Abstract
Regulator of G-protein signaling 4 (RGS4), an intracellular modulator of G-protein coupled receptor (GPCR)-mediated signaling, is regulated by multiple processes including palmitoylation and proteasome degradation. We found that co-expression of DHHC acyltransferases (DHHC3 or DHHC7), but not their acyltransferase-inactive mutants, increased expression levels of RGS4 but not its Cys2 to Ser mutant (RGS4C2S). DHHC3 interacts with and palmitoylates RGS4 but not RGS4C2S in vivo. Palmitoylation prolongs the half-life of RGS4 by over 8-fold and palmitoylated RGS4 blocked α1A-adrenergic receptor -stimulated intracellular Ca2+ mobilization. Together, our findings revealed that DHHC proteins could regulate GPCR-mediated signaling by increasing RGS4 stability.
Structured summary
MINT-8049215: Rgs4 (uniprotkb:P49799) physically interacts (MI:0915) with DHHC3 (uniprotkb:Q8R173) by anti tag coimmunoprecipitation (MI:0007)
Keywords: G-protein coupled receptor, Regulator of G-protein signaling 4, Proteasome degradation, Palmitoylation, DHHC acyltransferases, α1A-adrenergic receptor
1. Introduction
The basic unit of a G-protein coupled receptor (GPCR) signaling system contains four major components: receptor, G-protein, effector and regulator of G-protein signaling (RGS) protein [1]. G-proteins, classified into Gs, Gi, Gq and G12 subfamilies, stimulate intracellular signal proteins (effectors) when GTP binds to the G-protein in response to ligand-activation of GPCRs. Signaling ends when the G-protein hydrolyzes the bound GTP. RGS proteins increase GTP hydrolysis rates of G-proteins up to 1000-fold, thus profoundly inhibiting downstream consequences of GPCR activation.
Regulator of G-protein signaling 4 (RGS4) regulates signaling mediated by GPCR coupled to Gi and Gq, and plays an important role in regulation of the cardiovascular [2] and central nervous systems [3] as well as tumorigenesis [4]. We reported that RGS4 down-regulation in breast cancer cells is due to proteasome degradation, and that proteasome blockade increased RGS4 protein to levels that markedly inhibit breast cancer metastatic abilities [5]. Interestingly, RGS4 is not intrinsically unstable, but is made unstable by N-arginylation [6; 7] at the oxidized N-terminal Cys (Cys2) [8] and then ubiquitinated and degraded by proteasomes [6; 9]. Thus, identification of mechanisms underlying the regulation of oxidation-sensitive proteasome degradation of RGS4 is important.
RGS4 is also known to be palmitoylated, a reversible thioester attachment of palmitate to Cys in a protein. Previous studies demonstrated that the primary palmitoylation site of RGS4 was Cys2 [10;11]. Since oxidation of Cys2 is critical for RGS4 degradation [8], it would be important to determine whether palmitoylation regulates RGS4 protein stability.
Palmitoyl acyltransferases are integral membrane proteins with a conserved DHHC (Asp-His-His-Cys) motif, and are largely localized to Golgi [12]. The DHHC motif is crucial for enzyme activity since mutation of Cys to Ser eliminates palmitoyltransferase activity [13]. A recent study demonstrated that DHHC3 and DHHC7 can palmitoylate Gα to control Gα localization to the plasma membrane, thus regulating GPCR signaling [13]. In the present study, we sought to determine if DHHC proteins palmitoylate RGS4 and how this impacts RGS4 stability given the convergence of these processes on the Cys2 residue. Our data are the first to show that DHHC3 and DHHC7 are capable of palmitoylating RGS4 in vivo, which prolongs RGS4 half-life by interfering with its proteasome degradation, thus attenuating α1A-adrenergic receptor (α1A-AR)-stimulated intracellular Ca2+ mobilization.
2. Materials and methods
2.1. Cell lines and reagents
HEK293 cells and MDA-MB-231 breast cancer cells were from American Type Culture Collection, and maintained in Dulbecco's Modified Eagle's Medium (DMEM) with 10% fetal bovine serum (FBS, Invitrogen). Primary antibodies include mouse anti-HA (Covance), rabbit anti-Myc (ABM), and goat anti-β-actin (Santa Cruz Biotechnology). Hydroxylamine, 2-bromopalmitate (2-BP), dithiothreitol (DTT), cycloheximide, and MG132 were from Sigma-Aldrich. 17-Octadecynoic acid (17-ODYA) was from Cayman Chemical Company.
