Abstract
Objectives
To examine the relative contributions of systemic cardiovascular factors to retinal arteriolar and venular caliber in men and women and in whites and African-Americans.
Methods
In the Atherosclerosis Risk in Communities (ARIC) study, retinal arteriolar caliber (central retinal arteriolar equivalent, CRAE) and venular caliber (central retinal venular equivalent, CRVE) were measured from digitized retinal photographs for 8,794 participants.
Results
The main systemic determinants of narrower CRAE were, in order of decreasing relative contribution, higher current mean arterial blood pressure (MABP), lower serum albumin, current alcohol consumption and higher body mass index. The main systemic determinants of wider CRVE were current cigarette smoking and higher current MABP, followed by higher white cell count, body mass index and plasma LDL cholesterol levels. These associations were generally similar in whites and African-Americans and in men and women.
Conclusions
The major systemic determinant of narrower retinal arteriolar caliber is higher blood pressure, while those of wider retinal venular caliber are cigarette smoking, higher blood pressure, systemic inflammation and obesity. These data offer further insights into systemic processes influencing arteriolar and venular characteristics, and may help explain the observed associations of retinal vascular caliber and the risk of clinical cardiovascular disease.
Keywords: arteriolar caliber, venular caliber, arterioles, venules, retinal vessels
INTRODUCTION
The retina is a unique site where the small blood vessels of the human vasculature can be directly and non-invasively visualized. As such, it offers an opportunity for detailed in vivo study of the structure of small arterioles and venules and their association with systemic disease processes such as hypertension and diabetes.
Recent studies have investigated the relationship of systemic disease factors to retinal arteriolar and venular caliber. 1–9 However, several important issues remain unclear. First, few studies have examined the relative importance of risk factors in determining arteriolar and venular calibers.10 This information may improve understanding of microvascular disease pathology, and suggest the most appropriate approach to therapeutic options targeted at the microcirculation. Second, whether the risk factors of vessel caliber vary by racial/ethnic groups and by gender is unclear.4–6;11 Finally, it is unknown whether past exposures to major systemic factors (e.g., hypertension, hyperglycemia, cigarette smoking) influence retinal vessel caliber.
The current analysis of data from the Atherosclerosis Risk in Communities (ARIC) Study was designed to address all these issues. We examined the relative importance of both current and past (e.g. past blood pressure, past glucose levels, past smoking) systemic determinants of retinal arteriolar and venular caliber. We also examined if associations were similar in whites and African-Americans and in men and women.
METHODS
Study Population
The ARIC study is a population-based cohort study that included 15,792 women and men 45–64 years of age at recruitment in 1987 through 1989.12;13 The study sample is composed of individuals who participated at the third examination, when retinal photography was first performed.Of the 12,887 who presented for this examination we excluded 211 who were not present at the second examination and, 38 whose race was neither black nor white and 42 nonwhite residents in Minneapolis and Washington County (due to small numbers), 224 with no retinal photographs, 1774 with ungradeable photographs, 21 with retinal vein occlusions, 981 without data on carotid/intima media thickness and 802 without data on serum albumin, leaving 8,794 with data available for the current analysis. Persons excluded were slightly older (by 1 year), more likely to be black, and had higher mean arterial blood pressure (MABP) at visit 3 (by 2 mmHg) but did not differ by gender from persons included. Institutional review boards at each study site and at the Fundus Photograph Reading Center at the University of Wisconsin, Madison, approved the study. Informed consent was obtained from all participants and the study was conducted in accordance with the Declaration of Helsinki. Differences between participants and nonparticipants at the baseline examination are presented elsewhere.14
Assessment of Retinal Vascular Caliber
The retinal photography procedure and grading of retinal microvascular signs are described in detail elsewhere.15 Briefly, a 45-degree retinal photograph of one randomly selected eye of each participant was taken at visit 3 following 5 minutes of dark adaptation. This photograph was centered on the region of the optic disc and the macula, and was taken using an auto-focus camera.
Trained graders masked to participant characteristics used a computer-assisted approach to measure the calibers of all arterioles and venules coursing through a specified area surrounding the optic disc.15 Individual vessel measurements were combined into summary indices - the central retinal arteriole equivalent (CRAE) and central retinal venule equivalent (CRVE). These indices represent the estimated central retinal arteriolar and venular caliber of the eye, after taking into account the branching patterns.15 These measurements are reliable, with intra- and inter-grader reliability coefficients for CRAE and CRVE of 0.84 and 0.79, respectively. 15;16 Intraindividual reliability coefficients were also generally high.16
Definition of Systemic Variables
Participants underwent standardized evaluations at each examination and detailed methods and variable descriptions are provided elsewhere.12;13;17 At each examination, blood pressures were taken with a random-zero sphygmomanometer, and mean arterial blood pressure (MABP) was calculated as 2/3 of the diastolic plus 1/3 of the systolic value. Current MABP was defined as MABP from visit 3, while past MABP was defined as the average MABP from visits 1 and 2. Cigarette smoking and alcohol consumption were ascertained from questionnaire. Mean carotid/intima media thickness (IMT) was derived from standardized B-mode ultrasonograms.18 Total plasma cholesterol and triglycerides were measured by enzymatic methods, high density lipoprotein (HDL) cholesterol was measured after dextran-magnesium precipitation of the non-HDL lipoproteins, and low density lipoprotein (LDL) cholesterol was calculated by the Friedewald formula (LDL cholesterol = total cholesterol − HDL cholesterol − (triglycerides/5).19 Glucose was measured by the modified hexokinase/glucose-6-phosphate dehydrogenase procedure. Past serum glucose and plasma triglycerides were defined as glucose and triglycerides values from visit 1. White cell count, serum albumin, fibrinogen, factor VIII and von Willebrand factor (vWF) were measured at visit 1 by Coulter counter (white cell count and albumin), thrombin-time titration, clotting time with use of factor VIII-deficient plasma and by ELISA kits respectively.17;20 Height and weight were measured for calculation of body mass index.
