Abstract
G-protein-coupled receptors (GPCRs) represent the largest family of cell surface receptors, and have evolved to detect and transmit a large palette of extracellular chemical and sensory signals into cells. Activated receptors catalyze the activation of heterotrimeric G proteins, which modulate the propagation of second messenger molecules and the activity of ion channels. Classically thought to signal as monomers, different GPCRs often pair up with each other as homo- and heterodimers, which have been shown to modulate signaling to G proteins. Here, we discuss recent advances in GPCR heteromer systems involving the kinetics of the early steps in GPCR signal transduction, the dynamic property of receptor–receptor interactions, and how the formation of receptor heteromers modulate the kinetics of G-protein signaling.
Keywords: G-protein-coupled receptors, Heterodimers, Signaling
Introduction
G-protein-coupled receptors (GPCRs) constitute the main family of cell surface receptors for a large variety of chemical stimuli (hormones, neurotransmitters, chemoattractants, calcium ions and pain killers, among others molecules) and sensory stimuli (light, odorants and taste molecules). GPCRs are expressed in virtually every cell and consist of seven membrane-spanning α-helical structures with the N- and C-termini exposed to the extracellular and intracellular environment, respectively (Hanson and Stevens, 2009). Signal transduction begins when an extracellular agonist ‘ligand’ binds and switches the receptor from an inactive state to an active state conformation. Activated receptors then catalyze the exchange of GDP for GTP on the α-subunit of heterotrimeric G proteins (Gαβγ), which in turn engages conformational and/or dissociation events between the Gα and dimeric Gβγ subunits that are associated with G-protein activation (Hofmann et al., 2009). Both the GTP-bound Gα subunit and the Gβγ dimer can then initiate or suppress the activity of effector enzymes (e.g. adenylyl cyclases, phosphodiesterases, phospholipases) and ion channels. These ion channels in turn modulate the flow of secondary messengers such as cAMP, cGMP, diacylglycerol or inositol trisphosphate, which are involved in the regulation of multiple intracellular signaling pathways, and which modulate cell functions in body systems as diverse as the skeletal, endocrine, cardiovascular and nervous systems, among others (Fig. 1). Activated GPCRs can also interact with cytosolic arrestins (β-arrestin-1 and β-arrestin-2), which coordinate receptor–G-protein uncoupling in space and time, promote receptor internalization and modulate various distal signaling responses such the MAP kinase cascades (Pierce et al., 2002). Malfunction of GPCRs are involved in many human diseases (Schöneberg et al., 2004), for instance, diabetes insipidus and mellitus, hypercalcemia, obesity, hypertension, cancer, hypothyroidism, retinitis pigmentosa and psychotic disorders, and are targets of most of the available clinical drugs used in humans, such as β-blockers, antipsychotics and analgesics. Understanding the signaling and trafficking mechanisms of GPCRs is thus central for the development of new and safer therapies for many physiological and psychological disorders.
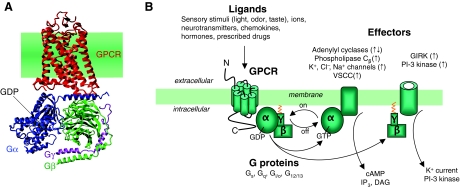
Fig. 1.
General principle of the GPCR signaling system. (A) Molecular representation of a GPCR in complex with a Gαβγ, based on crystal structures of rhodopsin (red; coordinates from PDB code 1GZM) and the inactive heterotrimeric Gi protein (from PDB 1GG2). (B) Following ligand binding, the receptor undergoes conformational changes, which promote the coupling with heterotrimeric G proteins (Gαβγ), and catalyzes the exchange of GDP for GTP on the α-subunit. This event triggers conformational and/or dissociation events between the α-subunit and βγ-subunit. GαS activates adenylyl cyclases, leading to cAMP synthesis, which in turn activates protein kinase A (PKA). Gαq activates phospholipase C, which cleaves phosphatidylinositol (4,5)-bisphosphate (PIP2) into diacylglycerol (DAG) and inositol (1,4,5)-trisphosphate (IP3). IP3 then diffuses through the cytosol and activates IP3-gated Ca2+ channels in the membranes of the endoplasmic reticulum, causing the release of stored Ca2+ into the cytosol. The increase of cytosolic Ca2+ promotes PKC translocation to the plasma membrane, and then activation by DAG. Activation of Gi blocks adenylyl-cyclase-mediated cAMP synthesis by its α-subunits, whereas Gβγ-mediated signaling processes such as activation of G-protein-regulated inwardly rectifying potassium (GIRK) channels. VSCC, voltage-sensitive Ca2+ channel.