2.2. Western blot analysis
Proteins were extracted from cells using 1x radioimmunoprecipitation assay lysis buffer (RIPA; Santa Cruz), subjected to 12% SDS-PAGE and transferred to Immobilon-FL membranes (Millipore). RGS4 was detected by its HA-tag while DHHC3 and DHHC7 were detected by their Myc-tags. IRDye700- or IRDye800-labeled secondary antibodies were used for protein band detection by a LI-COR Odyssey imaging system (LI-COR Biosciences).
2.3. 2-BP, DTT, and hydroxylamine treatment
HEK293 cells were transfected with RGS4 and DHHC3 for 30 hrs and then were treated without or with 2-BP (100 μM) for 4 hrs before extracting protein with RIPA buffer. For DTT or hydroxylamine treatment, cell lysate (30 μg) was incubated with DTT (200 μM), 0.5 M hydroxylamine (pH 7.0) or 0.5 M Tris-HCl (pH 7.0) at room temperature for 1 hr, respectively. The mixtures were then analyzed by western blot.
2.4. Hypoxia treatment and RGS4 degradation analysis [14]
Hypoxic conditions were established by evacuating oxygen from a sealed chamber with 5% CO2 and 95% N2. HEK293 cells transfected with RGS4 or RGS4C2S with or without DHHC3 were cultured under hypoxia for 24 hrs to accumulate RGS4 protein. Then, the cells were cultured under nomoxia in the presence of cycloheximide (100 μM). The disappearance of the accumulated RGS4 protein pool was followed over time by western blot.
2.5. Detection of palmitoylated RGS4 using Cu(I)-catalyzed azide-alkyne cycloaddition reaction (click chemistry)
HEK293 cells co-transfected with GFP-tagged DHHC3 and HA-tagged RGS4 were cultured in media containing 5% charcoal-stripped FBS for 24 hrs. The media was changed to serum-free DMEM for 1 hr and then labeled with the lipid 17-ODYA (100 μM in DMEM and 1% fatty-acid free BSA) for 8 hrs. Cells were lysed with buffer (50 mM Tris-HCl, pH7.4, 150 mM NaCl, 2% Triton X-100, protease inhibitors). RGS4 was immunoprecipitated using anti-HA affinity agarose beads and eluted with 200 μg/ml HA peptide (Sigma-Aldrich).
Octadecynoylated RGS4 protein in the immunoprecipitates was linked to biotin-azide reporter groups via click chemistry [15]. Samples were analyzed by western blot using anti-HA antibody to detect HA-tagged RGS4 and streptavidin-IRDye800 (LI-COR) to detect 17-ODYA labeled proteins via the attached biotin.
2.6. Co-immunoprecipitation assay
Myc-tagged DHHC3 was co-transfected with control vector, HA-tagged RGS4 or RGS4C2S mutant into HEK293 cells. After 30 hrs, cell lysates were immunoprecipitated with anti-HA affinity agarose beads. The immunoprecipitate was subjected to western blot analysis for RGS4 and DHHC3.
2.7. Measurement of intracellular Ca2+ in HEK293 cells expressing α1A-AR
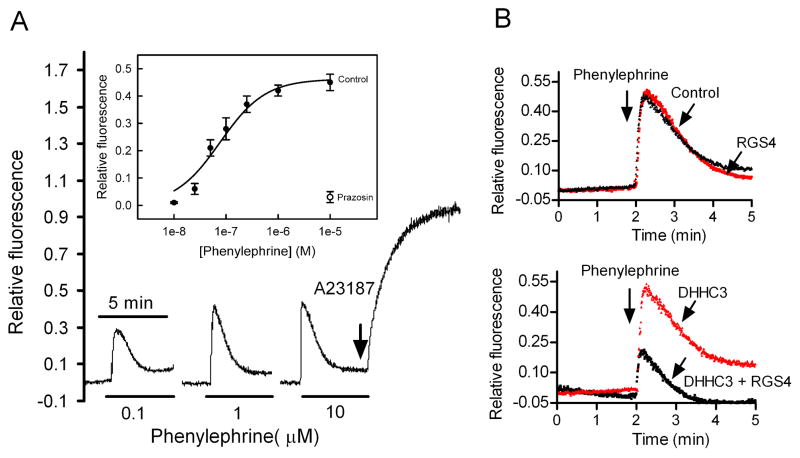
HEK293 cells were stably transfected with mouse α1A-AR and α1A-AR expression was verified with [3H]prazosin, as described [16]. Intracellular Ca2+ was measured using the fluorescent Ca2+ indicator Flur-4. Fluorescence of cell suspensions (1x106 cells) were measured on a Varian Cary Eclipse fluorescence spectrometer at 30°C using 485 nm excitation/515 nm emission. Maximum fluorescence was obtained with the calcium ionophore A23187 (2 μM). Changes in intracellular Ca2+ were quantified by the ratio of phenylephrine-induced peak fluorescence vs. the maximum fluorescence.