Statistical methods
To assess the determinants of CRAE and CRVE we used multiple linear regression models that adjusted for continuous variables (per interquartile range, IQR) and dichotomous variables (presence vs absence), and compared the partial R2, a measure of the contribution of each systemic factor to the total variance in CRAE or CRVE. We used the interquartile range to provide a standardized comparison between continuous variables as some were normally distributed while others were not (e.g. serum glucose). We constructed three multivariable models. Model 1 adjusted for the variables in Table 1. Narrower arteriolar caliber and wider venular caliber may be due to pathology, normal anatomical variation, or measurement error and we have previously shown anatomical variation and measurement error in arteriolar and venular caliber may lead to spurious associations.21;22 We attempted to control for these factors by including the fellow vessel caliber in the models as a covariable, as both arteriolar and venular calibers are influenced by these factors in the same manner (i.e. persons of smaller stature have narrower arterioles and venules, and measurement errors from ocular magnification and background pigmentation tend to over-or underestimate both arteriolar and venular calibers in a similar manner.) Model 2 thus adjusted for variables in Model 1 as well as the fellow vessel caliber (i.e. addition of CRAE in models for CRVE, and CRVE in models for CRAE). Model 3 adjusted for all the variables in Model 2 as well as past MABP, past serum glucose level, past plasma triglycerides, past smoking and past alcohol consumption. We report differences in CRAE and CRVE (μm), 95% confidence intervals (CI) and partial R2. We repeated these analyses stratified by race and gender. SAS (SAS institute, version 8.2, Cary, NC) was used for all analyses.
Table 1.
Description of study population
| Mean or Numbers (% total) | Interquartile range (25th to 75th percentile) | |
|---|---|---|
| Age, years, mean | 60.0 | 10 (55–65) |
| Male, n (%) | 3993 (45.4) | -- |
| African-American, n (%) | 1601 (18.2) | -- |
| Arteriolar caliber, CRAE, μm | 162.3 | 21.6 (151.4–173.0) |
| Venular caliber, CRVE, μm | 193.1 | 21.9 (181.8–203.7) |
| Current MABP, mmHg | 89.3 | 15.3 (81.3–96.7) |
| Current serum glucose level, mg/dL | 111.1 | 18 (93–111) |
| Body mass index, kg/m2 | 27.6 | 6.3 (24.0–30.3) |
| Current plasma triglycerides, mg/dL | 142.6 | 85 (87–172) |
| HDL cholesterol, mg/dL | 52.1 | 23 (39–62) |
| LDL cholesterol, mg/dL | 127.3 | 44.8 (103.8–148.6) |
| White cell count, ×109 | 6.0 | 2.2 (4.8–7.0) |
| Fibrinogen, mg/dL | 298.9 | 73 (258–331) |
| Serum albumin, g/L | 3.9 | 0.4 (3.7–4.1) |
| Von willebrand factor, mg/dL | 115.5 | 58 (82–140) |
| Factor VIII, mg/dL | 128.9 | 44 (104–148) |
| Current smoking, n (%) | 1481 (16.8) | -- |
| Current alcohol consumption, n (%) | 5034 (57.2) | -- |
CRAE refers to retinal arteriolar caliber; CRVE to retinal venular caliber; MABP: mean arterial blood pressure; HDL: high density lipoprotein; LDL: low density lipoprotein.
RESULTS
The distribution of vascular risk factors in the population is described in Table 1. Blacks had wider CRAE (approximately 3 μm) and CRVE (approximately 8–9 μm) than whites. Women had slightly wider CRAE (2 μm) and narrower CRVE (2 μm) than men; participants aged >60 years had slightly narrower (2–3 μm) CRAE and CRVE.(data not shown)
We examined the relationship of CRAE and CRVE with systemic factors according to model 1 (unadjusted for fellow vessel caliber) and model 2 (adjusted for fellow vessel caliber). In general, both models gave similar results but there were a few exceptions. Associations for CRVE with increasing MABP reversed in direction after additional adjustment in model 2, while associations for CRAE with serum glucose, white cell count and current smoking were reduced in magnitude and lost statistical significance. For example, with each interquartile range (IQR) increase in MABP (i.e, comparing a person with higher current MABP e.g. in the 75th percentile with a person with low current MABP e.g in the 25th percentile), CRAE decreased by 7.0 μm in model 1. This is interpreted to mean that a person with higher current MABP in the 75% percentile had CRAE that was on average 7.0 μm narrower (4.0% narrower) than a person with lower current MABP (25th percentile), and a similar interpretation should be applied for results in this and following tables. The MABP-CRAE association changed little with additional adjustment for CRVE. With each IQR increase in MABP, CRVE decreased by 1.0 μm in model 1. However, after additional adjustment for CRAE in model 2, the association per IQR increase in current MABP reversed in direction from −1.0 μm to 2.6 μm. The results after adjusting for model 2 are presented in Table 2.
Table 2.