GPCRs have traditionally been considered to exist and function as cell-surface-receptor monomers (Chabre and Le Maire, 2005; Chabre et al., 2009). Consistent with this view, recent studies characterized in reconstituted phospholipid bilayer systems demonstrated that purified receptors such as rhodopsin, β2-adrenergic and μ-opioid receptors can activate heterotrimeric G proteins as single GPCR monomers (Whorton et al., 2007; Whorton et al., 2008; Kuszak, 2008). These studies indicate that a single GPCR protomer is capable of activating G proteins, and that receptor oligomerization is not necessary to mediate G-protein activation. Several GPCRs can, however, assemble and signal in both homo-oligomer and hetero-oligomer complexes at the surface membrane of native and cultured cells (Prinster et al., 2005; Guo et al., 2008; Milligan, 2009) (Box 1). Two recent studies provided strong experimental evidence of the in vivo homodimerization of GPCRs. The first showed dimerization of the mouse luteinizing hormone receptor (LHR) by using a transgenetic approach to express binding and signaling variants of LHR together (Rivero-Muller et al., 2010), whereas the second study demonstrated the existence of native oxytocin receptor dimers by using a fluorescence resonance energy transfer (FRET) approach (Albizu et al., 2010). In this Commentary, we discuss the kinetics of the early steps involved in GPCR signaling (receptor activation, the receptor–G-protein interaction, G-protein activation), the stability of the receptor–receptor interaction, and how this interaction modulates the kinetics of G-protein signaling.
Box 1. Receptor heterodimer and receptor mosaic concepts
In the 1980s, Agnati and Fuxe introduced the concept of intermembrane receptor–receptor interactions between different types of GPCRs, based on the effects of neuropeptides on the binding characteristics of monoamine receptors in plasma membrane preparations from discrete regions of the brain (Agnati et al., 1980; Fuxe et al., 1981; Fuxe et al., 1983; Fuxe and Agnati, 1985). These results were in line with earlier findings (Limbird et al., 1975) showing negative cooperativity in β-ARs, which could be explained by the existence of receptor homodimers leading to site–site interactions. Additional in vivo studies, which examined the interactions of clonidine (a partial agonist for α2A-ARs) and neuropeptide Y (NPY; a 36 amino acid neurotransmitter) on sleep–wakefulness cycles and arterial blood pressure control in both normal and spontaneously hypertensive rats (Fuxe et al., 1989; Fuxe, 1990; Yang et al., 1994), further supported the existence of receptor–receptor interactions and their potential functional relevance. As a logical consequence for the indications of direct physical interactions between neuropeptide and monoamine receptors, the term heteromerization was introduced to describe a specific interaction between different types of GPCRs (Zoli et al., 1993). The concept of the GPCR heterodimer was later confirmed by studies reporting that two non-functional GPCR monomers, GABAB1 and GABAB2, can assemble in signaling heterodimers at the cell surface to transmit the inhibitory effect of the γ-aminobutyric acid (GABA) neurotransmitter (Marshall et al., 1999): when expressed alone, the GABAB1 receptor is retained in the endoplasmic reticulum, and is able to reach the cell surface and bind GABA only in the presence of its partner, the GABAB2 receptor.
In 1982, Agnati and colleagues suggested that clusters of more than two receptors, representing higher-order oligomers, termed receptor mosaics (RMs), in which each receptor represents a single tessera within the mosaic, could exist and interact with each other (i.e. through allosteric interactions) in the plasma membrane (Agnati et al., 1982; Zoli et al., 1993). It was proposed that an RM functions as an integrated unit with unique signaling properties that are modulated by receptor–receptor allosteric interactions. The integrative action of an RM is likely to be affected not only by its stoichiometry, but also by the topological arrangement of its individual receptors (Agnati et al., 2005; Agnati et al., 2007). A recent study by Fung and colleagues supports the RM concept by demonstrating that highly purified β2AR protomers reconstituted into phospholipid vesicles can assemble together to form a tetramer complex that is stabilized by the binding of inverse agonists (Fung et al., 2009). This study shows not only that a GPCR can indeed exhibit cooperativity by forming higher-order oligomers beyond just homodimers, but also that GPCRs can assemble together through direct receptor–receptor interactions. Indeed, most studies of GPCR oligomerization being performed in live cells with both optical and biochemical approaches [e.g. FRET and bioluminescence resonance energy transfer (BRET)-based techniques, co-immunoprecipitation] to determine the formation and modulation of receptor–receptor complexes do not rule out the possibility that receptor oligomers are held together by scaffold proteins, rather than engaging in direct receptor–receptor interactions. The highly purified nature of the study by Fung and co-workers convincingly demonstrated that the formation of a higher-order β2AR complex takes place without other proteins.
