOI] [PubMed] [Google Scholar]
- Keller U. Actinomycin synthetases. Multifunctional enzymes responsible for the synthesis of the peptide chains of actinomycin. J Biol Chem. 1987 Apr 25;262(12):5852–5856. [PubMed] [Google Scholar]
- Kleinkauf H., von Döhren H. Nucleic acid independent synthesis of peptides. Curr Top Microbiol Immunol. 1981;91:129–177. doi: 10.1007/978-3-642-68058-8_6. [DOI] [PubMed] [Google Scholar]
- Krause M., Marahiel M. A., von Döhren H., Kleinkauf H. Molecular cloning of an ornithine-activating fragment of the gramicidin S synthetase 2 gene from Bacillus brevis and its expression in Escherichia coli. J Bacteriol. 1985 Jun;162(3):1120–1125. doi: 10.1128/jb.162.3.1120-1125.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laland S. G., Zimmer T. L. The protein thiotemplate mechanism of synthesis for the peptide antibiotics produced by Bacillus brevis. Essays Biochem. 1973;9:31–57. [PubMed] [Google Scholar]
- Lee S. G., Lipmann F. Tyrocidine synthetase system. Methods Enzymol. 1975;43:585–602. doi: 10.1016/0076-6879(75)43121-4. [DOI] [PubMed] [Google Scholar]
- Lim S. M., Hooker A. L. Southern Corn Leaf Blight: Genetic Control of Pathogenicity and Toxin Production in Race T and Race O of COCHLIOBOLUS HETEROSTROPHUS. Genetics. 1971 Sep;69(1):115–117. doi: 10.1093/genetics/69.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipmann F. Attempts to map a process evolution of peptide biosynthesis. Science. 1971 Sep 3;173(4000):875–884. doi: 10.1126/science.173.4000.875. [DOI] [PubMed] [Google Scholar]
- Macko K. A., Hodos W. Near point of accommodation in pigeons. Vision Res. 1985;25(10):1529–1530. doi: 10.1016/0042-6989(85)90232-9. [DOI] [PubMed] [Google Scholar]
- Marahiel M. A., Krause M., Skarpeid H. J. Cloning of the tyrocidine synthetase 1 gene from Bacillus brevis and its expression in Escherichia coli. Mol Gen Genet. 1985;201(2):231–236. doi: 10.1007/BF00425664. [DOI] [PubMed] [Google Scholar]
- Mohr H., Kleinkauf H. Alamethicin biosynthesis: acetylation of the amino terminus and attachment of phenylalaninol. Biochim Biophys Acta. 1978 Oct 12;526(2):375–386. doi: 10.1016/0005-2744(78)90129-8. [DOI] [PubMed] [Google Scholar]
- Vater J., Kleinkauf H. Gramicidin S-synthetase. A further characterization of phenylalanine racemase, the light enzyme of gramicidin s-synthetase. Biochim Biophys Acta. 1976 May 13;429(3):1062–1072. doi: 10.1016/0005-2744(76)90351-x. [DOI] [PubMed] [Google Scholar]
- Walton J. D., Earle E. D., Gibson B. W. Purification and structure of the host-specific toxin from Helminthosporium carbonum race 1. Biochem Biophys Res Commun. 1982 Aug;107(3):785–794. doi: 10.1016/0006-291x(82)90592-7. [DOI] [PubMed] [Google Scholar]
- Zocher R., Keller U., Kleinkauf H. Enniatin synthetase, a novel type of multifunctional enzyme catalyzing depsipeptide synthesis in Fusarium oxysporum. Biochemistry. 1982 Jan 5;21(1):43–48. doi: 10.1021/bi00530a008. [DOI] [PubMed] [Google Scholar]
- Zocher R., Nihira T., Paul E., Madry N., Peeters H., Kleinkauf H., Keller U. Biosynthesis of cyclosporin A: partial purification and properties of a multifunctional enzyme from Tolypocladium inflatum. Biochemistry. 1986 Feb 11;25(3):550–553. doi: 10.1021/bi00351a005. [DOI] [PubMed] [Google Scholar]