Retinal arteriolar caliber (CRAE), retinal venular caliber (CRVE) and systemic factors, unadjusted and adjusted for fellow correlated vessel caliber
| Mean difference (95% CI) Retinal arteriolar caliber (CRAE) in μm Model 2 | Mean difference (95% CI) Retinal venular caliber (CRVE) in μm Model 2 | ||||
|---|---|---|---|---|---|
| Blood pressure | |||||
| Current MABP (mmHg) | Per IQR increase | −6.4 | (−6.8, −6.0) | 2.6 | (2.2, 3.1) |
| Metabolic | |||||
| Current serum glucose level | Per IQR increase | 0.05 | (−0.1, 0.2) | 0.3 | (0.1, 0.4) |
| Body mass index | Per IQR increase | −0.7 | (−1.1, −0.3) | 0.6 | (0.2, 1.0) |
| Lipids | |||||
| Current plasma triglycerides | Per IQR increase | −0.5 | (−0.9, −0.1) | 0.3 | (−0.06, 0.8) |
| HDL cholesterol | Per IQR increase | −0.5 | (−1.0, −0.09) | −0.05 | (−0.5, 0.4) |
| LDL cholesterol | Per IQR increase | −0.05 | (−0.4, 0.3) | 0.9 | (0.5, 1.2) |
| Inflammatory markers | |||||
| White cell count | Per IQR increase | −0.2 | (−0.6, 0.2) | 1.2 | (0.9, 1.6) |
| Fibrinogen | Per IQR increase | 0.09 | (−0.3, 0.5) | 0.7 | (0.3, 1.1) |
| Serum albumin | Per IQR increase | 1.5 | (1.0, 1.9) | −0.6 | (−1.1, −0.2) |
| Endothelial function markers | |||||
| Von willebrand factor | Per IQR increase | 0.9 | (0.4, 1.4) | −0.8 | (−1.3, −0.3) |
| Factor VIII | Per IQR increase | −0.4 | (−0.9, 0.08) | −0.07 | (−0.6, 0.4) |
| Systemic atherosclerosis | |||||
| Carotid Intima media thickness | Per IQR increase | −0.2 | (−0.5, 0.1) | 0.3 | (-0.03, 0.6) |
| Lifestyle | |||||
| Current smoking | Vs never | 0.7 | (−0.1, 1.5) | 5.7 | (4.9, 6.5) |
| Current alcohol consumption | Vs never | −1.2 | (−1.7, −0.6) | 0.9 | (0.3, 1.4) |
| Fellow retinal caliber (CRVE/CRAE) | Per SD increase | 8.9 | (8.6, 9.2) | 8.8 | (8.5, 9.1) |
| Total Adjusted R2 | 0.389 | 0.370 | |||
MABP: mean arterial blood pressure; HDL: high density lipoprotein; LDL: low density lipoprotein; IQR: interquartile range; SD refers to standard deviation; Model 1 adjusted for all the variables in the table; Model 2 adjusted for all the variables in the table plus either CRVE (for CRAE) or CRAE (for CRVE).
Bolded figures are significant at p<0.05
The greatest proportional determinants of narrower CRAE, as measured from partial R2 contribution to Model 2, were higher current MABP (0.089), lower serum albumin (0.003) and current alcohol consumption (0.002) (Table 3). The largest determinants of wider CRVE, by partial R2, were current smoking (0.026), higher current MABP (0.026), higher white cell count (0.006), BMI (0.002) and LDL cholesterol (0.002). Persons with higher values of current MABP (75th percentile) had CRVE that on average was 2.6 μm wider (1.4% wider) than persons with lower values of current MABP (25th percentile).
Table 3.
Retinal arteriolar caliber (CRAE), retinal venular caliber (CRVE) and relative contribution of systemic factors, adjusted for fellow vessel (Model 2).
| Rank by | Retinal arteriolar caliber (CRAE) | Retinal venular caliber (CRVE) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Partial R2 | Variable | Mean difference (95% CI), μm | P | Partial R2 | Variable | Mean difference (95% CI), μm | P | Partial R2 | ||
| 1 | Current MABP | Per IQR increase | −6.4 (−6.8, −6.0) | <0.0001 | 0.089 | Current smoking | Vs never | 5.7 (4.9, 6.5) | <0.0001 | 0.026 |
| 2 | Serum albumin | Per IQR increase | 1.5 (1.0, 1.9) | <0.0001 | 0.003 | Current MABP | Per IQR increase | 2.6 (2.2, 3.1) | <0.0001 | 0.026 |
| 3 | Current alcohol consumption | Vs never | −1.2 (−1.7, −0.6) | <0.0001 | 0.002 | White cell count | Per IQR increase | 0.9 (0.3, 1.4) | <0.0001 | 0.006 |
| 4 | Body mass index | Per IQR increase | −0.7 (−1.1, −0.3) | 0.001 | 0.001 | Body mass index | Per IQR increase | 0.6 (0.2, 1.0) | <0.0001 | 0.002 |
| 5 | Von willebrand factor | Per IQR increase | 0.9 (0.4, 1.4) | 0.001 | 0.001 | LDL cholesterol | Per IQR increase | 0.9 (0.5, 1.2) | <0.0001 | 0.002 |
| 6 | HDL cholesterol | Per IQR increase | −0.5 (−1.0, −0.09) | 0.02 | 0.001 | Current serum glucose level | Per IQR increase | 0.3 (0.1, 0.4) | 0.0002 | 0.001 |
| 7 | -- | -- | -- | -- | -- | Fibrinogen | Per IQR increase | 0.7 (0.3, 1.1) | 0.0002 | 0.001 |
| 8 | -- | -- | -- | -- | -- | Von willebrand factor | Per IQR increase | −0.8 (−1.3, −0.3) | 0.0002 | 0.001 |
MABP: mean arterial blood pressure; HDL: high density lipoprotein; LDL: low density lipoprotein; IQR: interquartile range; SD refers to standard deviation; Values are adjusted in Model 2 i.e. adjusted for all the variables in the table, including CRVE (for CRAE) and CRAE (for CRVE).