Initial steps in the GPCR signaling system
Biophysical and biochemical approaches have revealed that GPCR signaling systems involve a succession of events that initially take place at the cell membrane and modulate the production and propagation of second-messenger molecules inside the cell (Fig. 2) (Lamb, 1996; Ferrandon et al., 2009). These events are initiated by ligand binding to an inactive receptor that switches to an active conformation. This receptor switch is driven by intramolecular rearrangement between the receptor's transmembrane helices, particularly helices 3 and 6 (Bourne, 1997). Activation of the β2-adrenergic receptor (β2-AR), for example, proceeds through the release of an ‘ionic lock’ that holds together the cytoplasmic sides of helix 3 and helix 6 in the inactive conformation of the receptor, and a rotamer toggle switch mechanism that modulates the conformation of helix 6 (Yao et al., 2006). These conformational changes are considered to expose the intracellular receptor domains at the cytosolic side, which interact and activate G proteins. Our FRET approach (for a review, see Vilardaga et al., 2009; Lohse et al., 2008) revealed that intramolecular rearrangements associated with receptor activation in live cells proceed with fast kinetics (Vilardaga et al., 2003). The activation of a receptor by a small neurotransmitter such as the adenosine A2A receptor, α2A- and β1-adrenergic receptors, and muscarinic receptors takes place with a time constant τ≈40 mseconds, whereas activation of the receptor for the larger parathyroid hormone proceeds within τ≈1 second (Vilardaga et al., 2003; Vilardaga, 2010; Maier-Peuschel et al., 2010). One of the mechanistic reasons for this 25-fold difference in receptor activation kinetics is thought to depend on distinct modes of binding between peptide hormones and smaller molecules (Castro et al., 2005; Vilardaga, 2010).
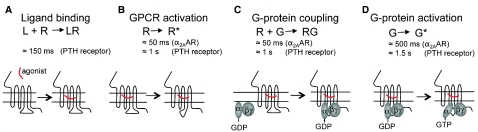
Fig. 2.
Kinetics of early reactions in the signaling cascade of GPCRs. Time constants (τ) of (A) ligand (L) association, (B) receptor (R) activation (R*), (C) receptor–G-protein association, and (D) G protein (G) activation (G*) are shown for two distinct GPCR systems: the parathyroid hormone receptor (PTHR) in response to the major endocrine regulator of Ca2+ homeostasis, PTH, and α2A-AR in response to the principal neurotransmitter of sympathetic nerves, norepinephrine.
Activated GPCRs then interact with heterotrimeric G proteins with kinetics that, in the context of the low level of G proteins, are not determined by the time course of receptor activation, but rather by a diffusion-limited collision process. At a high level of G-protein expression, however, a receptor–G-protein interaction can be as fast as receptor activation itself, with a time constant ranging from 40 mseconds to 1 second, depending on the nature of the receptor (Hein, 2005; Ferrandon et al., 2009). The step following the rapid interaction between an activated receptor and a G protein triggers the GDP–GTP exchange on the Gα subunit through conformational rearrangements or dissociation between the Gα and Gβγ subunits that occur with much slower kinetics, displaying a half-time (t1/2) of ~0.5–1 seconds for adrenergic and adenosine receptors, and t1/2≈1–2 seconds for the parathyroid hormone receptor (Bünemann et al., 2003; Nikolaev et al., 2006; Hein et al., 2006; Ferrandon et al., 2009). This step, as opposed to receptor activation and receptor–G-protein interactions, is thus the rate-limiting step for G-protein activation.
GPCR oligomers: static or dynamic interactions?
The family C GPCRs, which include the metabotropic glutamate receptors (mGluR1–mGluR5), the calcium-sensing receptor (CaSR) and the γ-aminobutyric acid receptors (GABABR), among others, differentiate themselves from the other GPCR families by large bilobed N-terminal extracellular domains known as the venus flytrap domains (Pin et al., 2003; Pin et al., 2005). These receptors associate with themselves and function as constitutive dimers. In many cases, receptor dimers are stabilized by disulfide bridges between Cys residues located in the venus flytrap domains (Romano et al., 1996; Ray et al., 1999; Ray and Hauschild, 2000). Most oliogomers of class A GPCRs, however, are formed through non-covalent interactions, and recent fluorescence recovery after photobleaching (FRAP) studies support the existence of a dynamic equilibrium between monomer and homodimer states of class A GPCRs, such as the dopamine D2 receptor (D2R) and the β1-AR (Dorsch et al., 2009; Fonseca and Lambert, 2009). The dynamics of a class A GPCR dimer has been further detailed by total internal reflection fluorescence (TIRF) microscopy, revealing that ~30% of recombinant muscarinic acetylcholine M1 receptor (M1R) expressed in CHO cells form transient homodimers with a life-time as short as 0.5 seconds (Hern et al., 2010). These studies suggest that at least some class A GPCRs, such as M1R, β1-AR and D2R, can transiently associate with each other, with half-lives presumably long enough to permit G-protein activation.
