SYNOPSIS
Assessment of patient reported outcomes (PROs) has been shown to provide important information to assist with clinical decision-making. There has been significant progress in the field of PROs over the last two decades with the introduction of a variety of validated disease- and symptom-specific instruments. The Functional Assessment of Cancer Therapy-Melanoma (FACT-M) is a melanoma-specific module to accompany the FACT-General which has been validated to assess health-related quality of life (HRQOL) for patients with all stages of melanoma. Melanoma-specific health state utilities, which are essential for calculating quality adjusted life years and performing cost-effectiveness studies, have also been reported from a number of studies. Assessment of PROs should be incorporated into routine clinical practice to inform clinicians and researchers of the patient perspective for clinical decision-making and to evaluate the effects of psychosocial and medical interventions.
Keywords: melanoma, patient reported outcomes, health-related quality of life, preferences, utilities, cancer
Introduction
Traditional outcome measures for cancer patients have been expressed in terms survival and recurrence, as these represent familiar domains of treatment effectiveness. While other treatment-related measures such as morbidity and toxicity are commonly evaluated, few would argue that these primarily quantitative measures represent the entirety of the disease experience. A recent systematic review of health-related quality of life (HRQOL) in cutaneous melanoma recognized that about 1/3 of patients with melanoma report clinically significant levels of distress – particularly around the time of diagnosis and immediately post-treatment.[1] It is now clear that patient reported outcomes (PROs) have emerged as a means of capturing important clinical information related to disease treatment and management – namely, the patient experience.[2] PROs have been defined in general as patient indicators of “well-being” which can be expressed as single or multi-dimensional measures, and HRQOL is the most commonly assessed PRO. PROs provide distinct prognostic information and have been found to be associated with survival in cancer patients,[3, 4] including those with melanoma.[5-7]
Instruments
There are numerous instruments available for assessing general HRQOL in addition to others targeting specific diseases or conditions. An inherent strength of generic instruments is that they facilitate comparisons of scores across populations and conditions, but they have been criticized for their lack of sensitivity to change for specific conditions.[8, 9] Conversely, disease specific instruments have increased sensitivity for detecting predicted differences among various subgroups of patients (e.g., according to disease stage) and demonstrating change over time but lack the comparability of more generalized instruments.[10, 11] It has been suggested that the most informative instruments for longitudinal assessments of PROs, therefore, would be a combination that includes both generic and disease-specific items to capitalize on the strengths of each.[12] Whether measurement is performed with generalized or disease-specific items or instruments or both combined, accurate assessment of PROs has been shown to provide important clinical information to assist with decisions regarding treatment protocols and other factors related to disease management.[1] This is particularly important when modest differences in treatment outcomes (i.e. survival, recurrence, morbidity, etc.) are expected.
Despite the increasing burden of melanoma as a public health problem, to date there have been only a limited number of studies examining HRQOL in melanoma patients. Among the available studies, most have employed generic instruments of HRQOL such as the Medical Outcomes Study Short Form 36 (SF-36)[13-17] and cancer-specific HRQOL instruments such as the Functional Assessment of Cancer Therapy (FACT-G),[10, 18] and more frequently, the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC-QLQ).[6, 19-23] Both the FACT-G and the EORTC-QLQ are validated instruments designed to assess HRQOL in patients with cancer, but they do not address uniquely relevant melanoma-specific constructs. There are no items in the FACT-G or the EORTC-QLQ related to surgical scarring, nor are there any items pertaining to lymphedema (e.g., post-operative extremity swelling), a common consequence of treatment for patients undergoing the surgical treatment of stage III melanoma. While the overall incidence of cancer-related lymphedema and its impact on HRQOL are poorly defined for melanoma patients, the consequences of this condition are known to have a significant decremental impact. Additionally, scarring and the cosmetic impact of surgery are important constructs for the assessment of HRQOL in melanoma patient populations, as has been shown in patients with early-stage melanoma.[24]
In 2003, an 11-item melanoma-specific HRQOL instrument was reported in the literature as a companion scale to the 36-item quality of life questionnaire from the EORTC-QLQ.[25] This instrument was validated in advanced-stage (stage IV) melanoma patients and reported that these patients experienced sensory dysfunction, dyspnea, and pain after 9 weeks of chemotherapy.[26] This melanoma-specific instrument contains two items assessing extremity numbness and swelling but does not include any items to assess perceptions related to scarring – a particularly relevant concern of early stage patients. Thus, the sensitivity of the instrument (often defined as the minimal change considered to be important by the persons with the health condition, their significant others, or their providers [27]) may be inadequate in discriminating between patients at different melanoma disease stages.
