Abstract
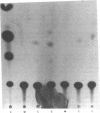
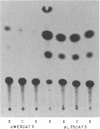
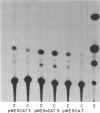
In previous studies, we showed that acute administration of iron to intact rats or to rat hepatoma cells in culture induces synthesis of the iron-storage protein ferritin by activating translation of inactive cytoplasmic ferritin mRNAs for both the heavy (H) and the light (L) subunits. In the course of activation, these ferritin mRNAs are recruited onto polysomes. To elucidate the structural features of these mRNAs involved in the translational response to iron, a chimera was constructed from the 5' and 3' untranslated regions (UTRs) of ferritin L subunit mRNA fused to the reading frame of the mRNA of bacterial chloramphenicol acetyltransferase (CAT). This chimera and deletion constructs derived from it were introduced into a rat hepatoma cell line by retrovirus-mediated gene transfer. The complete chimera showed increased CAT activity in response to iron enrichment of the medium, whereas deletion of the first 67 nucleotides of the 5' UTR, which contain a highly conserved sequence, caused loss of regulation by iron. Whereas cis-acting sequences located in the 5' flanking regions of many genes have been repeatedly implicated in modulating their transcriptional expression, we report here a specific regulatory translational sequence found within the 5' UTR of a eukaryotic mRNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aziz N., Munro H. N. Both subunits of rat liver ferritin are regulated at a translational level by iron induction. Nucleic Acids Res. 1986 Jan 24;14(2):915–927. doi: 10.1093/nar/14.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Choudary P. V., Tsuji S., Martin B. M., Guild B. C., Mulligan R. C., Murray G. J., Barranger J. A., Ginns E. I. The molecular biology of Gaucher disease and the potential for gene therapy. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):1047–1052. doi: 10.1101/sqb.1986.051.01.121. [DOI] [PubMed] [Google Scholar]
- Costanzo F., Colombo M., Staempfli S., Santoro C., Marone M., Frank R., Delius H., Cortese R. Structure of gene and pseudogenes of human apoferritin H. Nucleic Acids Res. 1986 Jan 24;14(2):721–736. doi: 10.1093/nar/14.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didsbury J. R., Theil E. C., Kaufman R. E., Dickey L. F. Multiple red cell ferritin mRNAs, which code for an abundant protein in the embryonic cell type, analyzed by cDNA sequence and by primer extension of the 5'-untranslated regions. J Biol Chem. 1986 Jan 15;261(2):949–955. [PubMed] [Google Scholar]
- Godefroy-Colburn T., Thivent C., Pinck L. Translational discrimination between the four RNAs of alfalfa mosaic virus. A quantitative evaluation. Eur J Biochem. 1985 Mar 15;147(3):541–548. doi: 10.1111/j.0014-2956.1985.00541.x. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling S. A., Gehrke L. Enhanced translation of chimaeric messenger RNAs containing a plant viral untranslated leader sequence. Nature. 1987 Feb 12;325(6105):622–625. doi: 10.1038/325622a0. [DOI] [PubMed] [Google Scholar]
- Korman A. J., Frantz J. D., Strominger J. L., Mulligan R. C. Expression of human class II major histocompatibility complex antigens using retrovirus vectors. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2150–2154. doi: 10.1073/pnas.84.8.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneluk R. G., Quan F., Gravel R. A. Rapid and reliable dideoxy sequencing of double-stranded DNA. Gene. 1985;40(2-3):317–323. doi: 10.1016/0378-1119(85)90055-1. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibold E. A., Aziz N., Brown A. J., Munro H. N. Conservation in rat liver of light and heavy subunit sequences of mammalian ferritin. Presence of unique octopeptide in the light subunit. J Biol Chem. 1984 Apr 10;259(7):4327–4334. [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Characterization and evolution of the expressed rat ferritin light subunit gene and its pseudogene family. Conservation of sequences within noncoding regions of ferritin genes. J Biol Chem. 1987 May 25;262(15):7335–7341. [PubMed] [Google Scholar]