Modulation of cell signaling by fast inter-conformational switches
During the past decade, studies recently reviewed by Rozenfeld and Devi (Rozenfeld and Devi, 2010) revealed that receptor heterodimerization modulates pharmacological, signaling and trafficking properties of individual parent receptors. Examples of such fascinating aspects of receptor heteromerization are found in the different combinations between the taste receptors T1R1, TIR2 and TIR3, which modulate the sensitivity of taste molecules – the T1R1–T1R3 heterodimer mediates the glutamate umami taste, whereas the T1R2–T1R3 heterodimer mediates the sweet taste (Nelson et al., 2001; Zhao et al., 2003; Mueller et al., 2005). Receptor heterodimers between the adenosine A2A and dopamine D2 receptors is another attractive example of how receptor heterodimerization can modulate receptor function. This receptor dimer is expressed in striatopallidal GABAergic neurons that are present in the basal ganglia, a region of the brain that is involved in sensory–motor integration (Canals et al., 2003). Adenosine inhibits dopamine-induced locomotor activity, and by exerting opposite effects in the basal ganglia, the A2A–D2 receptor heteromer contributes to the fine-tuning of neural activity. At the biochemical level, the activation of A2A receptors by adenosine or adenosine analogs affects the pharmacology of D2 receptors by modulating the binding characteristics of dopamine agonists, and counteracts D2-receptor-mediated intracellular responses (Agnati et al., 2003). The binding of ligand to one receptor altering the activation of the partner receptor in the heterodimer complex led to the hypothesis that conformational changes induced by ligand binding to one receptor might be transmitted to the receptor partner to modulate its function (Zoli et al., 1993).
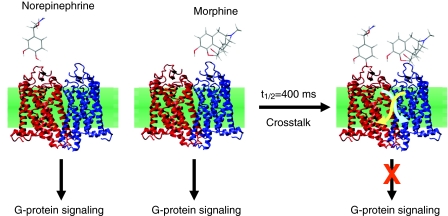
We confirmed this hypothesis with FRET studies in living cells, by showing that allosteric interactions between the α2A-AR and μ-opioid receptor (MOR) are mediated by direct cross-conformational changes between these receptors (Vilardaga et al., 2008). Norepinephrine and morphine, which are the native ligands for the α2A-AR and MOR, respectively, are well known to stimulate common inhibitory G protein (Gi)-mediated signaling pathways through their respective receptors, but the simultaneous action of norepinephrine and morphine produces a cellular response different from the expected additive effects (Jordan et al., 2003). In this case, morphine binding to the MOR triggers a rapid conformational change in the norepinephrine (NE)-bound α2A-AR that proceeds with a t1/2=400 mseconds, which is faster than the kinetics for G-protein activation (Fig. 3). This fast trans-conformational switch between receptors decreases both activation of Gi signaling and stimulation of MAP kinase phosphorylation. A trans-conformational switch in the reverse direction, from the α2A-AR towards the MOR, was not directly observed because FRET-based MOR biosensors are not functional. The inhibition of morphine-mediated G-protein activation by NE suggests, however, that such a conformational transfer also occurs. Thus, conformational crosstalk between receptors is bidirectional, and rapidly prevents overstimulation of signaling pathways by a combination of ligands acting on a receptor heteromer, by rapidly adjusting the extent of G-protein activation.
Fig. 3.
Modulation of G-protein signaling by receptor heterodimers. Representation of the α2A-AR (red)–MOR (blue) heterodimer complex based on the crystal structure of rhodopsin (coordinates from PDB code 1GZM), and chemical structures of norepinephrine and morphine. G-protein signaling mediated by the receptor heterodimer in response to norepinephrine or morphine is modulated by the simultaneous action of both signaling ligands. This modulation proceeds through conformational changes (arrows) that propagate from one receptor to the other with a half-life of 400 mseconds.
A fundamental property of ligands acting at a given GPCR is their capacity to stabilize different active receptor conformations that can each activate a unique signaling pathway. The term ‘functional selectivity’ was introduced as a parameter to express the ability of a ligand to produce a selective response (Kenakin and Miller, 2010). Functional selectivity in agonism is also linked with the GPCR heterodimer. For example, the Gi-coupled cannabinoid receptor (CB1R) forms a heterodimer complex with the GS-coupled A2AR and signals to Gi only when CB1 and A2A agonists are combined (Carriba et al., 2007). These findings suggest that the receptor–receptor interaction constrains the capacity of the CB1R to adopt an active conformation that can stimulate Gi activation in the CB1R–D2R heterodimer, and that this constraint is released only by the action of an A2A agonist. This release might be accomplished by means of inter-conformational switches between these two receptors. This hypothesis needs to be tested, but would indicate that functional selectivity at GPCR heteromers is modulated directly at the level of receptors through conformational crosstalk.