FACT-Melanoma
The Functional Assessment of Chronic Illness Therapy is a well-known catalogue of HRQOL assessment instruments that share a common core set of items representing patient well-being in the physical, functional, emotional, and social/family domains.[8] In the context of cancer disease management, physical well-being refers to symptoms related to disease (e.g., pain, fatigue, and nausea) and side effects of treatment. Functional well-being assesses the ability to carry out daily living activities (e.g., walking, bathing, and dressing oneself) and performing social roles and tasks. Emotional-well being refers to patients’ coping abilities and reflects their experience of emotions ranging from enjoyment to distress. Lastly, social well-being reflects the quality of relationships with family and friends and serves as a measure of social interaction.[28] These common core items facilitate comparisons among patients with different types of malignancies, but they are supplemented with disease-specific modules that increase the instrument’s sensitivity with items addressing concerns most relevant to disease-specific subpopulations. For patients with cancer, the common core items representing the physical, functional, social/family, and emotional domains are collectively known as the Functional Assessment of Cancer Therapy – General (FACT-G). A number of supplementary disease-specific FACT subscales have been developed,[29] including cancers of the breast,[30] colon and rectum,[31] and lung.[32]
A melanoma-specific module has also been developed to accompany the FACT-G to address limitations related to instrument sensitivity and discriminatory power for patients with all stages of melanoma.[33] When the melanoma-specific items are combined with the FACT-G, the expanded questionnaire is known as the FACT-Melanoma (FACT-M). The instrument was developed according to a standardized methodology for FACIT subscales similar to that used for the FACT-G scale with three distinct phases: item generation, item review and reduction, and scale construction.[8, 28, 33] Following an extensive review of data gathered from four primary sources: the literature; the FACIT item bank; healthcare providers; and melanoma patients, a total of 23 items distinct from the FACT-G were identified for consideration. Additional items were generated through questionnaires seeking expert opinion from 20 melanoma researcher/healthcare providers at the Melanoma and Skin Center at the University of Texas MD Anderson Cancer Center. With IRB approval, a pilot study was of 40 patients diagnosed within the previous three years with stages I, II, III, and IV melanoma was carried out to evaluate the relevance of the items using semi-structured interviews to assess item comprehension, relevance, and overall content. Additional content and linguistic revisions were made based on reviews of the linguistic team at the Center on Outcomes, Research, and Education of Evanston Northwestern Healthcare. The FACT-M supplements version 4 of the FACT-G with 24 items (Box 1) representing three of the four HRQOL domains of the FACT-G.
Box 1. Melanoma specific items of the FACT-M*.
Melanoma Subscale
I have pain at my melanoma site or surgical site
I have noticed new changes in my skin (lumps, bumps, color)
I worry about the appearance of surgical scars
I have been short of breath
I have to limit my physical activity because of my condition
I have had headaches
I have had fevers
I have swelling or cramps in my stomach area
I have a good appetite
I have aches and pains in my bones
I have noticed blood in my stool
I have to limit my social activity because of my condition
I feel overwhelmed by my condition
I isolate myself from others because of my condition
I have difficulty thinking clearly (remembering, concentrating)
I feel fatigued
Melanoma Surgery Subscale
I have swelling at my melanoma site
I have swelling as a result of surgery
I am bothered by the amount of swelling
Movement of my swollen area is painful
Swelling keeps me from doing the things I want to do
Swelling keeps me from wearing the clothes or shoes that I want to wear
I feel numbness at my surgical site
I have good range of motion in my arm or leg
*Data from: Cormier JN, Davidson L, Xing Y, et al: Measuring quality of life in patients with melanoma: development of the FACT-melanoma subscale. J Support Oncol 3:139, 2005; Cormier JN, Ross MI, Gershenwald JE, et al: Prospective assessment of the reliability, validity, and sensitivity to change of the Functional Assessment of Cancer Therapy-Melanoma questionnaire. Cancer 112:2249, 2008
Additional studies have been performed to establish the FACT-M as a valid and reliable measure of patient-reported HRQOL.[10] Specifically, the psychometric properties of the FACT-M have been examined with respect to the following properties:[10, 28, 34]
Face validity reflecting how well the items represent the specific construct of interest
Construct validity demonstrating the relationships between FACT-M scores and other related PRO variables
Criterion validity examining the ability of the FACT-M to reflect known differences between disease subgroups
Convergent and Divergent validity demonstrated as positive and negative associations between FACT-M scores and scores from related constructs
Internal consistency demonstrating the extent to which scale items assess a single underlying concept
Discrimination representing the ability of the scale to differentiate between known subgroups (e.g., different stages of disease or according to treatment status - active treatment vs. surveillance)
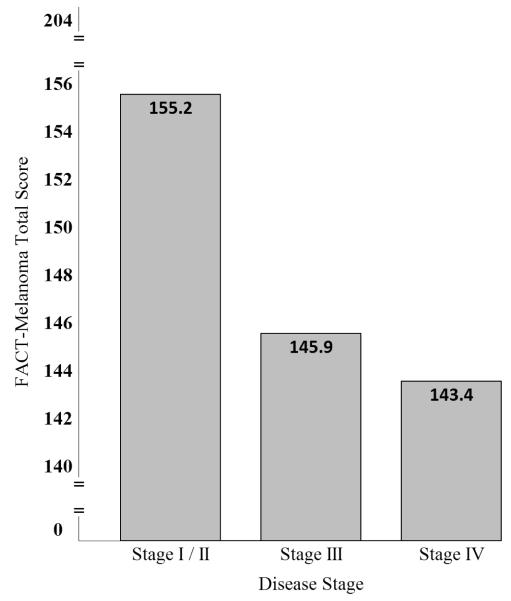
Significant differences in FACT-M scores were found between disease stage subgroups with decrements in HRQOL for patients with advanced (stage III and IV) melanoma (Figure 1).[10] FACT-M responsiveness and sensitivity to change were also examined and confirmed through longitudinal comparisons of patient disease stage according to American Joint Committee Cancer melanoma staging criteria[35] and performance status as rated by patients and providers using the Eastern Cooperative Oncology Group Performance Status Rating (ECOG-PSR)[36] and the Karnofsky Performance Scale (KPS).[37]
Figure 1.
