Abstract
Objective
Lack of dose adjustment for renally cleared drugs in the presence of poor renal function is a common problem in the hospital setting. The absence of a clinical decision support system (CDSS) from direct clinician workflows such as computerized provider order entry (CPOE) hinders the uptake of CDSS. This study implemented CDSS in an environment independent of CPOE, introduced to prescribers via academic detailing, to address the dosing of renally cleared drugs.
Design
GFR+ was designed to automatically calculate and update renal function, doses of key drugs adjusted for renal function, and highlight clinically significant decreases in renal function. Prescribers were made aware of GFR+, its navigation, and surrounding clinical issues, using academic detailing.
Measurement
The rate of dosing conformity and management for key renally cleared drugs in hospitalized patients, before and after GFR+ implementation.
Results
Improvements were seen in dosing conformity for enoxaparin (from 68% to 86%, p=0.03), gentamicin (63–87%, p=0.01), and vancomycin (47–77%, p=0.07), as well as the appropriate use of gentamicin therapeutic drug monitoring (70–90%, p=0.02). During episodes of acute renal impairment, renally cleared drugs were held on 38% of instances in the pre-intervention period compared with 62% post-intervention (p=0.01).
Conclusion
Clinical decision support implemented with academic detailing improved dosing conformity and management of key renally cleared drugs in a hospitalized population. Academic detailing should be strongly considered to facilitate the introduction of CDSS systems that cannot be placed directly into the clinician workflow.
Keywords: Academic detailing, clinical decision support, drug dosing, enoxaparin, gentamicin, vancomycin
Dose adjustment of renally cleared drugs according to renal function is an essential element of prescribing. Renal function declines slowly with age, but this is poorly recognized by those prescribing renally cleared drugs and is reflected in the subsequent lack of dose adjustment.1 2 This may cause confusion with dose adjustment, and result in inadvertent overdosing in older patients, resulting in an excess of adverse drug events.3 4 In fact, the most common specific factor associated with prescribing errors is a decline in renal function requiring alteration of drug therapy.5 Non-compliance for renal drug dosing within the hospital setting is common, with inappropriate prescribing in 19–67% of patients.6 Furthermore, a lack of clear dose adjustment guidelines for renally cleared medications is an impediment to this process.7 This clinical issue was successfully addressed by Chertow et al,8 who utilized a clinical decision support system (CDSS) for dosing of renally cleared drugs. This was incorporated as part of a computerized provider order entry (CPOE) and thus a mandatory part of the workflow for prescribers. Apart from this there is little published data on the use of CDSS to address the dosing of renally cleared drugs, especially as a CDSS outside the framework of CPOE.
Poor prescribing of renally cleared drugs within our hospital was identified as a source of avoidable serious adverse drug events. In response to this we developed a computerized CDSS (named GFR+) aimed at the clinical utilization of sophisticated dosing approaches for key renally cleared drugs. Our hospital does not have the facility for CPOE and thus GFR+ could not be incorporated directly into the clinician workflow, which is literally a mandatory requirement for any CDSS to be effective. This led to concerns that GFR+ may be underutilized. We employed an academic detailing process to introduce and familiarize clinicians with GFR+, and the dosing issues inherent to this area, and thus facilitate the interaction between people, technology and organizational workflow around this clinical issue. Academic detailing (also known as educational visiting) has been identified as a practice that improves the prescribing practice of clinicians.9 This involves face-to-face sessions, defining clear educational and behavioral objectives, establishing credibility with respect to objectivity, stimulating physician interaction, key messages, educational materials and providing positive re-enforcement with follow-up visits.10
Aims
To determine the impact of a CDSS focusing on the dosing of renally cleared drugs (GFR+) in the hospital setting. This CDSS was outside the direct clinician workflow, but was introduced through an academic detailing process.
To determine the relative contribution of academic detailing to the uptake of GFR+.