Systemic associations of CRAE were similar in whites and blacks. Most associations with CRAE in men and women were similar. For example, men with higher current MABP (75th percentile) had CRAE that was 6.5 μm narrower (4.1% narrower) than men with lower current MABP (25th percentile), while women with higher current MABP had CRAE that was 6.3 μm narrower than womener with low current MABP (4.0%). There were some sex differences, particularly with HDL cholesterol, endothelial function markers and current alcohol consumption.(data not shown)
Persons with higher current MABP (75th percentile) had CRAE that was 4.8 μm narrower (3.0% narrower) than persons with lower MABP (25th percentile), whereas persons with higher past MABP (75th percentile) had CRAE that was only 2.6 μm narrower (1.6% narrower) than persons with lower past MABP (25th percentile) (Table 4). Higher current and past MABP were both associated with wider CRVE, as were current and past smoking.
Table 4.
Comparison of current and past systemic variables on retinal arteriolar caliber (CRAE) and retinal venular caliber (CRVE)
| Variable | Retinal Arteriolar Caliber (CRAE) in μm | Retinal Venular Caliber (CRVE) in μm | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean difference (95% CI) | P | Partial R2 | Mean difference (95% CI) | P | Partial R2 | ||||
| Blood pressure | |||||||||
| Current MABP | Per IQR increase | −4.8 (−5.3, −4.3) | <0.0001 | 0.089 | 1.8 (1.3, 2.3) | <0.0001 | 0.025 | ||
| Past MABP | Per IQR increase | −2.6 (−3.2, −2.1) | <0.0001 | 0.008 | 1.5 (0.9, 2.0) | <0.0001 | 0.003 | ||
| Metabolic | |||||||||
| Current serum glucose level | Per IQR increase | 0.08 (−0.1, 0.3) | 0.39 | Neg | 0.2 (0.05, 0.4) | 0.01 | Neg | ||
| Past serum glucose level | Per IQR increase | 0.01 (−0.2, 0.2) | 0.90 | Neg | 0.04 (−0.1, 0.2) | 0.67 | Neg | ||
| Lipids | |||||||||
| Current plasma triglycerides | Per IQR increase | −0.5 (-1.0, −0.03) | 0.03 | Neg | 0.3 (−0.2, 0.8) | 0.18 | Neg | ||
| Past plasma triglycerides | Per IQR increase | 0.05 (−0.7, 0.8) | 0.89 | Neg | −0.05 (−0.8, 0.7) | 0.90 | Neg | ||
| Lifestyle | |||||||||
| Current smoking | Vs never | 0.3 (−0.6, 1.2) | 0.47 | Neg | 6.9 (6.0, 7.8) | <0.0001 | 0.026 | ||
| Past smoking | Vs never | −0.2 (−0.8, 0.5) | 0.61 | Neg | 1.8 (1.1, 2.4) | <0.0001 | 0.002 | ||
| Current alcohol consumption | Vs never | −1.5 (−2.3, −0.8) | <0.0001 | 0.001 | 0.7 (−0.06, 1.4) | 0.07 | Neg | ||
| Past alcohol consumption | Vs never | −0.8 (−1.7, 0.05) | 0.06 | Neg | 0.2 (−0.7, 1.1) | 0.66 | Neg | ||
| Fellow retinal caliber (CRVE/CRAE) | Per SD increase | 8.9 (8.6, 9.2) | <0.0001 | 0.284 | 8.8 8.5, 9.1 | <0.0001 | 0.284 | ||
| Adjusted R2 | 0.397 | 0.374 | |||||||
Values are adjusted in Model 3 i.e. adjusted for all the variables in the table plus those in Model 2 (see table 4) plus either CRVE (for CRAE) or CRAE (for CRVE).
MABP: mean arterial blood pressure; Neg: negligible (<0.001)
In a supplementary analysis, we excluded the 2297 participants taking antihypertensive treatments. The association of narrower CRAE with current MABP strengthened slightly, with CRAE narrowing by 7.4 μm per IQR increase in MABP in model 1.
In summary, most associations of CRAE and CRVE were similar using model 1 or model 2. The major proportional determinants of narrower CRAE were higher current MABP, lower serum albumin and current alcohol consumption. The major determinants of wider CRVE were current smoking, higher current MABP, higher white cell count, BMI and LDL cholesterol. Associations were largely similar across race and gender.