Stage-specific FACT-Melanoma total scores*
*Data from: Cormier JN, Ross MI, Gershenwald JE, et al: Prospective assessment of the reliability, validity, and sensitivity to change of the Functional Assessment of Cancer Therapy-Melanoma questionnaire. Cancer 112:2249, 2008
Minimal Important Differences
In longitudinal assessments of patient HRQOL, it is important to determine which changes in questionnaire scores can be considered clinically meaningful. This is particularly so in the context of clinical trial research in terms of defining benchmarks of change (e.g., for responder analysis) and in the clinical setting for medical decision making. Multiple statistical techniques have been employed to assess minimal important differences (MIDs) in HRQOL, including distribution-based methods,[38, 39] anchor-based methods,[40] mixed modeling procedures,[41] or combinations of methods.[42, 43] Distribution-based methods make use of distributional measures to establish minimum thresholds of change. While distributional measures such as the Standard Error of Measurement (SEM) and effect-size correlates of 1/2 and 1/3 standard deviation have been shown to approximate the MID,[39, 44, 45] it is important to note that these methods provide no direct information about the clinical meaning of change-score thresholds.[46, 47] In contrast, anchor-based methods make use of known clinically relevant indicators of patient change such as functional performance status. With anchor-based methods, relevant differences in performance status scores are used to create patient groups, and the differences in HRQOL scores between groups is used to quantify an MID. Mixed modeling procedures facilitate visit-level analysis (vs. patient-level) and often use clinical anchors as independent variables and HRQOL scales scores as dependent variables. A particular advantage to this technique is that it allows statistical control of known covariates in the model. Whichever techniques are chosen, a comprehensive assessment of MIDs makes use of multiple approaches to triangulate on the minimum score or range of scores to be considered clinically meaningful thresholds of change.[47] MIDs have been determined for a number cancer-specific HRQOL instruments.[48-50] MIDs for the FACT-M have been reported as 4 to 6 points.[44]
Response Burden
HRQOL instruments are often employed when studying the very young, elderly, and those with advanced disease, and the burden associated with completing prolonged assessments can be difficult to tolerate in these populations.[51] Respondent burden has been defined as, “the time, effort, and other demands placed on those to whom the instrument is administered,” [52] and excessive instrument burden can reduce both response rates and data quality.[53] Particularly for oncology research, instruments with fewer items are highly desirable to minimize the biases associated respondent burden,[53, 54] and for melanoma in particular investigations are currently underway to reduce respondent burden of the FACT-M.