Methods
GFR+ was developed as a decision support module to address renally cleared drug dosing at the Repatriation General Hospital, a 300-bed teaching hospital specializing in care for the elderly. Functions incorporated into GFR+ included: automatic updating of renal function estimates; dose calculation of renally cleared drugs; highlighting episodes of acute renal failure (ARF).
The module was linked through local databases to enable the prepopulation of fields and automatic updating of required variables. GFR+ was not part of an electronic prescribing process, or in the direct workflow for prescribers, who were under no obligation to use it.
The study was approved by the local hospital ethics committee. General medical and surgical patients who were receiving or commenced on designated renally cleared drugs of interest during the admission were studied. Psychiatry and day surgery patients were excluded.
Baseline data were collected for a 6-month period in the total absence of GFR+. GFR+ was then put on-line and introduced to prescribers using an academic detailing format, and a further 5 months of data were collected. Data were acquired prospectively from case notes and drug charts at discharge for each patient. The academic detailing incorporated a 15-min session with the clinician by a trained operator, and was performed within 2 weeks of the clinician commencing at the hospital. The session included directions on the navigation of GFR+ and background on the clinical issues that prompted the development of the system. Academic detailing was performed by the same person throughout the study.
GFR+ targeted renally cleared drugs commonly used at this hospital that were associated with low therapeutic windows or other locally identified prescribing issues. Targeted drugs included allopurinol, angiotensin-converting enzyme inhibitors (ACEI), digoxin, enoxaparin, gentamicin, lithium, metformin, spironolactone, and vancomycin. Enoxaparin and gentamicin in particular were targeted as key drugs due to their potential for severe toxicity as evidenced by local death review audit data.
Development of dosing guidelines
Guidelines incorporating quantitative dose adjustment according to the degree of renal impairment were developed. Specific hospital-approved dosing models were developed for enoxaparin and gentamicin to use in GFR+. These sophisticated dosing approaches differed from the simple dosing approaches in use at the hospital during the pre-intervention period. Enoxaparin dosing was based on significant primary literature published over the past few years indicating that enoxaparin doses should be more gradually reduced in line with renal function commencing at 80 ml/min, and a loading dose is required for patients with significant renal impairment.11 This has subsequently been shown to significantly reduce bleeding rates with no obvious loss of efficacy.12 Gentamicin dosing was based on a model derived from local population kinetics utilizing a gentamicin clearance estimation from the estimated renal function, aiming for an initial area under the curve of 80 mg.h/l and maintaining a peak gentamicin concentration of 10 mg/l for those patients with renal impairment by early extension of the dosing interval. Both these dosing approaches used in GFR+ were a significant departure from those in place beforehand.
Renal function estimation
This was calculated using an optimized version of the Cockcroft–Gault approach. This utilizes the lesser of ideal or actual body weight, and caps serum creatinine at a minimum 60 μmol/l.13
Prescribing parameters
Enoxaparin, gentamicin and vancomycin doses were nominally considered appropriate if they were within one-third above or below the appropriate dose. In the pre-intervention phase, this was the dose as determined by the local hospital guidelines, and in the post-intervention phase it was the dose as indicated by GFR+. Renal function was calculated using creatinine recorded immediately before the commencement of therapy. For allopurinol, metformin and ACEI, a dose of one-third or less above the maximum recommended dose with no lower limit was considered appropriate, as lower doses may still provide satisfactory therapeutic efficacy.
We examined whether designated drugs (allopurinol, ACEI, digoxin, furosemide, lithium, metformin, and spironolactone) were held during episodes of ARF, which was defined as a greater than 40% decrease in renal function across any of the five previous serum creatinine measurements dating back to a maximum of 3 years earlier.
Therapeutic drug monitoring (gentamicin, vancomycin)
Our hospital utilizes trough sampling and this is performed only for patients expected to require at least 3 days of therapy. For those patients who received 3 days or more of therapy, sampling was considered appropriate if the sample was drawn 4 h or less before any dose administered in the first 3 days of therapy.