3. Results and discussion
3.1. Cys2 is critical for oxidation-sensitive proteasome degradation of RGS4 in cells
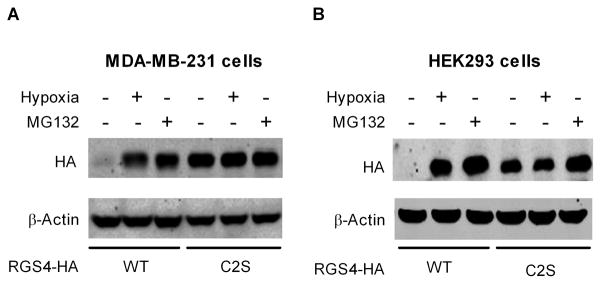
Breast cancer MDA-MB-231 cells have very low levels of endogenous RGS4 because of rapid proteasome degradation [5]. As shown in Fig. 1A, MDA-MB-231 cells transfected with C-terminal HA-tagged wild-type (WT) RGS4 also have very low levels of recombinant RGS4 unless grown under hypoxic conditions, when RGS4 levels approximate those seen in the presence of the proteasome inhibitor MG132. Mutating the Cys2 residue to Ser in RGS4 (RGS4C2S) eliminates susceptibility to oxidation such that RGS4 levels are high and similar during normoxia, hypoxia or proteasome blockade. Fig. 1B shows that this effect is not confined to MDA-MB-231 cells since similar results are seen in HEK293 cells. Thus, we confirmed that the Cys2 residue is critical for oxidation-sensitive proteasome degradation of RGS4 in cells [8].
Figure 1.
Cys2 is critical for oxidation-sensitive proteasome degradation of RGS4 protein in MDA-MB-231 cells (A) and HEK293 cells (B). Cells were transfected with 1 μg plasmids encoding HA-tagged WT RGS4 or RGS4C2S mutant. Cells under hypoxia (+) or nomoxia (-) for 24 hrs were treated without or with 20 μM MG132 for 4 hrs. Proteins in cell lysate were subjected to western blot analysis with anti-HA and anti-β-actin antibodies. Images shown are representatives of four separate experiments.
3.2. Co-expression of active DHHC proteins increases expression levels of RGS4 but not RGS4C2S mutant
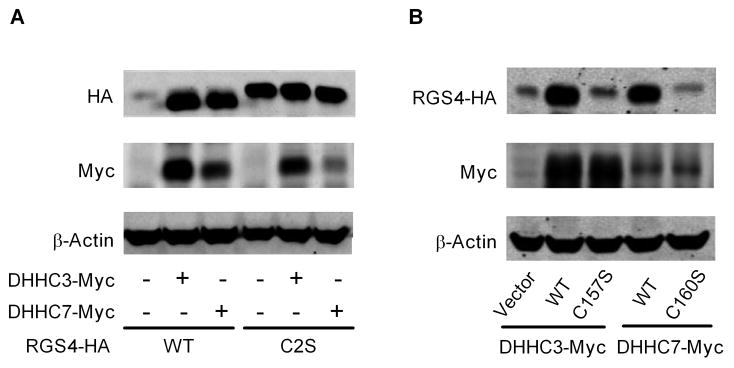
The Cys2 residue of RGS4 also is the primary target for palmitoylation [10; 11]. We investigated whether palmitoylation affects RGS4 degradation. Palmitoylation of RGS4 in vivo has been shown in mammalian cells, but the acyltransferase for RGS4 palmitoylation is unknown. Recently, a family of acyltransferases characterized by a DHHC domain has been identified as enzymes for protein palmitoylation [17]. Fig. 2A shows the effects of Myc-tagged DHHC3 or DHHC7 expression on co-expressed HA-tagged RGS4 or RGS4C2S mutant in HEK293 cells. For WT RGS4 (left), expression of DHHC3 or DHHC7 led to large increases in RGS4 protein. In contrast, RGS4C2S protein is readily detected, and there was no effect of DHHC3 or DHHC7 expression on its levels (right). Interestingly, a comparison of RGS4 mobility on the SDS-PAGE gel reveals that co-expression of DHHC3 or DHHC7 causes RGS4 but not RGS4C2S mutant protein to migrate more rapidly during electrophoresis (lanes 2 and 3 vs. lanes 5 and 6).