Multiple psychometric methods have been developed and applied to various HRQOL instruments in an attempt to reduce respondent burden while maintaining instrument validity, including item response theory and computer adaptive testing.[51, 52, 54, 55] The Patient-Reported Outcomes Measurement Information System (PROMIS) is a National Institute of Health funded project designed to create a publically available set of standardized instruments that capitalize on the advantages of these techniques to reduce respondent burden and accomplish other related measurement objectives.[56, 57] By developing an extensive item bank, researchers in the PROMIS network have been able to create multiple HRQOL instruments that include short forms, long forms, and computer adapted tests for many domains, including pain, fatigue, emotional distress, physical functioning, and social role participation.[58] Instruments from the PROMIS network are field tested and calibrated such that patient scores from all items for a particular domain are represented on a single uni-dimensional scale (e.g., difficulty getting out of bed vs difficulty running for long distances).[58] These scales are then standardized to the U.S. population (mean score = 0, standard deviation = 1) to facilitate broad comparisons across disease, condition, and other population specific subsets. There are a number of advantages associated with the PROMIS system, and expansion into other HRQOL domains is expected to continue.[56, 59, 60]
Health Utilities
Health utilities are distinct yet related measures of HRQOL that assess patient and population preferences for specific health states.[61] In the context of cancer care, curative treatment can often be accompanied by serious decrements in patient HRQOL, and health state preferences help quantify the value associated with these and other compromised health states.[62] Health utilities are particularly important in the context of cost-benefit and comparative effectiveness analyses, as they facilitate the calculation of quality-adjusted life years (QALY) – a measure created by multiplying years of survival by the value (percent) associated with the health states experienced during those years.[63] The health utility coefficients and the subsequent measures of QALY facilitate comparisons of the costs and benefits associated with treatment protocols and can often inform policy decisions regarding resource allocation.[63]
There are multiple methods available to derive health utility coefficients, including use of a visual analog scale, standard gamble techniques, and time tradeoff evaluations.[64] The visual analog scale is one of the most commonly used methods of preference elicitation, in which respondents are asked to visually rank multiple states of compromised health on a 0 (worst) −100 (best) point line scale.[63] With this method, both the rank-order of states is established along with strength of preference for given health states.[63] The standard gamble is a choice-based technique of eliciting health utilities where the respondent is asked to hypothetically choose between a certain health state for a specific number of years and a given probability of achieving a preferred health state (from a presumed treatment or decision) for the same number of years.[65] Similar choices are presented in series with varying probability for the preferred state until the respondent is indifferent to the choice (i.e. when the options are of equal value to the respondent). This final probability value represents the respondent’s preference for the health state under consideration.[65] For the time tradeoff method, respondents are asked to sequentially compare a specific period of time in a compromised health state with an increasingly shorter span of time in a perfect health state.[62] When the respondent is indifferent to the choice, the percentage of time in perfect health in relation to its initial value represents the preference value for the health state.[62]
Multi-attribute utility measures assess preferences for health states covering multiple HRQOL domains. The EuroQol EQ-5D is a well-known multi-attribute utility measure used to assess quality of life and has been used for cost-benefit analyses.[66] For the EQ-5D, the utility function is derived from large population-based samples of respondents, and the scores represent summary utility coefficients ranging from 0 (death) to 1 (perfect health).[67] Health utilities like the EQ-5D have been positively associated with relevant clinical factors such as symptom burden in cancer patients.[68, 69]
Melanoma-specific utilities have been reported for a number of health states using techniques involving standard gamble, time tradeoff estimation, and visual analog scales. Most of the reported studies have used these utilities to estimate the cost-effectiveness of treatment strategies by incorporating measures of cost, survival, quality adjusted life years, among other factors.[21, 70-77] Directly elicited utilities ranged from 0.52 to 0.97 for patients with stages I to IV melanoma and included a number of health states ranging from disease-free to advanced disease under treatment. Stage specific melanoma utilities have also been collected using the EQ-5D in the context of a longitudinal study designed to validate the FACT-M.[10] The results are summarized in Table 1.
Table 1.
Summary of melanoma-specific studies eliciting patient-derived health utilities
| Author, year | Melanoma population |
Source / Elicitation techniques |
Health Statea | Utilityb |
|---|---|---|---|---|
| Beusterien,[78] 2009 |
Advanced melanoma |
Standard gamble |
Clinical response states | |
| Partial response | 0.88 | |||
| Stable disease | 0.80 | |||
| Progressive disease | 0.52 | |||
| Best supportive care | 0.52 | |||
|
| ||||
| Dixon,[21] 2006 | Stage III | EQ-5D | Treatment with Interferon | 0.76 |
| Controls | 0.79 | |||
|
| ||||
| Crott,[73] 2004 | Stage III | Time trade- off |
Treatment with Interferon | 0.52 |
| Recurrence | 0.23 | |||
|
| ||||
| Kilbridge,[79] 2001 | Early stage melanoma |
Standard gamble |
Interferon with no side effects | 0.92 |
| Interferon with mild-moderate side effects | 0.88 | |||
| Interferon with laboratory side effects | 0.86 | |||
| Interferon with severe side effects | 0.81 | |||
| Interferon, recurrence | 0.62 | |||
| Recurrence | 0.61 | |||
| Disease free survival | 0.96 | |||
|
| ||||
| Chen,[80] 2004 | Stages I, II, III |
Time trade- off |
Stage I (Actual / Hypothetical) | 0.93 / 0.94 |
| Stage II (Actual / Hypothetical) | 0.97 / 0.73 | |||
| Stage III (Actual / Hypothetical) | 0.52 / 0.50 | |||
| FACT-M validation study |
EQ-5D | Stage I | 0.90 | |
| Stage II | 0.87 | |||
| Stage III | 0.82 | |||
| Stage IV | 0.82 | |||
Abbreviations: EQ-5D, EuroQol EQ-5D
The health states presented in this table do not reflect the entirety of health states estimated in these studies.
