Abstract
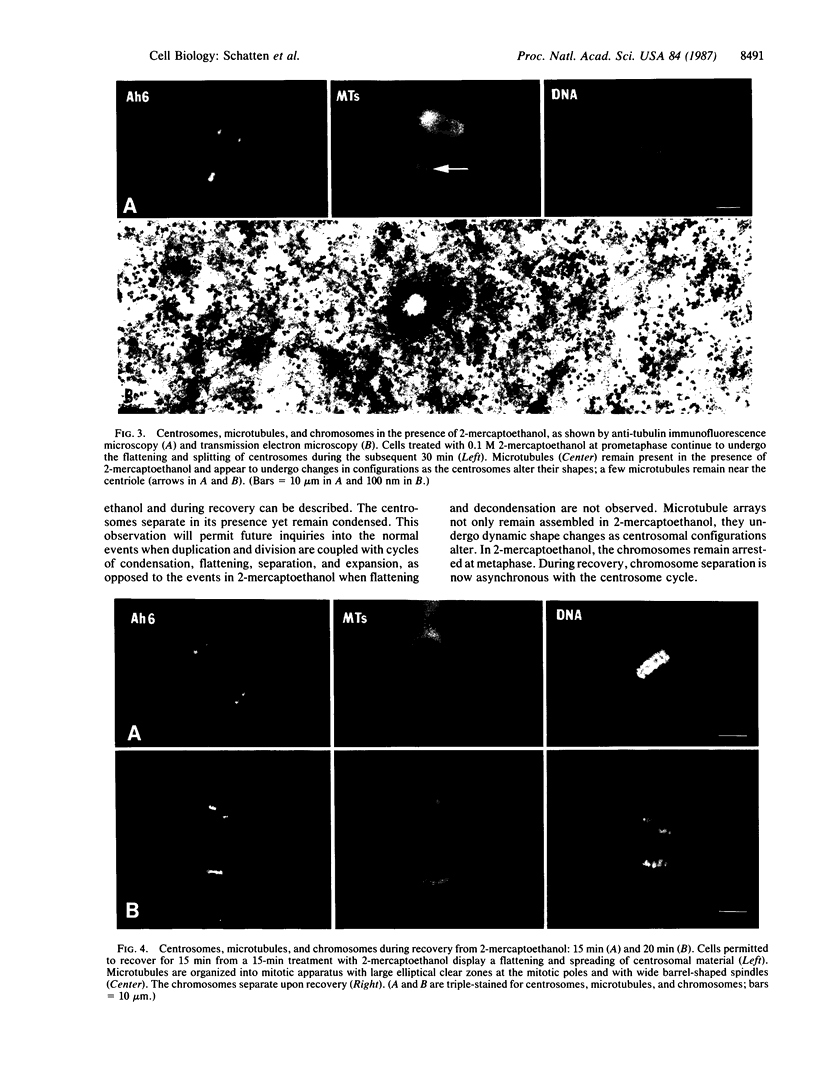
A mouse monoclonal antibody generated against Drosophila intermediate filament proteins (designated Ah6/5/9 and referred to herein as Ah6) is found to cross-react specifically with centrosomes in sea urchin eggs and with a 68-kDa antigen in eggs and isolated mitotic apparatus. When preparations stained with Ah6 are counterstained with a human autoimmune serum whose anti-centrosome activity has been established, the immunofluorescence images superimpose exactly. A more severe test of the specificity of the antibody demands that it display all of the stages of the centrosome cycle in the cell cycle: the flattening and spreading of the compact centrosomes followed by their division and the establishment of two compact poles. The test was made by an experimental design that uses a period of exposure of the eggs to 2-mercaptoethanol. This treatment allows observation of the stages of the centrosome cycle--separation, division, and bipolarization--while the chromosomes are arrested in metaphase. Mitosis is arrested in the presence of 0.1 M 2-mercaptoethanol. Chromosomes remain in a metaphase configuration while the centrosomes divide, producing four poles perpendicular to the original spindle axis. Microtubules are still present in the mitotic apparatus, as indicated by immunofluorescence and transmission electron microscopy. When 2-mercaptoethanol is removed, the chromosomes reorient to the poles of a tetrapolar (sometimes tripolar) mitotic apparatus. During the following cycle, the blastomeres form a monopolar mitotic apparatus. The observations of the centrosome cycle with the Ah6 antibody display very clearly all the stages that have been seen or deduced from work with other probes. The 68-kDa antigen that reacts with the Ah6 monoclonal antibody to Drosophila intermediate filament proteins must be a constant component of sea urchin centrosomes because it is present at all stages of the centrosome cycle.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht-Buehler G. Is cytoplasm intelligent too? Cell Muscle Motil. 1985;6:1–21. doi: 10.1007/978-1-4757-4723-2_1. [DOI] [PubMed] [Google Scholar]
- Calarco-Gillam P. D., Siebert M. C., Hubble R., Mitchison T., Kirschner M. Centrosome development in early mouse embryos as defined by an autoantibody against pericentriolar material. Cell. 1983 Dec;35(3 Pt 2):621–629. doi: 10.1016/0092-8674(83)90094-6. [DOI] [PubMed] [Google Scholar]
- Clayton L., Black C. M., Lloyd C. W. Microtubule nucleating sites in higher plant cells identified by an auto-antibody against pericentriolar material. J Cell Biol. 1985 Jul;101(1):319–324. doi: 10.1083/jcb.101.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly J. A., Kalnins V. I. Visualization of centrioles and basal bodies by fluorescent staining with nonimmune rabbit sera. J Cell Biol. 1978 Nov;79(2 Pt 1):526–532. doi: 10.1083/jcb.79.