DISCUSSION
The ARIC study has well documented data on a range of systemic cardiovascular risk factors and digitized retinal images, from which we measured retinal arteriolar (CRAE) and venular caliber (CRVE). In an earlier report, our group examined the association of cardiovascular risk factors with arteriole to venule ratio, a measure of arteriolar narrowing which has since been shown to have several limitations.21 Our findings are consistent with those from our earlier report and provide further insight into the vascular processes driving changes in the arteriole to venule ratio
Our study expands on earlier work examining the specific relationships of cardiovascular, metabolic, inflammatory, and lifestyle risk factors with retinal vascular caliber,17 including reports from the Multi-Ethnic Study of Atherosclerosis (MESA),3 Beaver Dam Eye Study (BDES),4 Rotterdam Study,5 Blue Mountains Eye Study (BMES),6;11 Cardiovascular Health Study,7 and the Funagata study.8 All studies consistently found that high blood pressure was associated with narrower arteriolar caliber, and that smoking and body mass index were associated with wider venular caliber. Some studies have also reported high blood pressure associated with narrower venular caliber, and smoking associated with wider arteriolar caliber, which our analyses now show disappear with adjustment for natural anatomic variation and measurement error.5 In the case of both MABP and smoking, findings from models with additional adjustment may be more congruent with clinical experience and existing biological knowledge. For example, wider retinal venules with increasing MABP may be explained as a response to chronic retinal hypoxia or hypoperfusion 23;24 from chronic hypertensive damage to the microcirculation, whereas it is less biologically apparent why narrower venules should be associated with increasing MABP. Similarly, a lack of influence of smoking on arteriolar caliber is more consistent with the literature that smoking has little effect on arteriolar caliber, or causes only mild arteriolar narrowing25;26 rather than widening.
An important concern in controlling for anatomy and measurement error using the fellow vessel caliber is the potential for over-adjustment where anatomic factors common to both arteriolar and venular caliber may be inappropriately adjusted for. This is particularly relevant in determining the associations of age, gender and race on vascular caliber. Increasing age was a major contributor to narrower arteriolar and venular caliber. Other researchers have reported that men have narrower arterioles than women,3;27 which could be related to the higher blood pressure in men (with higher arteriolar resistance).27 In animal experiments, male gender is associated with narrower arterioles than female gender at the same blood pressure level.28;29 The associations of male gender and black race with wider venular caliber were reported in the BDES4 and MESA,3 respectively, but the underlying mechanisms are not known. These differences may reflect genetic or cardiovascular influences not examined in this report.
We observed that the main determinants of narrower arteriolar caliber (other than demographic factors) were higher current MABP, lower serum albumin, and current alcohol consumption. In longitudinal analyses, both current and past MABP were strongly associated with narrower arteriolar caliber. All studies to date have consistently demonstrated that current,3–8 and past blood pressure30;31 are strongly associated with narrower arteriolar caliber, confirming retinal arteriolar narrowing as a marker of current and longer-term blood pressure levels. The association of lower serum albumin with narrower retinal arterioles was also observed in the BDES,4 although it was not statistically significant in that population. Lower serum albumin may be a non-specific marker of inflammation or chronic kidney disease, and the association with narrower arterioles may reflect these disease processes The markers of endothelial dysfunction examined in this report, vWF and factor VIII levels, made at most small or negligible contributions to arteriolar caliber, consistent with results from the BDES,4 MESA3 and Hoorn studies32 which found no association of retinal arteriolar caliber with other more specific serum markers of endothelial dysfunction (e.g. serum soluble intercellular adhesion molecule-1, sICAM-1). However, we did not have data on other markers of endothelial dysfunction, such as plasma asymmetric dimethylarginine.33 The association with current alcohol consumption has been reported in some studies (MESA),3 but not others (Rotterdam),5 and may reflect impaired vasodilation with chronic alcohol ingestion, which is associated with impaired vasodilation in the cerebral 34;35 and systemic microcirculations.36
The main determinants of wider venular caliber in this population (other than demographic factors) were current smoking and higher current MABP, followed by increasing white cell count. Smoking has consistently been associated with wider retinal venules in the Rotterdam study, 5 BDES,4 MESA3 the Wisconsin Epidemiologic Study of Diabetic Retinopathy,10 BMES,37, and we now additionally show a temporal association of past smoking with wider retinal venules that is independent of current smoking status. Retinal venule widening in smokers has been observed clinically, and may be due to reduced oxyhemoglobin and tissue hypoxia, nicotine-induced changes in vessel autoregulation, and secondary polycythaemia.38;39 Chronic smoking also promotes inflammation (see below) and endothelial dysfunction,40–42 which may lead to disruptions of microvascular autoregulatory vasomotor function and venular widening.43;44 Wider retinal venules may thus be a lasting microvascular marker of damage from long-standing smoking.
Higher white cell count was a major contributor to wider venular caliber, with lesser contributions from other nonspecific markers of inflammation (fibrinogen and serum albumin).3–5 A range of inflammatory markers has now been consistently linked with wider retinal venules in all the major population-based studies,3–5;45 strongly implicating a role for inflammation in influencing retinal venular caliber. Inflammation may cause venular widening through mechanisms such as damage to the venular endothelial surface layer,5;45 resulting in loss of glycocalyx (and hence a wider blood column detected on retinal photography) or increased vasodilation.
We also examined for possible racial/ethnic and gender differences in associations. Associations were largely similar, but some systemic determinants appeared to vary by race or gender e.g. BMI, alcohol consumption and markers of endothelial dysfunction. These findings should be interpreted with caution as they may be chance associations, were not specified apriori, most interactions were not strongly statistically significant, and the mechanisms which may underlie them are not known.
The vascular risk factors examined in this report explain more of the variation in retinal arteriolar caliber than in retinal venular caliber, highlighting the lack of knowledge about the determinants of venular caliber. It was earlier assumed that venular calibers remain relatively static in the presence of vascular disease, but this is now shown to be clearly not the case. Some recent studies indicate that venular widening may be a marker of other processes such as endothelial dysfunction,3 hypoperfusion and cerebral hypoxia.46 However, it should be borne in mind that associations with venular widening may actually reflect associations with arteriolar widening, but due to the strong concomitant association of arteriolar narrowing with blood pressure this possibility is difficult to determine, and our method of adjusting for the fellow vessel caliber may be an overadjustment.