When separate utility coefficients were estimated for multiple time points, the earliest time point was presented.
Summary
Melanoma-specific HRQOL instruments are important for characterizing melanoma patient populations and in evaluating the effects of psychosocial and medical interventions. The FACT-M is a melanoma-specific module which has been validated to assess health-related quality of life (HRQOL) for patients with all stages of melanoma. Stage specific differences in FACT-M scores have been shown with statistically significant decrements in HRQOL for patients with advanced (stages III and IV) disease. Melanoma-specific utilities have also been published for a number of health states and are available for calculating quality-adjusted life years and performing cost-effectiveness studies. Current PRO research is focused on utilizing publicly available item banks and reducing respondent burden by using computer adaptive testing. PROs should be incorporated into routine clinical practice to inform clinicians and researchers of the patient perspective for clinical decision-making.
Acknowledgments
Part of the work presented was supported by Grant No. 5-K12-CA088084 from the National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no disclosures.
References
- 1.Cornish D, Holterhues C, van de Poll-Franse LV, et al. A systematic review of health-related quality of life in cutaneous melanoma. Ann Oncol. 2009;20(Suppl 6):vi51–58. doi: 10.1093/annonc/mdp255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliver A, Greenberg CC. Measuring outcomes in oncology treatment: the importance of patient-centered outcomes. Surg Clin North Am. 2009;89:17–25. vii. doi: 10.1016/j.suc.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gotay CC, Kawamoto CT, Bottomley A, Efficace F. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol. 2008;26:1355–1363. doi: 10.1200/JCO.2007.13.3439. [DOI] [PubMed] [Google Scholar]
- 4.Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes. 2009;7:102. doi: 10.1186/1477-7525-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butow PN, Coates AS, Dunn SM. Psychosocial predictors of survival in metastatic melanoma. J Clin Oncol. 1999;17:2256–2263. doi: 10.1200/JCO.1999.17.7.2256. [DOI] [PubMed] [Google Scholar]
- 6.Lehto US, Ojanen M, Dyba T, et al. Baseline psychosocial predictors of survival in localized melanoma. J Psychosom Res. 2007;63:9–15. doi: 10.1016/j.jpsychores.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Chiarion-Sileni V, Del Bianco P, De Salvo GL, et al. Quality of life evaluation in a randomised trial of chemotherapy versus bio-chemotherapy in advanced melanoma patients. Eur J Cancer. 2003;39:1577–1585. doi: 10.1016/s0959-8049(03)00372-1. [DOI] [PubMed] [Google Scholar]
- 8.Cella DF, Tulsky DS. Quality of life in cancer: definition, purpose, and method of measurement. Cancer Invest. 1993;11:327–336. doi: 10.3109/07357909309024860. [DOI] [PubMed] [Google Scholar]
- 9.Langenhoff BS, Krabbe PF, Wobbes T, Ruers TJ. Quality of life as an outcome measure in surgical oncology. Br J Surg. 2001;88:643–652. doi: 10.1046/j.1365-2168.2001.01755.x. [DOI] [PubMed] [Google Scholar]
- 10.Cormier JN, Ross MI, Gershenwald JE, et al. Prospective assessment of the reliability, validity, and sensitivity to change of the Functional Assessment of Cancer Therapy-Melanoma questionnaire. Cancer. 2008;112:2249–2257. doi: 10.1002/cncr.23424. [DOI] [PubMed] [Google Scholar]
- 11.Lipscomb J, Gotay CC, Snyder C. Outcomes assessment in cancer : measures, methods, and applications. Cambridge University Press; Cambridge, UK; New York: 2005. [Google Scholar]
- 12.Both H, Essink-Bot ML, Busschbach J, Nijsten T. Critical review of generic and dermatology-specific health-related quality of life instruments. J Invest Dermatol. 2007;127:2726–2739. doi: 10.1038/sj.jid.5701142. [DOI] [PubMed] [Google Scholar]
- 13.Devine D, Parker PA, Fouladi RT, Cohen L. The association between social support, intrusive thoughts, avoidance, and adjustment following an experimental cancer treatment. Psychooncology. 2003;12:453–462. doi: 10.1002/pon.656. [DOI] [PubMed] [Google Scholar]
- 14.McHorney CA, Ware JE, Jr., Rogers W, et al. The validity and relative precision of MOS short- and long-form health status scales and Dartmouth COOP charts. Results from the Medical Outcomes Study. Med Care. 1992;30:MS253–265. doi: 10.1097/00005650-199205001-00025. [DOI] [PubMed] [Google Scholar]