Figure 2.
Co-expression of active DHHC proteins increases expression levels of RGS4 but not RGS4C2S mutant. HEK293 cells were transfected with HA-tagged WT RGS4 or RGS4C2S mutant in the absence or presence of Myc-tagged DHHC3, DHHC7 or their inactive C157S or C160S mutants. Proteins in cell lysate were analyzed by western blot using anti-HA, anti-Myc and anti-β-actin antibodies. Images shown are representatives of three separate experiments. (A). Co-expression of DHHC3 or DHHC7 only caused WT RGS4 to accumulate in cells and migrate more rapidly during electrophoresis (B). Acyltransferase activity is critical for DHHC3 and DHHC7 to modulate RGS4.
The C157S mutation of DHHC3 or the C160S mutation of DHHC7 renders these proteins unable to palmitoylate [13]. As shown in Fig. 2B, WT Myc-tagged DHHC3 or DHHC7 maintained RGS4 protein levels and the increased rate of RGS4 band migration in comparison to the acyltransferase-inactive DHHC mutants. This suggests that the effects of DHHC proteins on RGS4 were probably caused by palmitoylation.
3.3. DHHC3 palmitoylates RGS4 in HEK293 cells
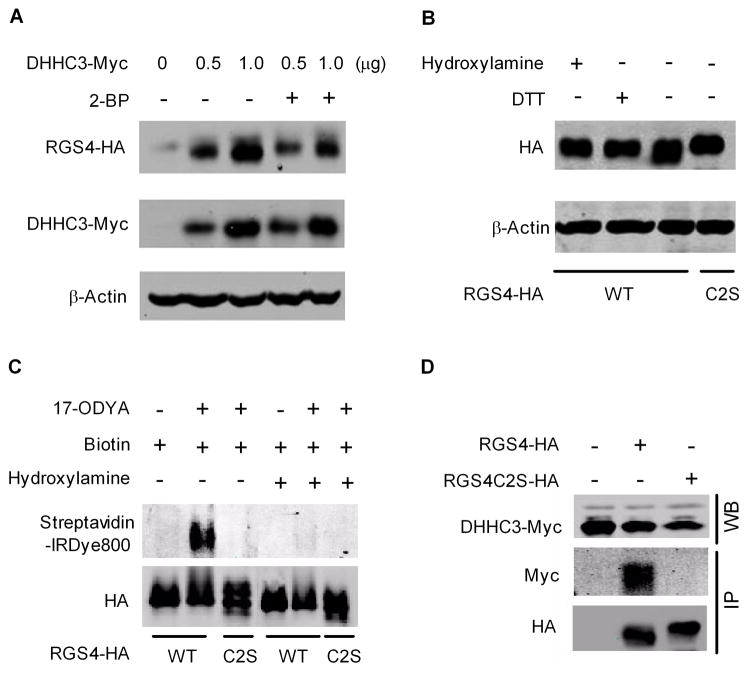
Fig. 3A shows DHHC3 dose effects on HA-tagged RGS4 band intensity and mobility on SDS-PAGE, effects that can be antagonized by treatment of cells for 4 hrs with 2-BP, a competitive inhibitor of palmitoylation. We also treated protein from DHHC3-transfected cells with DTT or hydroxylamine to cleave the thioester bond of palmitoylated proteins. As shown in Fig. 3B, DTT or hydroxylamine reduces mobility of the prominent faster migrating RGS4 band (lane 3 vs. lanes 1 and 2) to the same position as the RGS4C2S mutant (lane 4). This suggests that the mobility shift of RGS4 was probably due to palmitoylation,
Figure 3.