Statistics
Chi-square was used for comparison of non-parametric data, with use of Fisher's exact and Yates' correction when appropriate. The Student's t test was used for parametric data. The level of significance was 5%.
Results
Pre-intervention data were collected over a 6-month period (March to August 2004) followed by a 5-month collection period post GFR+ implementation (July to November 2005). Academic detailing was then constantly available to all clinicians over the post-intervention period. Direct links to GFR+ were available on all clinically located computers. During the study period access to GFR+ was available to five medical teams and four surgical teams, generally consisting of one active consultant, one registrar, and one to two junior doctors each, as well as clinical pharmacists. At any one time there was a transient pool of 30–40 clinicians with access to GFR+. Forty-four doctors and 12 clinical pharmacists underwent academic detailing during the study period. All doctors responsible for the medical or surgical team patients included in the study underwent academic detailing within 2 weeks of commencing at the hospital. Post-intervention patients had lower renal function at admission, were more likely to be taking furosemide, and less likely to be commenced on gentamicin or vancomycin during their admission (see table 1).
Table 1.
Patient characteristics
| Pre-intervention | Post-intervention | |
| N | 509 | 492 |
| No of men | 292 | 300 |
| Age (years) | 77.9±11.2 | 78.6±12.4 |
| Weight (kg) | 73.9±17.8 | 72.7±19.9 |
| Length of stay (median, range) | 7 (1–154) | 7 (1–84) |
| Co-morbidities (median, range) | 3 (0–12) | 3 (0–9) |
| Renal function at admission* (ml/min) | 47.4+24.1 | 43.3+22.7 |
| ARF (n) | 24 | 16 |
| ACEI (n) | 258 | 252 |
| Allopurinol (n) | 50 | 54 |
| Digoxin (n) | 98 | 80 |
| Enoxaparin (n) | 44 | 58 |
| Furosemide (n)* | 204 | 233 |
| Gentamicin (n)* | 77 | 38 |
| Lithium (n) | 1 | 1 |
| Metformin (n) | 64 | 60 |
| Vancomycin (n)* | 37 | 17 |
Mean (±1 SD) unless otherwise stated.
p<0.05 for difference between pre and post-intervention period.
ACEI, angiotensin-converting enzyme inhibitor; ARF, acute renal failure.
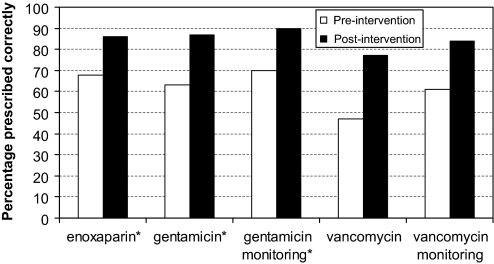
Enoxaparin
Dosing conformity improved from a baseline of 68% (n=44) to 86% (n=58) after GFR+ implementation (p=0.03, figure 1). This was despite the increased complexity of the dosing approach used in GFR+ compared with that used in the non-GFR+ baseline period. GFR+ was accessed in 61% of instances for dosage calculation for this group. In order to eliminate the possibility that the improvements seen across the pre and post-intervention phases were chance findings due to the different dosing approaches used in each phase, we separately examined the changes using only manufacturers' guidelines across both phases. There were no changes in dosing accuracy when the manufacturers' guidelines were used to assess both pre and post-intervention prescribing accuracy (68% vs 62%, p=0.5). However, when the new dosing guidelines developed for GFR+ were used to assess pre and post-intervention prescribing accuracy there was a marked improvement in the rate of dosing conformity (52% vs 86%, p<0.001). This indicates that the improved prescribing accuracy was driven by GFR+, and not the use of different dosing approaches in each phase.
Figure 1.
Prescribing of key drugs and therapeutic drug monitoring. *p<0.05 for difference.