- 15.Trask PC, Griffith KA. The identification of empirically derived cancer patient subgroups using psychosocial variables. J Psychosom Res. 2004;57:287–295. doi: 10.1016/j.jpsychores.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Trask PC, Paterson AG, Griffith KA, et al. Cognitive-behavioral intervention for distress in patients with melanoma: comparison with standard medical care and impact on quality of life. Cancer. 2003;98:854–864. doi: 10.1002/cncr.11579. [DOI] [PubMed] [Google Scholar]
- 17.Trask PC, Paterson AG, Hayasaka S, et al. Psychosocial characteristics of individuals with non-stage IV melanoma. J Clin Oncol. 2001;19:2844–2850. doi: 10.1200/JCO.2001.19.11.2844. [DOI] [PubMed] [Google Scholar]
- 18.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 19.Al-Shakhli H, Harcourt D, Kenealy J. Psychological distress surrounding diagnosis of malignant and nonmalignant skin lesions at a pigmented lesion clinic. J Plast Reconstr Aesthet Surg. 2006;59:479–486. doi: 10.1016/j.bjps.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Avril MF, Aamdal S, Grob JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. J Clin Oncol. 2004;22:1118–1125. doi: 10.1200/JCO.2004.04.165. [DOI] [PubMed] [Google Scholar]
- 21.Dixon S, Walters SJ, Turner L, Hancock BW. Quality of life and cost-effectiveness of interferon-alpha in malignant melanoma: results from randomised trial. Br J Cancer. 2006;94:492–498. doi: 10.1038/sj.bjc.6602973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rataj D, Jankowiak B, Krajewska-Kulak E, et al. Quality-of-life evaluation in an interferon therapy after radical surgery in cutaneous melanoma patients. Cancer Nurs. 2005;28:172–178. doi: 10.1097/00002820-200505000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Lehto US, Ojanen M, Kellokumpu-Lehtinen P. Predictors of quality of life in newly diagnosed melanoma and breast cancer patients. Ann Oncol. 2005;16:805–816. doi: 10.1093/annonc/mdi146. [DOI] [PubMed] [Google Scholar]
- 24.Cassileth BR, Lusk EJ, Tenaglia AN. Patients’ perceptions of the cosmetic impact of melanoma resection. Plast Reconstr Surg. 1983;71:73–75. doi: 10.1097/00006534-198301000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Sigurdardottir V, Bolund C, Brandberg Y, Sullivan M. The impact of generalized malignant melanoma on quality of life evaluated by the EORTC questionnaire technique. Qual Life Res. 1993;2:193–203. doi: 10.1007/BF00435223. [DOI] [PubMed] [Google Scholar]
- 26.Sigurdardottir V, Bolund C, Sullivan M. Quality of life evaluation by the EORTC questionnaire technique in patients with generalized malignant melanoma on chemotherapy. Acta Oncol. 1996;35:149–158. doi: 10.3109/02841869609098495. [DOI] [PubMed] [Google Scholar]
- 27.Lohr K, Aaronson NK, Alonso J, et al. Scientific Advisory Committee: Instrument Review Criteria. Medical Outcomes Trust. 1997:1–5. [Google Scholar]
- 28.Cella D, Nowinski CJ. Measuring quality of life in chronic illness: the functional assessment of chronic illness therapy measurement system. Arch Phys Med Rehabil. 2002;83:S10–17. doi: 10.1053/apmr.2002.36959. [DOI] [PubMed] [Google Scholar]
- 29.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15:974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 31.Ward WL, Hahn EA, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) quality of life instrument. Qual Life Res. 1999;8:181–195. doi: 10.1023/a:1008821826499. [DOI] [PubMed] [Google Scholar]
- 32.Cella DF, Bonomi AE, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer. 1995;12:199–220. doi: 10.1016/0169-5002(95)00450-f. [DOI] [PubMed] [Google Scholar]
- 33.Cormier JN, Davidson L, Xing Y, et al. Measuring quality of life in patients with melanoma: development of the FACT-melanoma subscale. J Support Oncol. 2005;3:139–145. [PubMed] [Google Scholar]
- 34.Doward LC, McKenna SP. Defining patient-reported outcomes. Value Health. 2004;7(Suppl):S4–8. doi: 10.1111/j.1524-4733.2004.7s102.x. [DOI] [PubMed] [Google Scholar]
- 35.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 36.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 37.Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherpeutic agents in cancer. In: Macleod CM, editor. Evaluation of chemotherapeutic agents in cancer. Columbia University Press; New York: 1949. pp. 191–205. Edition. [Google Scholar]
- 38.Rejas J, Pardo A, Ruiz MA. Standard error of measurement as a valid alternative to minimally important difference for evaluating the magnitude of changes in patient-reported outcomes measures. J Clin Epidemiol. 2008;61:350–356. doi: 10.1016/j.jclinepi.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 39.Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol. 1999;52:861–873. doi: 10.1016/s0895-4356(99)00071-2. [DOI] [PubMed] [Google Scholar]