DHHC3 palmitoylates RGS4 in HEK293 cells. Cell lysates were analyzed by western blot. Images are representatives of 3–6 separate experiments. (A). DHHC3 increased RGS4 protein in a dose-dependent manner, which was blocked by 2-BP (100 μM). (B). DTT or hydroxylamine reversed DHHC3-induced increase in the mobility of RGS4. (C). HEK293 cells co-transfected with GFP-tagged DHHC3 and RGS4 or RGS4C2S were metabolically labeled with 17-ODYA. RGS4 and RGS4C2S were immunoprecipitated and biotin labeled. Samples were treated with Tris-HCl (lanes 1–3) or hydroxylamine (lanes 4–6) for 1 hr prior to western blot analysis using anti-HA antibody and streptavidin-IRDye800. (D). DHHC3 forms complex with RGS4 but not RGS4C2S in HEK293 cells. Expression of DHHC3-Myc was analyzed by western blot (upper panel). HA-tagged RGS4, RGS4C2S, and associated DHHC3-Myc were immunoprecipitated using anti-HA affinity agarose beads and detected by western blot using anti-HA and anti-Myc antibodies, respectively (lower panel).
Protein palmitoylation has historically been detected by incorporation of radiolabeled palmitate, but acetylene-fatty acids such as 17-ODYA have recently been shown to function as a metabolically incorporated probe for profiling protein palmitoylation in mammalian cells [15]. As shown in Fig 3C, click chemistry-detected 17-ODYA incorporation into RGS4 was entirely dependent on Cys2 since this band was eliminated by the C2S mutation (lane 3 vs. lane 2), and by hydroxylamine (lane 5 vs. 2).
Co-immunoprecipitation of Myc-tagged DHHC3 with HA-tagged RGS4 was performed using anti-HA antibody-agarose beads. Data in Fig. 3D indicates that DHHC3 interacts with RGS4, but not RGS4C2S, again showing the importance of the Cys2 residue for DHHC3-dependent palmitoylation of RGS4.
3.4. DHHC3-dependent palmitoylation protects RGS4 from proteasome degradation
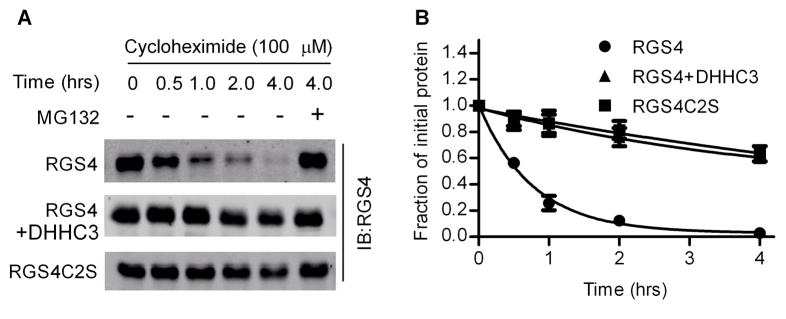
We next determined if increased RGS4 protein levels seen with palmitoylation were due to prolongation of RGS4 half-life. HEK293 cells with hypoxia-induced RGS4 accumulation were transferred to normoxia in the presence of cycloheximide to block new protein synthesis. The disappearance of pre-accumulated RGS4 was then followed over time by western blot. Fig. 4A shows a representative western blot indicating that RGS4 was degraded rapidly and that MG132 blocked degradation. In contrast, degradation of oxidation-resistant RGS4C2S mutant is much slower. Interestingly, co-expression of DHHC3 slowed the degradation rate of RGS4. RGS4 had a half-life of approximately 30 minutes while the half-lives of RGS4C2S mutant and RGS4 that was palmitoylated by DHHC3 exceeded 4 hrs (Fig. 4B). Thus, DHHC3- dependent palmitoylation protects RGS4 from proteasome degradation and extends its half-life by over 8-fold.
Figure 4.
Palmitoylation protects RGS4 from oxidation-sensitive degradation. HEK293 cells were transfected with RGS4C2S, RGS4 or RGS4 and DHHC3, cultured under hypoxia for 24 hrs and then transferred to normoxia in the presence of cycloheximide (100 μM). Oxidation-induced degradation of pre-accumulated RGS4 or its mutant was examined over time by western blot assays. (A). Representative western blot. MG132 blocked RGS4 degradation. (B). Time-course of RGS4 degradation obtained from three independent experiments. RGS4 protein was quantified by densitometry and initial optical density units in each group were set as 1. Points, mean; bars, SE.