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner F. G., Saumweber H., Biessmann H. Two Drosophila melanogaster proteins related to intermediate filament proteins of vertebrate cells. J Cell Biol. 1981 Oct;91(1):175–183. doi: 10.1083/jcb.91.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon N. D., Porter K. R., McNiven M. A. Three dimensional structure of the cell center revealed by computer graphics methodology. Biophys J. 1986 Jan;49(1):65–66. doi: 10.1016/S0006-3495(86)83594-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosti-Testu F., Marty M. C., Berges J., Maunoury R., Bornens M. Identification of centrosomal proteins in a human lymphoblastic cell line. EMBO J. 1986 Oct;5(10):2545–2550. doi: 10.1002/j.1460-2075.1986.tb04533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosti F., Marty M. C., Courvalin J. C., Maunoury R., Bornens M. Centrosomal proteins and lactate dehydrogenase possess a common epitope in human cell lines. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1000–1004. doi: 10.1073/pnas.84.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R., Borisy G. G. Identification of molecular components of the centrosphere in the mitotic spindle of sea urchin eggs. J Cell Biol. 1985 Aug;101(2):524–530. doi: 10.1083/jcb.101.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZIA D., ZIMMERMAN A. M. SH compounds in mitosis. II. The effect of mercaptoethanol on the structure of the mitotic apparatus in sea urchin eggs. Exp Cell Res. 1958 Aug;15(1):138–153. doi: 10.1016/0014-4827(58)90070-3. [DOI] [PubMed] [Google Scholar]
- Maro B., Howlett S. K., Webb M. Non-spindle microtubule organizing centers in metaphase II-arrested mouse oocytes. J Cell Biol. 1985 Nov;101(5 Pt 1):1665–1672. doi: 10.1083/jcb.101.5.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunoury M. R. Localisation immunocytochimique de la centrosphère de cellules tumorales humaines par utilisation d'anticorps naturels de lapin. C R Acad Sci Hebd Seances Acad Sci D. 1978 Feb 13;286(6):503–506. [PubMed] [Google Scholar]
- Mazia D. Centrosomes and mitotic poles. Exp Cell Res. 1984 Jul;153(1):1–15. doi: 10.1016/0014-4827(84)90442-7. [DOI] [PubMed] [Google Scholar]
- Mazia D., Harris P. J., Bibring T. The Multiplicity of the Mitotic Centers and the Time-Course of Their Duplication and Separation. J Biophys Biochem Cytol. 1960 Feb 1;7(1):1–20. doi: 10.1083/jcb.7.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazia D., Paweletz N., Sluder G., Finze E. M. Cooperation of kinetochores and pole in the establishment of monopolar mitotic apparatus. Proc Natl Acad Sci U S A. 1981 Jan;78(1):377–381. doi: 10.1073/pnas.78.1.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazia D. The chromosome cycle and the centrosome cycle in the mitotic cycle. Int Rev Cytol. 1987;100:49–92. doi: 10.1016/s0074-7696(08)61698-8. [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Schäfer G., Hilz H., Eppenberger H. M. Cyclic-AMP-dependent protein kinase type II is associated with the Golgi complex and with centrosomes. Cell. 1985 Jul;41(3):1039–1051. doi: 10.1016/s0092-8674(85)80084-2. [DOI] [PubMed] [Google Scholar]