- 40.Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol. 1994;47:81–87. doi: 10.1016/0895-4356(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 41.Brant R, Sutherland L, Hilsden R. Examining the minimum important difference. Stat Med. 1999;18:2593–2603. doi: 10.1002/(sici)1097-0258(19991015)18:19<2593::aid-sim392>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 42.Barrett B, Brown R, Mundt M. Comparison of anchor-based and distributional approaches in estimating important difference in common cold. Qual Life Res. 2008;17:75–85. doi: 10.1007/s11136-007-9277-2. [DOI] [PubMed] [Google Scholar]
- 43.Kulkarni AV. Distribution-based and anchor-based approaches provided different interpretability estimates for the Hydrocephalus Outcome Questionnaire. J Clin Epidemiol. 2006;59:176–184. doi: 10.1016/j.jclinepi.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 44.Askew RL, Xing Y, Palmer JL, et al. Evaluating Minimal Important Differences for the FACT-Melanoma Quality of Life Questionnaire. Value Health. 2009 doi: 10.1111/j.1524-4733.2009.00570.x. [DOI] [PubMed] [Google Scholar]
- 45.Cohen J. Statistical power analysis for the behavioral sciences. Academic Press; New York: 1977. [Google Scholar]
- 46.Hays RD, Farivar SS, Liu H. Approaches and recommendations for estimating minimally important differences for health-related quality of life measures. Copd. 2005;2:63–67. doi: 10.1081/copd-200050663. [DOI] [PubMed] [Google Scholar]
- 47.Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61:102–109. doi: 10.1016/j.jclinepi.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 48.Eton DT, Cella D, Yost KJ, et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol. 2004;57:898–910. doi: 10.1016/j.jclinepi.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 49.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. doi: 10.1186/1477-7525-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yost KJ, Cella D, Chawla A, et al. Minimally important differences were estimated for the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) instrument using a combination of distribution- and anchor-based approaches. J Clin Epidemiol. 2005;58:1241–1251. doi: 10.1016/j.jclinepi.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 51.Bjorner JB, Chang CH, Thissen D, Reeve BB. Developing tailored instruments: item banking and computerized adaptive assessment. Quality of Life Research. 2007;16(Suppl 1):95–108. doi: 10.1007/s11136-007-9168-6. [DOI] [PubMed] [Google Scholar]
- 52.Science Advisory Committee of the Medical Outcomes Trust Assessing health status and quality-of-life instruments: Attributes and review criteria. Quality of Life Research. 2002;11:193–205. doi: 10.1023/a:1015291021312. [DOI] [PubMed] [Google Scholar]
- 53.Bernhard J, Cella DF, Coates AS, et al. Missing quality of life data in cancer clinical trials: serious problems and challenges. Stat Med. 1998;17:517–532. doi: 10.1002/(sici)1097-0258(19980315/15)17:5/7<517::aid-sim799>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 54.Ballatori E. Unsolved problems in evaluating the quality of life of cancer patients. Ann Oncol. 2001;12(Suppl 3):S11–13. doi: 10.1093/annonc/12.suppl_3.s11. [DOI] [PubMed] [Google Scholar]
- 55.Coste J, Guillemin F, Pouchot J, Fermanian J. Methodological approaches to shortening composite measurement scales. J Clin Epidemiol. 1997;50:247–252. doi: 10.1016/s0895-4356(96)00363-0. [DOI] [PubMed] [Google Scholar]
- 56.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45:S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcia SF, Cella D, Clauser SB, et al. Standardizing patient-reported outcomes assessment in cancer clinical trials: a patient-reported outcomes measurement information system initiative. J Clin Oncol. 2007;25:5106–5112. doi: 10.1200/JCO.2007.12.2341. [DOI] [PubMed] [Google Scholar]
- 58.Reeve BB, Hays RD, Bjorner JB, et al. Psychometric evaluation and calibration of health-related quality of life item banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS) Med Care. 2007;45:S22–31. doi: 10.1097/01.mlr.0000250483.85507.04. [DOI] [PubMed] [Google Scholar]
- 59.Gershon R, Rothrock NE, Hanrahan RT, et al. The development of a clinical outcomes survey research application: Assessment Center. Qual Life Res. 19:677–685. doi: 10.1007/s11136-010-9634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McNeil C. Quality of life researchers have new tool and new focus on measurement. J Natl Cancer Inst. 2008;100:234–236. doi: 10.1093/jnci/djn031. [DOI] [PubMed] [Google Scholar]