3.5. Palmitoylated RGS4 attenuates α1A-AR-dependent intracellular Ca2+ mobilization
α1A-AR predominantly couple to Gq-mediated phosphoinositide hydrolysis pathways which mobilize intracellular Ca2+ [18]. As shown in Fig. 5A, in HEK293 cells stably expressing α1A-AR, the α1A-AR agonist phenylephrine caused a transient increase in the Ca2+ indicator Flur-4 fluorescence that lasted approximately 1 min. Phenylephrine increased intracellular Ca2+ in a concentration-dependent manner (Fig. 5A, inset) with an EC50 of 77±15 nM. The α1-AR antagonist prazosin blocked phenylephrine-stimulated Ca2+ mobilization. Expression of RGS4 alone had little effect, presumably due to rapid degradation of expressed RGS4 protein. In contrast, co-transfection of RGS4 with DHHC3 resulted in 60% reduction of phenylephrine-stimulated intracellular Ca2+ as compared to cells expressing DHHC3 alone (Fig. 5B). Thus, palmitoylated RGS4 protein can attenuate α1A-AR dependent Ca2+ mobilization in vivo.
Figure 5.
Palmitoylated RGS4 attenuates α1A-AR-stimulated intracellular Ca2+ mobilization. HEK293 cells stably expressing α1A-AR were loaded with Flur-4. Elevations in intracellular Ca2+ were quantified by the ratio of phenylephrine-induced peak fluorescence vs. maximum fluorescence in the presence of A23187 (2 μM). (A). Phenylephrine concentration-response curve in the absence (Control) or presence of prazosin (1 μM) (n=4). (B). Co-expression of RGS4 with DHHC3 attenuated phenylephrine-stimulated elevation in intracellular Ca2+. Data are typical of three experiments.
4. Conclusion
We confirmed that RGS4 protein levels are regulated by the N-end rule for proteasomal degradation via a Cys2-dependent oxidation of RGS4 [6–9]. In addition, our study provides the first evidence that RGS4 is palmitoylated by DHHC3 and DHHC7 acyltransferases in vivo and that Cys2 of RGS4 is essential for this palmitoylation. Palmitoylation usually increases protein affinity for cell membranes as a possible regulatory mechanism. However, palmitoylation of RGS4 is not required for its attachment to the plasma membrane [19]. We demonstrated that the palmitoylation of RGS4 in vivo prolongs RGS4 half-life by interfering with its oxidation-dependent degradation, and that this palmitoylated RGS4 can attenuate functions of GPCRs such as α1A-AR in cells. Thus, the present findings revealed a novel mechanism by which DHHC acyltransferase-mediated protein palmitoylation. Moreover, our data raise the interesting possibility that in addition to controlling G-protein localization [13], DHHCs may also modulate GPCR signaling events through regulation of RGS4 stability.
Supplementary Material
Acknowledgments
Supported by National Institute of Health (R011CA125661), Nebraska State LB595, National Basic Research Program of China (2004CB720000) and the National Natural Science Foundation of China (30900246).