Strengths of this study include the use of well-measured cardiovascular risk factors, adjustment for many potential confounders and large sample size permitting subgroup analyses. As the main analyses were cross-sectional, care must be taken in interpreting the temporal sequence of effect. Although many associations were observed, most contributed only marginally to differences in vascular caliber. Although a high proportion of subjects had ungradeable photographs, we do not believe this influenced the associations we observed, as it is unlikely that persons with ungradeable photographs had retinal vessel calibers different from persons with gradeable photographs.
In summary, findings from the ARIC population suggest distinct patterns of systemic associations with arteriolar and venular caliber. The major systemic determinants of narrower arteriolar caliber were higher current blood pressure, lower serum albumin and current alcohol consumption. The major determinants of wider venular caliber were current smoking and higher blood pressure, with contributions from inflammatory processes. Past blood pressure and past smoking were associated with narrower arteriolar caliber and wider venular caliber respectively. Our results also indicate that spurious associations from shared anatomy and measurement error may occur with regards to associations with blood pressure and smoking. However, with regards to other systemic variables, spurious associations do not appear to be a major issue. These data show clearly the deleterious effects of elevated blood pressure, smoking, alcohol, obesity, dyslipidemia and inflammation on the microcirculation and emphasize again the importance of controlling these risk factors in preventing and treating cardiovascular disease. More research is needed into whether close monitoring of the retinal microvasculature using retinal imaging techniques permits clinicians to quantify the efficacy of treatments for elevated blood pressure and other vascular risk factors.
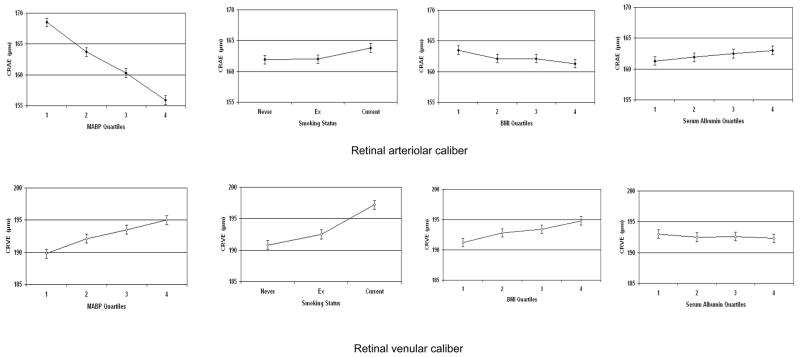
Figure 1.
Relationship of retinal arteriolar (CRAE) and venular (CRVE) calibers to current mean arterial blood pressure (MABP), smoking, body mass index (BMI) and serum albumin. Vertical bars indicate 95% confidence intervals. CRAE and CRVE are adjusted for the factors in model 2 (please see text).
Acknowledgments
This study was supported by contracts N01-HC-35125, N01-HC-35126, N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022 from the National Heart, Lung, and Blood Institute, Bethesda, MD. Additional support was provided by the National Heart Foundation, the Science Technology Innovation Grant, Victoria and the Sylvia and Charles Viertel Clinical Investigator Award (TYW). The authors thank the staff and participants of the ARIC study for their important contributions. Tien Wong had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Wong TY, Mitchell P. Hypertensive retinopathy. N Engl J Med. 2004;351:2310–2317. doi: 10.1056/NEJMra032865. [DOI] [PubMed] [Google Scholar]
- 2.Wong TY, Mitchell P. The eye in hypertension. Lancet. 2007;369:425–435. doi: 10.1016/S0140-6736(07)60198-6. [DOI] [PubMed] [Google Scholar]
- 3.Wong TY, Islam FM, Klein R, Klein BE, Cotch MF, Castro C, et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multi-ethnic study of atherosclerosis (MESA) Invest Ophthalmol Vis Sci. 2006;47:2341–2350. doi: 10.1167/iovs.05-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Knudtson MD, Wong TY, Tsai MY. Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch Ophthalmol. 2006;124:87–94. doi: 10.1001/archopht.124.1.87. [DOI] [PubMed] [Google Scholar]
- 5.Ikram MK, de Jong FJ, Vingerling JR, Witteman JC, Hofman A, Breteler MM, et al. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci. 2004;45:2129–2134. doi: 10.1167/iovs.03-1390. [DOI] [PubMed] [Google Scholar]
- 6.Leung H, Wang JJ, Rochtchina E, Wong TY, Klein R, Mitchell P. Dyslipidaemia and microvascular disease in the retina. Eye. 2005;19:861–868. doi: 10.1038/sj.eye.6701668. [DOI] [PubMed] [Google Scholar]
- 7.Wong TY, Hubbard LD, Klein R, Marino EK, Kronmal R, Sharrett AR, et al. Retinal microvascular abnormalities and blood pressure in older people: the Cardiovascular Health Study. Br J Ophthalmol. 2002;86:1007–1013. doi: 10.1136/bjo.86.9.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawasaki R, Wang JJ, Rochtchina E, Taylor B, Wong TY, Tominaga M, et al. Cardiovascular risk factors and retinal microvascular signs in an adult Japanese population: the Funagata Study. Ophthalmology. 2006;113:1378–1384. doi: 10.1016/j.ophtha.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 9.Taylor B, Rochtchina E, Wang JJ, Wong TY, Heikal S, Saw SM, et al. Body mass index and its effects on retinal vessel diameter in 6-year-old children. Int J Obes (Lond) 2007 doi: 10.1038/sj.ijo.0803674. [DOI] [PubMed] [Google Scholar]