Perspectives and Conclusions
We have recently shown that signal transduction mediated by GPCRs involves a series of reversible reactions (ligand binding, receptor activation and deactivation, receptor–G-protein interactions, and G protein activation and deactivation) that initially take place at the plasma membrane (for a review see Vilardaga, 2010). These reactions, which are conveyed by interactions between ligands, receptors and G proteins, and conformational changes associated with the activation–deactivation process of each protein, can be modulated by dynamic receptor–receptor interactions. The capacity of GPCR heterodimers to modulate receptor function leads to the concept that dysregulation of receptor heterodimer expression and function might be implicated in several diseases (Prinster et al., 2005; Dalrymple et al., 2008; Rozenfeld and Devi, 2010). Recent studies show that A2AR, D2R and mGlu5R form a receptor heterocomplex in the striatal spine module (Cabello et al., 2009), a site that has a crucial role in controlling motor activity, motor learning and some forms of associative and visual learning. Imbalance of these activities caused by a gradual and progressive loss of dopaminergic neurons, which are implicated in glutamatergic, dopaminergic and adenosinergic neurotransmission, is thought to be one of the crucial determinants of Parkinson's disease. In recent years, the A2AR–D2R–mGlu5R heteromer has become a principal target for the treatment of Parkinson's disease, because antagonists of both A2AR and mGlu5R enhance D2R function (for a review, see Fuxe et al., 2007). The finding that adenosine and glutamate ligand binding to their respective receptors reduces (in the case of agonists) or increases (in the case of antagonists) dopamine D2R signaling suggests that allosteric interactions within the A2AR–D2R–mGlu5R heteromer regulate basal ganglia neurotransmission through a presumably fast inter-conformational switch. An extension of this theme implies that the integrative receptor–receptor interactions through an assembly of several distinct GPCRs, named higher-order receptor heteromers or receptor mosaics (Agnati et al., 1982), increases the complexity by which extracellular stimuli transduce signals into the cell (Box 1). Future studies will be needed to better understand this complexity by establishing the molecular details of the interface between receptors, and how conformational crosstalk between receptors modulates functional selectivity. The findings should generate new information relevant to the design of selective and bivalent synthetic ligands that target GPCR heteromers, which would be a promising approach to the development of new drugs. Examples of this approach are found in the work of Whistler, Franco and colleagues. The Whistler group showed that the agonist 6′-guanidinoaltrindole (6′-GNTI) selectively activates the heterodimer of δ- and κ-opioid receptors to produce an analgesic response in mice (Waldhoer et al., 2005), and the Franco group designed hetero-bivalent ligands containing both a D2R agonist and an A2AR antagonists coupled by chemical spacer to target the A2AR–D2R hetrodimer as a new strategy for the treatment of Parkinson's disease (Soriano et al., 2009).
Acknowledgments
This work was supported by the National Institutes of Health grant R01DK087688 (to J.-P.V). Deposited in PMC for release after 12 months.
References
- Agnati L. F., Fuxe K., Zini I., Lenzi P., Hokfelt T. (1980). Aspects on receptor regulation and isoreceptor identification. Med. Biol. 58, 182-187 [PubMed] [Google Scholar]
- Agnati L. F., Fuxe K., Zoli M., Rondanini C., Ogren S. O. (1982). New vistas on synaptic plasticity: the receptor mosaic hypothesis of the engram. Med Biol. 60, 183-190 [PubMed] [Google Scholar]
- Agnati L. F., Ferre S., Lluis S., Franco R., Fuxe K. (2003). Molecular mechanisms and therapeutical implications of intramembrane receptor/receptor interactions among heptahelical receptors with examples from the striatopallidal GABA neurons. Pharmacol. Rev. 55, 509-550 [DOI] [PubMed] [Google Scholar]
- Agnati L. F., Fuxe K., Ferre S. (2005). How receptor mosaics decode transmitter signals. Possible relevance of cooperativity. Trends Biochem. Sci. 30, 188-193 [DOI] [PubMed] [Google Scholar]
- Agnati L. F., Guidolin D., Leo G., Fuxe K. (2007). A boolean network modelling of receptor mosaics relevance of topology and cooperativity. J. Neural. Transm. 114, 77-92 [DOI] [PubMed] [Google Scholar]