Abbreviations
- GPCR
G-protein coupled receptor
- RGS4
Regulator of G-protein signaling 4
- RGS4C2S
RGS4 Cys2 to Ser mutant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- 2.Cifelli C, Rose RA, Zhang H, Voigtlaender-Bolz J, Bolz SS, Backx PH, Heximer SP. RGS4 regulates parasympathetic signaling and heart rate control in the sinoatrial node. Circ Res. 2008;103:527–535. doi: 10.1161/CIRCRESAHA.108.180984. [DOI] [PubMed] [Google Scholar]
- 3.Hooks SB, Martemyanov K, Zachariou V. A role of RGS proteins in drug addiction. Biochem Pharmacol. 2008;75:76–84. doi: 10.1016/j.bcp.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 4.Hurst JH, Hooks SB. Regulator of G-protein signaling (RGS) proteins in cancer biology. Biochem Pharmacol. 2009;78:1289–1297. doi: 10.1016/j.bcp.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 5.Xie Y, Wolff DW, Wei T, Wang B, Deng C, Kirui JK, Jiang H, Qin J, Abel PW, Tu Y. Breast cancer migration and invasion depend on proteasome degradation of regulator of G-protein signaling 4. Cancer Res. 2009;69:5743–5751. doi: 10.1158/0008-5472.CAN-08-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davydov IV, Varshavsky A. RGS4 is arginylated and degraded by the N-end rule pathway in vitro. J Biol Chem. 2000;275:22931–22941. doi: 10.1074/jbc.M001605200. [DOI] [PubMed] [Google Scholar]
- 7.Tasaki T, Mulder LC, Iwamatsu A, Lee MJ, Davydov IV, Varshavsky A, Muesing M, Kwon YT. A family of mammalian E3 ubiquitin ligases that contain the UBR box motif and recognize N-degrons. Mol Cell Biol. 2005;25:7120–7136. doi: 10.1128/MCB.25.16.7120-7136.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu RG, Sheng J, Qi X, Xu Z, Takahashi TT, Varshavsky A. The N-end rule pathway as a nitric oxide sensor controlling the levels of multiple regulators. Nature. 2005;437:981–986. doi: 10.1038/nature04027. [DOI] [PubMed] [Google Scholar]
- 9.Lee MJ, Tasaki T, Moroi K, An JY, Kimura S, Davydov IV, Kwon YT. RGS4 and RGS5 are in vivo substrates of the N-end rule pathway. Proc Natl Acad Sci U S A. 2005;102:15030–15035. doi: 10.1073/pnas.0507533102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srinivasa SP, Bernstein LS, Blumer KJ, Linder ME. Plasma membrane localization is required for RGS4 function in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1998;95:5584–5589. doi: 10.1073/pnas.95.10.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tu Y, Popov S, Slaughter C, Ross EM. Palmitoylation of a conserved cysteine in the regulator of G protein signaling (RGS) domain modulates the GTPase-activating activity of RGS4 and RGS10. J Biol Chem. 1999;274:38260–38267. doi: 10.1074/jbc.274.53.38260. [DOI] [PubMed] [Google Scholar]
- 12.Linder ME, Deschenes RJ. Model organisms lead the way to protein palmitoyltransferases. J Cell Sci. 2004;117:521–526. doi: 10.1242/jcs.00989. [DOI] [PubMed] [Google Scholar]
- 13.Tsutsumi R, Fukata Y, Noritake J, Iwanaga T, Perez F, Fukata M. Identification of G protein alpha subunit-palmitoylating enzyme. Mol Cell Biol. 2009;29:435–447. doi: 10.1128/MCB.01144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sundvall M, Korhonen A, Paatero I, Gaudio E, Melino G, Croce CM, Aqeilan RI, Elenius K. Isoform-specific monoubiquitination, endocytosis, and degradation of alternatively spliced ErbB4 isoforms. Proc Natl Acad Sci USA. 2008;105:4162–4167. doi: 10.1073/pnas.0708333105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin BR, Cravatt BF. Large-scale profiling of protein palmitoylation in mammalian cells. Nat Methods. 2009;6:135–138. doi: 10.1038/nmeth.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiao X, Gonzalez-Cabrera PJ, Xiao L, Bradley ME, Abel PW, Jeffries WB. Tonic inhibitory role for cAMP in alpha(1a)-adrenergic receptor coupling to extracellular signal-regulated kinases 1/2. J Pharmacol Exp Ther. 2002;303:247–256. doi: 10.1124/jpet.102.037747. [DOI] [PubMed] [Google Scholar]
- 17.Tsutsumi R, Fukata Y, Fukata M. Discovery of protein-palmitoylating enzymes. Pflugers Arch. 2008;456:1199–1206. doi: 10.1007/s00424-008-0465-x. [DOI] [PubMed] [Google Scholar]
- 18.Graham RM, Perez DM, Hwa J, Piascik MT. alpha 1-adrenergic receptor subtypes. Molecular structure, function, and signaling. Circ Res. 1996;78:737–749. doi: 10.1161/01.res.78.5.737. [DOI] [PubMed] [Google Scholar]
- 19.Bernstein LS, Grillo AA, Loranger SS, Linder ME. RGS4 binds to membranes through an amphipathic alpha -helix. J Biol Chem. 2000;275:18520–18526. doi: 10.1074/jbc.M000618200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.