- 10.Klein R, Klein BE, Moss SE, Wong TY, Sharrett AR. Retinal vascular caliber in persons with type 2 diabetes: the Wisconsin Epidemiological Study of Diabetic Retinopathy: XX. Ophthalmology. 2006;113:1488–1498. doi: 10.1016/j.ophtha.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Wang JJ, Mitchell P, Leung H, Rochtchina E, Wong TY, Klein R. Hypertensive retinal vessel wall signs in a general older population: the Blue Mountains Eye Study. Hypertension. 2003;42:534–541. doi: 10.1161/01.HYP.0000090122.38230.41. [DOI] [PubMed] [Google Scholar]
- 12.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 13.NHLBI. Atherosclerosis Risk in Communities Study: Operations Manual No. 2: Cohort Component Procedures. Version 2.0. Chapel Hill, NC: ARIC Coordinating Center, School of Public Health, University of North Carolina; 1988. [Google Scholar]
- 14.Jackson R, Chambless LE, Yang K, Byrne T, Watson R, Folsom A, et al. Differences between respondents and nonrespondents in a multicenter community-based study vary by gender ethnicity. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. J Clin Epidemiol. 1996;49:1441–1446. doi: 10.1016/0895-4356(95)00047-x. [DOI] [PubMed] [Google Scholar]
- 15.Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 16.Couper DJ, Klein R, Hubbard LD, Wong TY, Sorlie PD, Cooper LS, et al. Reliability of retinal photography in the assessment of retinal microvascular characteristics: the Atherosclerosis Risk in Communities Study. Am J Ophthalmol. 2002;133:78–88. doi: 10.1016/s0002-9394(01)01315-0. [DOI] [PubMed] [Google Scholar]
- 17.Klein R, Sharrett AR, Klein BE, Chambless LE, Cooper LS, Hubbard LD, et al. Are retinal arteriolar abnormalities related to atherosclerosis?: The Atherosclerosis Risk in Communities Study. Arterioscler Thromb Vasc Biol. 2000;20:1644–1650. doi: 10.1161/01.atv.20.6.1644. [DOI] [PubMed] [Google Scholar]
- 18.High-resolution B-mode ultrasound scanning methods in the Atherosclerosis Risk in Communities Study (ARIC) The ARIC Study Group. J Neuroimaging. 1991;1:68–73. [PubMed] [Google Scholar]
- 19.Sharrett AR, Patsch W, Sorlie PD, Heiss G, Bond MG, Davis CE. Associations of lipoprotein cholesterols, apolipoproteins A-I and B, and triglycerides with carotid atherosclerosis and coronary heart disease. The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb. 1994;14:1098–1104. doi: 10.1161/01.atv.14.7.1098. [DOI] [PubMed] [Google Scholar]
- 20.Nelson JJ, Liao D, Sharrett AR, Folsom AR, Chambless LE, Shahar E, et al. Serum albumin level as a predictor of incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2000;151:468–477. doi: 10.1093/oxfordjournals.aje.a010232. [DOI] [PubMed] [Google Scholar]
- 21.Liew G, Sharrett AR, Kronmal R, Klein R, Wong TY, Mitchell P, et al. Measurement of retinal vascular caliber: issues and alternatives to using the arteriole to venule ratio. Invest Ophthalmol Vis Sci. 2007;48:52–57. doi: 10.1167/iovs.06-0672. [DOI] [PubMed] [Google Scholar]
- 22.Liew G, Wong TY, Mitchell P, Wang JJ. Are narrower or wider retinal venules associated with incident hypertension? Hypertension. 2006;48:e10. doi: 10.1161/01.HYP.0000231652.97173.4c. [DOI] [PubMed] [Google Scholar]
- 23.Saldivar E, Cabrales P, Tsai AG, Intaglietta M. Microcirculatory changes during chronic adaptation to hypoxia. Am J Physiol Heart Circ Physiol. 2003;285:H2064–H2071. doi: 10.1152/ajpheart.00349.2003. [DOI] [PubMed] [Google Scholar]
- 24.Klijn CJ, Kappelle LJ, van Schooneveld MJ, Hoppenreijs VP, Algra A, Tulleken CA, et al. Venous stasis retinopathy in symptomatic carotid artery occlusion: prevalence, cause, and outcome. Stroke. 2002;33:695–701. doi: 10.1161/hs0302.104619. [DOI] [PubMed] [Google Scholar]
- 25.Black HR, Zeevi GR, Silten RM, Walker Smith GJ. Effect of heavy cigarette smoking on renal and myocardial arterioles. Nephron. 1983;34:173–179. doi: 10.1159/000183005. [DOI] [PubMed] [Google Scholar]
- 26.Auerbach O, Carter HW, Garfinkel L, Hammond EC. Cigarette smoking and coronary artery disease. A macroscopic and microscopic study. Chest. 1976;70:697–705. doi: 10.1378/chest.70.6.697. [DOI] [PubMed] [Google Scholar]
- 27.Huang A, Kaley G. Gender-specific regulation of cardiovascular function: estrogen as key player. Microcirculation. 2004;11:9–38. doi: 10.1080/10739680490266162. [DOI] [PubMed] [Google Scholar]