- Albizu L., Cottet M., Kralikova M., Stoev S., Seyer R., Brabet I., Roux T., Bazin H., Bourrier E., Lamarque L., et al. (2010). Time-resolved FRET between GPCR ligands reveals oligomers in native tissues. Nat. Chem. Biol. 6, 587-594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R. (1997). How receptors talk to trimeric G proteins. Curr. Opin. Cell Biol. 9, 134-142 [DOI] [PubMed] [Google Scholar]
- Bünemann M., Frank M., Lohse M. J. (2003). Gi protein activation in intact cells involves subunit rearrangement rather than dissociation. Proc. Natl. Acad. Sci. USA 100, 16077-16082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello N., Gandía J., Bertarelli D. C., Watanabe M., Lluís C., Franco R., Ferré S., Luján R., Ciruela F. (2009). Metabotropic glutamate type 5, dopamine D2 and adenosine A2a receptors form higher-order oligomers in living cells. J. Neurochem. 109, 1497-1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals M., Marcellino D., Fanelli F., Ciruela F., de Benedetti P., Goldberg S. R., Neve K., Fuxe K., Agnati L. F., Woods A. S., et al. (2003). Adenosine A2A-dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J. Biol. Chem. 278, 46741-46749 [DOI] [PubMed] [Google Scholar]
- Carriba P., Ortiz O., Patkar K., Justinova Z., Stroik J., Themann A., Müller C., Woods A. S., Hope B. T., Ciruela F., et al. (2007). Striatal adenosine A2A and cannabinoid CB1 receptors form functional heteromeric complexes that mediate the motor effects of cannabinoids. Neuropsychopharmacology 32, 2249-2259 [DOI] [PubMed] [Google Scholar]
- Castro M., Nikolaev O. V., Palm D., Lohse M. J., Vilardaga J.-P. (2005). Turn-on switch in parathyroid hormone receptor by a two-step PTH binding mechanism. Proc. Natl. Acad. Sci. USA 102, 16084-16089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabre M., Le Maire M. (2005). Monomeric G-protein-coupled receptors as a functional unit. Biochemistry 44, 9395-9403 [DOI] [PubMed] [Google Scholar]
- Chabre M., Deterre P., Antonny B. (2009). The apparent cooperativity of some GPCRs does not necessarily imply dimerization. Trends Pharmacol Sci. 30, 182-187 [DOI] [PubMed] [Google Scholar]
- Dalrymple M. B., Pfleger K. D. G., Eidne K. A. (2008). G protein-coupled receptor dimmers: functional consequences, disease states and drug targets. Pharmacol. Ther. 118, 359-371 [DOI] [PubMed] [Google Scholar]
- Dorsch S., Klotz K. N., Engelhardt S., Lohse M. J., Bünemann M. (2009). Analysis of receptor oligomerization by FRAP microscopy. Nat. Methods 6, 225-230 [DOI] [PubMed] [Google Scholar]
- Ferrandon S., Feinstein T. N., Castro C., Bouley R., Potts J. T., Gardella T. J., Vilardaga J.-P. (2009). Parathyroid hormone mediates sustained cyclic AMP production by endocytosis of ligand-receptor-G protein complexes. Nat. Chem. Biol. 5, 734-742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca J. M., Lambert N. A. (2009). Instability of a class A G protein-coupled receptor interface. Mol. Pharmacol. 75, 1296-1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung J. J., Deupi X., Pardo L., Yao X. J., Velez-Ruiz G. A., Devree B. T., Sunahara R. K., Kobilka B. K. (2009). Ligand-specific regulation of beta(2)-adrenoceptors in a model lipid bilayer. EMBO J. 28, 3315-3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K., Agnati L. F. (1985). Receptor-receptor interactions in the central nervous system. A new integrative mechanism in synapses. Med. Res. Rev. 5, 441-482 [DOI] [PubMed] [Google Scholar]
- Fuxe K., Agnati L. F., Benfenati F., Cimmino M., Algeri S., Hökfelt T., Mutt V. (1981). Modulation by cholecystokinins of 3H-spiroperidol binding in rat striatum: evidence for increased affinity and reduction in the number of binding sites. Acta Physiol. Scand. 113, 567-569 [DOI] [PubMed] [Google Scholar]
- Fuxe K., Agnati L. F., Benfenati F., Celani M., Zini I., Zoli M., Mutt V. (1983). Evidence for the existence of receptor-receptor interactions in the central nervous system. Studies on the regulation of monoamine receptors by neuropeptides. J. Neural. Transm. 18, 165-179 [PubMed] [Google Scholar]
- Fuxe K., von Euler G., van der Ploeg I., Fredholm B. B., Agnati L. F. (1989). Pertussis toxin treatment counteracts the cardiovascular effects of neuropeptide Y and clonidine in the awake unrestrained rat. Neurosci. Lett. 101, 337-341 [DOI] [PubMed] [Google Scholar]