Gentamicin
Dosing conformity improved from 63% (n=73) to 87% (n=38) (p=0.01, figure 1). Appropriate therapeutic drug monitoring also improved, from 70% (n=71) at baseline to 90% (n=38) in the post-intervention period (p=0.02, figure 1). GFR+ was accessed in 68% of instances for dosage calculation for this group. Similar to enoxaparin, in order to eliminate the possibility that the improvements seen across the pre and post-intervention phases were chance findings due to the different dosing approaches used in each phase, we separately examined the changes using only hospital guidelines across both phases. Assessing both phases according to hospital guidelines led to a decreased rate of dosing conformity (66% vs 42%, p=0.015). When both phases were compared for dosing conformity using the new guidelines developed for GFR+, however, there was a marked improvement in the rate of appropriate prescribing (66% vs 87%, p<0.01). Again this indicates that the improved dosing conformity was driven by GFR+, not the use of different dosing approaches in each phase.
Vancomycin
Dosing conformity showed a non-significant improvement from 47% (n=34) at baseline to 77% (n=17) in the post-intervention period (p=0.07, figure 1). At the same time appropriate therapeutic drug monitoring showed a non-significant improvement from 61% (n=33) at baseline to 84% (n=17) (p=0.17, figure 1). GFR+ was accessed in 53% of instances for dosage calculation of vancomycin.
Management of renally cleared drugs during periods of ARF
At baseline, ARF was experienced by 41 patients taking a total of 65 renally cleared drugs. These were appropriately held 38% of the time compared with 62% in the post-intervention group, in which 32 patients were receiving a total of 52 renally cleared drugs (p=0.01, see table 2).
Table 2.
Management of renally cleared drugs during periods of ARF
| Pre-intervention | Post-intervention | |||
| Drug held | Not held | Drug held | Not held | |
| ACEI | 12 | 12 | 9 | 3 |
| Digoxin | 2 | 14 | 4 | 3 |
| Furosemide | 8 | 11 | 12 | 9 |
| Metformin | 1 | 2 | 1 | 3 |
| Spironolactone | 2 | 1 | 6 | 2 |
| Total | 25 | 40 | 32 | 20 |
ACEI, angiotensin-converting enzyme inhibitor; ARF, acute renal failure.
Impact of academic detailing and sustainability of GFR+
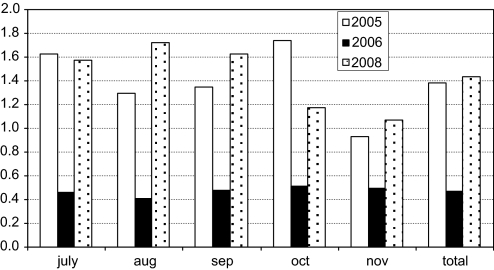
During the post-intervention study period (July to November 2005) there were on average 1.38 hits per patient via GFR+ (see figure 2). On study completion, GFR+ remained available to clinicians, but in the absence of academic detailing. In the July to November period of the following year (2006), in the absence of academic detailing, the average hits per patient decreased significantly to 0.47 (66% decrease, p<0.01). In this latter period, it is estimated that less than 25% of doctors who had received earlier academic detailing were still working within the hospital, as this hospital only represents a small number of hospital beds in the region in which the training medical staff rotate and rotations are 3-monthly. Academic detailing on GFR+ was subsequently instigated as a routine clinical service at the hospital during 2007. During the period July to November in 2008, the average number of hits per patient was 1.43. This was a significant 304% increase compared with the use of GFR+ during the non-academic detailing period in 2006 (p<0.01), and equivalent to the number of hits per patient seen when academic detailing was used under study conditions. This pattern remained intact when the utilization of GFR+ was calculated as the number of hits per month unadjusted for the patient load.
Figure 2.