- 28.Huang A, Sun D, Kaley G, Koller A. Estrogen maintains nitric oxide synthesis in arterioles of female hypertensive rats. Hypertension. 1997;29:1351–1356. doi: 10.1161/01.hyp.29.6.1351. [DOI] [PubMed] [Google Scholar]
- 29.Littleton-Kearney MT, Agnew DM, Traystman RJ, Hurn PD. Effects of estrogen on cerebral blood flow and pial microvasculature in rabbits. Am J Physiol Heart Circ Physiol. 2000;279:H1208–H1214. doi: 10.1152/ajpheart.2000.279.3.H1208. [DOI] [PubMed] [Google Scholar]
- 30.Sharrett AR, Hubbard LD, Cooper LS, Sorlie PD, Brothers RJ, Nieto FJ, et al. Retinal arteriolar diameters and elevated blood pressure: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1999;150:263–270. doi: 10.1093/oxfordjournals.aje.a009997. [DOI] [PubMed] [Google Scholar]
- 31.Leung H, Wang JJ, Rochtchina E, Wong TY, Klein R, Mitchell P. Impact of current and past blood pressure on retinal arteriolar diameter in an older population. J Hypertens. 2004;22:1543–1549. doi: 10.1097/01.hjh.0000125455.28861.3f. [DOI] [PubMed] [Google Scholar]
- 32.van Hecke MV, Dekker JM, Nijpels G, Stolk RP, Henry RM, Heine RJ, et al. Are retinal microvascular abnormalities associated with large artery endothelial dysfunction and intima-media thickness? The Hoorn Study. Clin Sci (Lond) 2006;110:597–604. doi: 10.1042/CS20050270. [DOI] [PubMed] [Google Scholar]
- 33.Hemminki V, Laakso J, Kahonen M, Turjanmaa V, Uusitalo H, Lehtimaki T, et al. Plasma asymmetric dimethylarginine and retinal vessel diameters in middle-aged men. Metabolism. 2007;56:1305–1310. doi: 10.1016/j.metabol.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Mayhan WG, Didion SP. Effect of chronic alcohol consumption on responses of cerebral arterioles. Alcohol Clin Exp Res. 1996;20:538–542. doi: 10.1111/j.1530-0277.1996.tb01089.x. [DOI] [PubMed] [Google Scholar]
- 35.Altura BM, Altura BT. Alcohol, the cerebral circulation and strokes. Alcohol. 1984;1:325–331. doi: 10.1016/0741-8329(84)90056-9. [DOI] [PubMed] [Google Scholar]
- 36.Potter JF, Beevers DG. The possible mechanisms of alcohol associated hypertension. Ann Clin Res. 1984;16 (Suppl 43):97–102. [PubMed] [Google Scholar]
- 37.Kifley A, Liew G, Wang JJ, Kaushik S, Smith W, Wong TY, et al. Long Term Effects of Smoking on Retinal Microvascular Caliber. Am J Epidemiol. doi: 10.1093/aje/kwm255. In press. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg ML, Chan DG, Francis IC, Coroneo MT. Smokers' veins: a useful clinical sign - response. Clin Experiment Ophthalmol. 2005;33:676. doi: 10.1111/j.1442-9071.2004.00968.x. [DOI] [PubMed] [Google Scholar]
- 39.Wimpissinger B, Resch H, Berisha F, Weigert G, Schmetterer L, Polak K. Response of retinal blood flow to systemic hyperoxia in smokers and nonsmokers. Graefes Arch Clin Exp Ophthalmol. 2005;243:646–652. doi: 10.1007/s00417-004-1083-8. [DOI] [PubMed] [Google Scholar]
- 40.Michaud SE, Dussault S, Haddad P, Groleau J, Rivard A. Circulating endothelial progenitor cells from healthy smokers exhibit impaired functional activities. Atherosclerosis. 2006;187:423–432. doi: 10.1016/j.atherosclerosis.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 41.Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation. 2004;109:II27–II33. doi: 10.1161/01.CIR.0000129501.88485.1f. [DOI] [PubMed] [Google Scholar]
- 42.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111:2684–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 43.Dalla VL, Palombo C, Ciardetti M, Porta A, Milani O, Kozakova M, et al. Contrasting effects of acute and chronic cigarette smoking on skin microcirculation in young healthy subjects. J Hypertens. 2004;22:129–135. doi: 10.1097/00004872-200401000-00022. [DOI] [PubMed] [Google Scholar]
- 44.Lova RM, Miniati B, Macchi C, Gulisano M, Gheri G, Catini C, et al. Morphologic changes in the microcirculation induced by chronic smoking habit: a videocapillaroscopic study on the human labial mucosa. Am Heart J. 2002;143:658. doi: 10.1067/mhj.2002.121461. [DOI] [PubMed] [Google Scholar]
- 45.De Jong FJ, Ikram MK, Witteman JC, Hofman A, De Jong PT, Breteler MM. Retinal vessel diameters and the role of inflammation in cerebrovascular disease. Ann Neurol. 2007;61:491–495. doi: 10.1002/ana.21129. [DOI] [PubMed] [Google Scholar]
- 46.De Jong FJ, Vernooij MW, Ikram MK, Ikram MA, Hofman A, Krestin GP, et al. Arteriolar Oxygen Saturation, Cerebral Blood Flow, and Retinal Vessel Diameters The Rotterdam Study. Ophthalmology. 2007 doi: 10.1016/j.ophtha.2007.06.036. [DOI] [PubMed] [Google Scholar]