- Fuxe K., Marcellino D., Genedani S., Aganti L. F. (2007). Adenosine A2A receptors, dopamine D2 receptors and their interactions in Parkinson's disease. Mov. Disord. 22, 1990-2014 [DOI] [PubMed] [Google Scholar]
- Guo W., Urizar E., Kralikova M., Mobarec J. C., Shi L., Filizola M., Javitch J. A. (2008). Dopamine D2 receptor form higher order oligomers at physiological expression levels. EMBO J. 27, 2293-2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M. A., Stevens R. C. (2009). Discovery of New GPCR biology – one receptor structure at a time. Structure 17, 8-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein P., Rochais F., Hoffmann C., Dorsch S., Nikolaev V. O., Engelhardt S., Berlot C. H., Lohse M. J., Bünemann M. (2006). Gs activation is time-limiting in initiating receptor-mediated signaling. J. Biol. Chem. 281, 33345-33351 [DOI] [PubMed] [Google Scholar]
- Hofmann K. P., Scheerer P., Hildebrand P. W., Choe H. W., Park J. H., Heck M., Ernst O. P. (2009). A G protein-coupled receptor at work: the rhodopsin model. Trends Biochem. Sci. 34, 540-552 [DOI] [PubMed] [Google Scholar]
- Jordan B. A., Gomes C., Rios J., Filipovska J., Devi L. A. (2003). Functional interaction between μ-opioid and α2A-adrenergic receptors receptors. Mol. Pharmacol. 64, 1317-1324 [DOI] [PubMed] [Google Scholar]
- Kenakin T., Miller L. J. (2010). Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol. Rev. 62, 265-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuszak A. J., Pitchiaya S., Anand J. P., Mosberg H. I., Walter N. G., Sunahara R. K. (2008). Purification and functional reconstitution of monomeric μ-opioid receptors: allosteric modulation of agonist binding by Gi2. J. Biol. Chem. 284, 26732-26741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. (1996). Gain and kinetics of activation in the G-protein cascade of phototransduction. Proc. Natl. Acad. Sci. USA 93, 566-570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbird L. E., Meyts P. D., Lefkowitz R. J. (1975). Beta-adrenergic receptors: evidence for negative cooperativity. Biochem. Biophys. Res. Commun. 64, 1160-1168 [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Nikolaev N. O., Hein P., Hoffmann C., Vilardaga J.-P., Bünemann M. (2008). Optical techniques to analyze real-time activation and signaling of G-protein-coupled receptors. Trends Pharmacol. Sci. 29, 159-165 [DOI] [PubMed] [Google Scholar]
- Maier-Peuschel M., Frolich N., Dees C., Hommers L. G., Hoffmann C., Nikolaev V. O., Lohse M. J. (2010). A FRET-based M2 muscarinic receptor sensor reveals rapid kinetics of allosteric modulation. J. Biol. Chem. 285, 8793-8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall F. H., Jones K. A., Kaupmann K., Bettler B. (1999). GABAB receptors-the first 7TM heterodimers. Trends Pharmacol. Sci. 20, 396-399 [DOI] [PubMed] [Google Scholar]
- Milligan G. (2009). G protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br. J. Pharmacol. 158, 5-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller K. L., Hoon M. A., Erlenbach I., Chandrashekar J., Zuker C. S., Ryba N. J. P. (2005). The receptors and coding for bitter taste. Nature 434, 225-229 [DOI] [PubMed] [Google Scholar]
- Nelson G., Hoon M. A., Chandrashekar J., Zhang Y., Ryba N. J. P., Zuker C. S. (2001). Mammalian sweet taste receptors. Cell 106, 381-390 [DOI] [PubMed] [Google Scholar]
- Nikolaev O. V., Hoffmann C., Bünemann M., Lohse M. J., Vilardaga J.-P. (2006). Molecular basis of partial agonism at the neurotransmitter α2A-adrenergic receptor and Gi-protein heterotrimer. J. Biol. Chem. 281, 24506-24511 [DOI] [PubMed] [Google Scholar]
- Pierce K. L., Premont R. T., Lefkowitz R. J. (2002). Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 3, 639-650 [DOI] [PubMed] [Google Scholar]
- Pin J.-P., Galvez T., Prezeau L. (2003). Evolution, structure and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol. Ther. 98, 325-354 [DOI] [PubMed] [Google Scholar]
- Pin J.-P., Kniazeff J., Liu J., Binet V., Goudet C., Rondard P., Prezeau L. (2005). Allosteric functioning of dimeric class C G-protein-coupled receptors. FEBS J. 272, 2947-2955 [DOI] [PubMed] [Google Scholar]
- Prinster S. C., Hague C., Hall R. A. (2005). Heterodimerization of G protein-coupled receptors: specificity and functional significance. Pharmacol. Rev. 57, 289-298 [DOI] [PubMed] [Google Scholar]
- Ray K., Hauschild B. C. (2000). Cys-140 is critical for metabotropic glutamate receptr-1 (mGlu-1) dimerization. J. Biol. Chem. 275, 34245-34251 [DOI] [PubMed] [Google Scholar]