- 61.Brauer CA, Rosen AB, Greenberg D, Neumann PJ. Trends in the measurement of health utilities in published cost-utility analyses. Value Health. 2006;9:213–218. doi: 10.1111/j.1524-4733.2006.00116.x. [DOI] [PubMed] [Google Scholar]
- 62.Fayers PM, Machin D. Quality of life : the assessment, analysis, and interpretation of patient-reported outcomes. J. Wiley; Chichester; Hoboken, NJ: 2007. [Google Scholar]
- 63.Feeny D. Preference-based measures: utility and quality adjusted life years. In: Fayers PM, Hays RD, editors. Assessing quality of life in clinical trials. Edition 2nd Oxford University Press; New York: 2005. pp. 405–429. [Google Scholar]
- 64.Stiggelbout AM, de Haes JC. Patient preference for cancer therapy: an overview of measurement approaches. J Clin Oncol. 2001;19:220–230. doi: 10.1200/JCO.2001.19.1.220. [DOI] [PubMed] [Google Scholar]
- 65.Feeny DH. The roles for preference-based measures in support of cancer research and policy. In: Lipscomb J, Gotay CC, Snyder CF, editors. Outcomes assessment in cancer. Cambridge University Press; Cambridge, UK: 2005. pp. 69–92. Edition. [Google Scholar]
- 66.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 67.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43:203–220. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 68.Cantor SB, Volk RJ, Cass AR, et al. Psychological benefits of prostate cancer screening: the role of reassurance. Health Expect. 2002;5:104–113. doi: 10.1046/j.1369-6513.2002.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shih YC, Wang XS, Cantor SB, Cleeland CS. The association between symptom burdens and utility in Chinese cancer patients. Qual Life Res. 2006;15:1427–1438. doi: 10.1007/s11136-006-0011-2. [DOI] [PubMed] [Google Scholar]
- 70.Morton RL, Howard K, Thompson JF. The cost-effectiveness of sentinel node biopsy in patients with intermediate thickness primary cutaneous melanoma. Ann Surg Oncol. 2009;16:929–940. doi: 10.1245/s10434-008-0164-z. [DOI] [PubMed] [Google Scholar]
- 71.Cashin RP, Lui P, Machado M, et al. Advanced cutaneous malignant melanoma: a systematic review of economic and quality-of-life studies. Value Health. 2008;11:259–271. doi: 10.1111/j.1524-4733.2007.00243.x. [DOI] [PubMed] [Google Scholar]
- 72.Cormier JN, Xing Y, Ding M, et al. Cost effectiveness of adjuvant interferon in node-positive melanoma. J Clin Oncol. 2007;25:2442–2448. doi: 10.1200/JCO.2007.10.7284. [DOI] [PubMed] [Google Scholar]
- 73.Crott R, Ali F, Burdette-Radoux S. Cost-utility of adjuvant high-dose interferon alpha therapy in stage III cutaneous melanoma in Quebec. Value Health. 2004;7:423–432. doi: 10.1111/j.1524-4733.2004.74005.x. [DOI] [PubMed] [Google Scholar]
- 74.Hengge UR, Wallerand A, Stutzki A, Kockel N. Cost-effectiveness of reduced follow-up in malignant melanoma. J Dtsch Dermatol Ges. 2007;5:898–907. doi: 10.1111/j.1610-0387.2007.06454.x. [DOI] [PubMed] [Google Scholar]
- 75.Lafuma A, Dreno B, Delaunay M, et al. Economic analysis of adjuvant therapy with interferon alpha-2a in stage II malignant melanoma. Eur J Cancer. 2001;37:369–375. doi: 10.1016/s0959-8049(00)00411-1. [DOI] [PubMed] [Google Scholar]
- 76.Losina E, Walensky RP, Geller A, et al. Visual screening for malignant melanoma: a cost-effectiveness analysis. Arch Dermatol. 2007;143:21–28. doi: 10.1001/archderm.143.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilson LS, Reyes CM, Lu C, et al. Modelling the cost-effectiveness of sentinel lymph node mapping and adjuvant interferon treatment for stage II melanoma. Melanoma Res. 2002;12:607–617. doi: 10.1097/00008390-200212000-00011. [DOI] [PubMed] [Google Scholar]
- 78.Beusterien KM, Szabo SM, Kotapati S, et al. Societal preference values for advanced melanoma health states in the United Kingdom and Australia. Br J Cancer. 2009;101:387–389. doi: 10.1038/sj.bjc.6605187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kilbridge KL, Weeks JC, Sober AJ, et al. Patient preferences for adjuvant interferon alfa-2b treatment. J Clin Oncol. 2001;19:812–823. doi: 10.1200/JCO.2001.19.3.812. [DOI] [PubMed] [Google Scholar]
- 80.Chen S, Bendeck S, Hadley J, et al. Can melanoma patients predict the quality of life impact of an alternate melanoma stage?. 26th Annual Meeting of the Society for Medical Decision Making; Atlanta, GA. 2004; Edition. [Google Scholar]