- Logan J., Shenk T. Adenovirus tripartite leader sequence enhances translation of mRNAs late after infection. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3655–3659. doi: 10.1073/pnas.81.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry T. J., Lindquist S. The preferential translation of Drosophila hsp70 mRNA requires sequences in the untranslated leader. Cell. 1985 Oct;42(3):903–911. doi: 10.1016/0092-8674(85)90286-7. [DOI] [PubMed] [Google Scholar]
- Murray M. T., White K., Munro H. N. Conservation of ferritin heavy subunit gene structure: implications for the regulation of ferritin gene expression. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7438–7442. doi: 10.1073/pnas.84.21.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
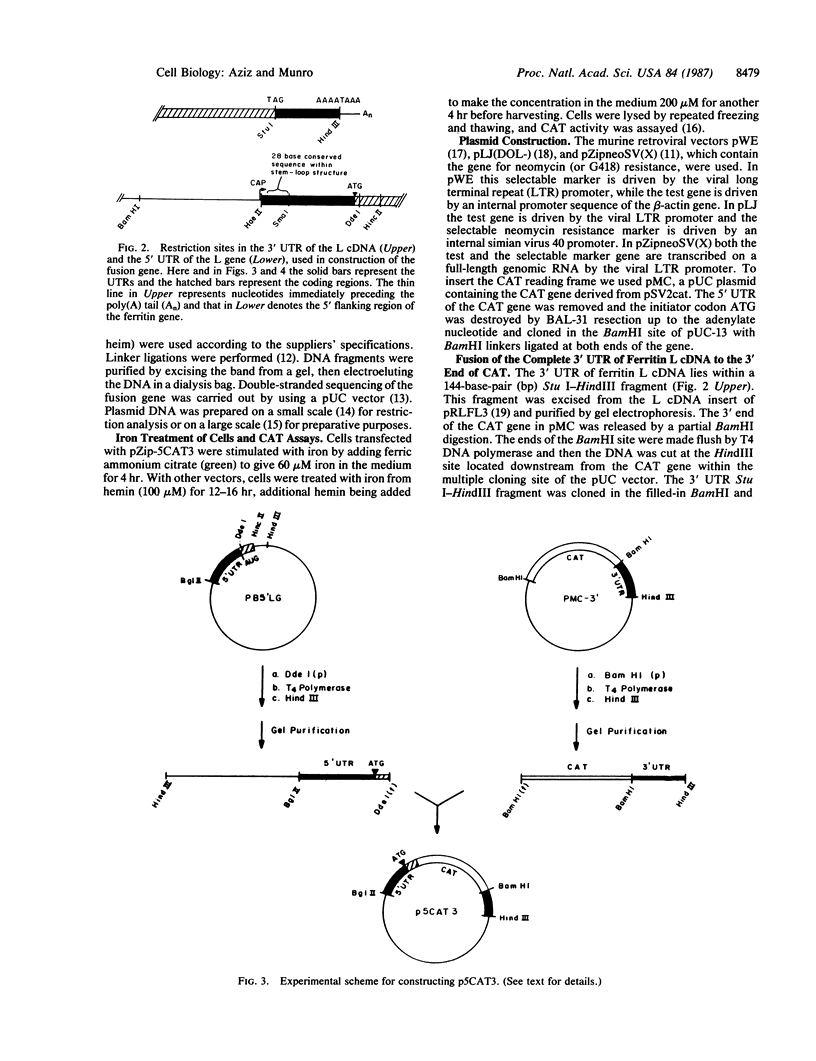
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5' noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell. 1985 Mar;40(3):515–526. doi: 10.1016/0092-8674(85)90200-4. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Munro H. Translation of ferritin light and heavy subunit mRNAs is regulated by intracellular chelatable iron levels in rat hepatoma cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2277–2281. doi: 10.1073/pnas.84.8.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Hayday A. C., Wiman K., Hayward W. S., Tonegawa S. Activation of the c-myc gene by translocation: a model for translational control. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7476–7480. doi: 10.1073/pnas.80.24.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro C., Marone M., Ferrone M., Costanzo F., Colombo M., Minganti C., Cortese R., Silengo L. Cloning of the gene coding for human L apoferritin. Nucleic Acids Res. 1986 Apr 11;14(7):2863–2876. doi: 10.1093/nar/14.7.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P., Verma I. M. Modulation of c-fos gene transcription by negative and positive cellular factors. Nature. 1987 Apr 2;326(6112):507–510. doi: 10.1038/326507a0. [DOI] [PubMed] [Google Scholar]
- Sonenberg N., Guertin D., Cleveland D., Trachsel H. Probing the function of the eucaryotic 5' cap structure by using a monoclonal antibody directed against cap-binding proteins. Cell. 1981 Dec;27(3 Pt 2):563–572. doi: 10.1016/0092-8674(81)90398-6. [DOI] [PubMed] [Google Scholar]
- Stevens P. W., Dodgson J. B., Engel J. D. Structure and expression of the chicken ferritin H-subunit gene. Mol Cell Biol. 1987 May;7(5):1751–1758. doi: 10.1128/mcb.7.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerton J., Atkins T., Bestwick R. A rapid method for preparation of bacterial plasmids. Anal Biochem. 1983 Aug;133(1):79–84. doi: 10.1016/0003-2697(83)90224-5. [DOI] [PubMed] [Google Scholar]
- Thireos G., Penn M. D., Greer H. 5' untranslated sequences are required for the translational control of a yeast regulatory gene. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5096–5100. doi: 10.1073/pnas.81.16.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zähringer J., Baliga B. S., Munro H. N. Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc Natl Acad Sci U S A. 1976 Mar;73(3):857–861. doi: 10.1073/pnas.73.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]