- Paweletz N., Mazia D., Finze E. M. The centrosome cycle in the mitotic cycle of sea urchin eggs. Exp Cell Res. 1984 May;152(1):47–65. doi: 10.1016/0014-4827(84)90229-5. [DOI] [PubMed] [Google Scholar]
- Petzelt C., Hafner M. Visualization of the Ca-transport system of the mitotic apparatus of sea urchin eggs with a monoclonal antibody. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1719–1722. doi: 10.1073/pnas.83.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon E. D., Segall R. R. Calcium-labile mitotic spindles isolated from sea urchin eggs (Lytechinus variegatus). J Cell Biol. 1980 Aug;86(2):355–365. doi: 10.1083/jcb.86.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatten H., Schatten G., Mazia D., Balczon R., Simerly C. Behavior of centrosomes during fertilization and cell division in mouse oocytes and in sea urchin eggs. Proc Natl Acad Sci U S A. 1986 Jan;83(1):105–109. doi: 10.1073/pnas.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R. B. Mitosis in sand dollar embryos is inhibited by antibodies directed against the calcium transport enzyme of muscle. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4302–4306. doi: 10.1073/pnas.83.12.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G., Begg D. A. Control mechanisms of the cell cycle: role of the spatial arrangement of spindle components in the timing of mitotic events. J Cell Biol. 1983 Sep;97(3):877–886. doi: 10.1083/jcb.97.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G., Rieder C. L. Centriole number and the reproductive capacity of spindle poles. J Cell Biol. 1985 Mar;100(3):887–896. doi: 10.1083/jcb.100.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turksen K., Aubin J. E., Kalnins V. I. Identification of a centriole-associated protein by antibodies present in normal rabbit sera. Nature. 1982 Aug 19;298(5876):763–765. doi: 10.1038/298763a0. [DOI] [PubMed] [Google Scholar]
- Vandre D. D., Davis F. M., Rao P. N., Borisy G. G. Phosphoproteins are components of mitotic microtubule organizing centers. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4439–4443. doi: 10.1073/pnas.81.14.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M. F., Biessmann H. Intermediate-sized filaments in Drosophila tissue culture cells. J Cell Biol. 1984 Oct;99(4 Pt 1):1468–1477. doi: 10.1083/jcb.99.4.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick S. M. Immunofluorescence microscopy of tubulin and microtubule arrays in plant cells. III. Transition between mitotic/cytokinetic and interphase microtubule arrays. Cell Biol Int Rep. 1985 Apr;9(4):357–371. doi: 10.1016/0309-1651(85)90031-1. [DOI] [PubMed] [Google Scholar]