- Ray K., Hauschild B. C., Steinbach P. J., Goldsmith P. K., Hauache O., Spiegel A. M. (1999). Identification of the cysteine residues in the amino-terminal extracellular domain of the human Ca2+ receptor critical for dimerization. Implications for function of monomeric Ca2+ receptor. J. Biol. Chem. 274, 27642-27650 [DOI] [PubMed] [Google Scholar]
- Rivero-Muller A., Chou Y. Y., Ji I., Lajic S., Hanyaloglu A. C., Jonas K., Rahman N., Ji T. H., Huhtaniemi I. (2010). Rescues of defective G protein-coupled receptor function in vivo by intermolecular cooperation. Proc. Natl. Acad. Sci. USA 107, 2319-2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C., Yan W.-L., O'Malley K. L. (1996). Metabotropic glutamate receptor 5 is a disulfide-linked dimer. J. Biol. Chem. 271, 28612-28616 [DOI] [PubMed] [Google Scholar]
- Rozenfeld R., Devi L. A. (2010). Receptor heteromerization and drug discovery. Trends Pharmacol. Sci. 31, 124-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöneberg T., Schulz A., Biebermann H., Hermsdorf T., Rompler H., Sangkuhl K. (2004). Mutant G protein-coupled receptord as a cause of human diseases. Pharmacol. Ther. 104, 173-206 [DOI] [PubMed] [Google Scholar]
- Soriano A., Ventura R., Molero A., Hoen R., Casadó V., Cortés A., Fanelli F., Albericio F., Lluís C., Franco R., et al. (2009). Adenosine A2A receptor-antagonist/dopamine D2 receptor-agonist bivalent ligands as pharmacological tools to detect A2A-D2 receptor heteromers. J. Med. Chem. 52, 5590-5602 [DOI] [PubMed] [Google Scholar]
- Vilardaga J.-P. (2010). Theme and variations on kinetics of GPCR activation/deactivation. J. Recept. Signal Transduct. Res. 30, 304-312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilardaga J. P., Bünemann M., Krasel C., Castro M., Lohse M. J. (2003). Measurement of the millisecond activation switch of G protein-coupled receptors in living cells. Nat. Biotechnol. 21, 807-812 [DOI] [PubMed] [Google Scholar]
- Vilardaga J.-P., Nikolaev O. V., Lorentz K., Ferrandon S., Zhuang Z., Lohse M. J. (2008). Direct inhibition of G protein signaling by cross-conformational switches between α2A-adrenergic and μ-opioid receptors. Nat. Chem. Biol. 4, 126-131 [DOI] [PubMed] [Google Scholar]
- Vilardaga J.-P., Bünemann M., Feinstein T. N., Lambert N., Nikolaev V. O., Engelhardt S., Lohse M. J., Hoffmann C. (2009). GPCR and G proteins: drug efficacy and activation in live cells. Mol. Endocrinol. 23, 590-599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilardaga J.-P., Romero G., Friedman P. A., Gardella T. J. (2010). Molecular basis of parathyroid hormone receptor signaling and trafficking: a family B GPCR paradigm. Cell. Mol. Life Sci. Epub ahead of print. PMID: 20703892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhoer M., Fong J., Jones R. M., Lunzer M. M., Sharma S. K., Kostenis E., Portoghese P. S., Whistler J. L. (2005). heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc. Natl. Acad. Sci. USA 102, 9050-9055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorton M. R., Bokoch M. P., Rasmussen S. G., Huang B., Zare R. N., Kobilka B., Sunahara R. K. (2007). A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc. Natl. Acad. Sci. USA 104, 7682-7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorton M. R., Jastrzebska B., Park P. S., Fotiadis D., Engel A., Palczewski K., Sunahara R. K. (2008). Efficient coupling of transducin to monomeric rhodopsin in a phospholipid bilayer. J. Biol. Chem. 283, 4387-4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. N., Fior D. R., Hedlund P. B., Agnati L. F., Fuxe K. (1994). Antagonistic regulation of alpha 2-adrenoceptors by neuropeptide Y receptor subtypes in the nucleus tractus solitarii. Eur. J. Pharmacol. 271, 201-212 [DOI] [PubMed] [Google Scholar]
- Yao X., Parnot C., Deupi X., Ratnala V. R., Swaminath G., Farrens D., Kobilka B. (2006). Coupling ligand structure to specific conformational switches in the beta2-adrenoreceptor. Nat. Chem. Biol. 2, 417-422 [DOI] [PubMed] [Google Scholar]
- Zhao G. Q., Zhang Y., Hoon M. A., Chandrashekar J., Erlenbach I., Ryba N. J. P., Zuker C. S. (2003). Mammalian sweet taste receptors. Cell 115, 255-266 [DOI] [PubMed] [Google Scholar]
- Zoli M., Agnati L. F., Hedlund P. B., Li X. M., Ferre S., Fuxe K. (1993). Receptor-receptor interactions as an integrative mechanism in nerve cells. Mol. Neurobiol. 7, 293-334 [DOI] [PubMed] [Google Scholar]