Monthly number of hits per patient on GFR+. 2005—study period (GFR+ with academic detailing performed as part of the study); 2006—GFR+ available, no academic detailing performed, regular use by clinical pharmacists only; 2008—GFR+ available, academic detailing performed as routine clinical practice. p<0.01 for the number of monthly hits per patient in 2005 versus 2006, and 2005 versus 2008. p=0.8 for 2005 versus 2008. Note, data for 2007 were incomplete.
Discussion
The introduction of computerized clinical decision support with academic detailing significantly improved a number of aspects of the management of renally cleared drugs for hospitalized patients. GFR+ allowed the use of sophisticated dosing algorithms, notably for enoxaparin and gentamicin, which would not be possible outside the setting of computerized CDSS. This allowed the incorporation of dose adjustment for both renal function and weight, including dose adjustment for the degree of obesity.
The lack of appropriate dose adjustment of renally cleared drugs in this elderly population is driven largely by a general lack of recognition of declining renal function. The resultant overdosing exposes patients to higher drug concentrations and the increased likelihood of an adverse drug event.3 Two drugs of particular concern, as evidenced by local audit data, were enoxaparin and gentamicin. They are used for potentially life-threatening conditions, have a low therapeutic index, require adjustment for impaired renal function, and commonly cause serious adverse drug events in the elderly. The dosing accuracy of both these drugs improved significantly. Enoxaparin in particular requires specific dosing.14 15 Patients receiving lower than recommended doses are associated with higher re-infarction rates and increased mortality, whereas those receiving higher than recommended doses have a significantly increased incidence of bleeding and death. An unexpected benefit for gentamicin management was the improvement in therapeutic drug monitoring, driven by the presence of the monitoring recommendation given with the dose on the decision support module. Vancomycin prescribing and monitoring also improved markedly but the small patient numbers recruited failed to reach statistical significance. GFR+ assisted clinicians' decision-making around those drugs that need to be held when renal function decreases. This was reflected in an improvement from 38% of target drugs held during episodes of ARF increasing to 62% in the presence of GFR+. This study did not attempt to assess the direct impact on patient safety. Anecdotally, however, before the implementation of GFR+, the hospital death review audit received three to four cases each year implicating either enoxaparin or gentamicin as adversely affecting patient outcomes. In the 3 years since GFR+ has been in operation, there has not been a single further case involving these two drugs.
The introduction of CDSS into a clinical workplace is a complex interaction between people, technology and organizational workflow.16 The individual elements of these sociotechnical systems are highly interrelated, with changes in one element affecting the other. The introduction of CDSS into health care should be viewed as a problem in changing thinking around workflows as much as a change in technology. The burden of achieving these benefits generally falls on the front-end junior staff already struggling under a huge workload. Further to this, there are several well-recognized elements of CDSS implementation that are more likely to lead to success.17 These include the provision of support at the time and location of decision-making, recommendations rather than assessments, and computer-based support. The most important, however, is the automatic provision of CDSS as part of the clinician workflow. For issues relating to prescribing of medication, the automatic provision of CDSS as part of the clinician workflow invariably requires the co-existence of CPOE. Most hospitals do not have the degree of IT sophistication to allow this, as evidenced by a recent paper by Jha et al.18 In America, only 17% of hospitals have CPOE fully implemented around medication ordering, a further 11% have it fully implemented in at least one unit, and 72% of hospitals do not have CPOE. Our hospital had the resources to develop an independent CDSS, but like many others without CPOE, lacked the appropriate IT framework to incorporate this directly into the prescriber workflow. It is likely that many hospitals will be in a similar position, in which they can either develop or buy some form of CDSS, but are unable to incorporate it directly into the workflow and therefore risk underutilizing it.
While this study was not originally designed to measure the impact of academic detailing on the uptake of GFR+, we were able to determine its impact on GFR+ uptake by comparing it with the same period in subsequent years, after completion of the formal study. GFR+ was available without academic detailing in the year following the study period (2006), thus opportunistically serving as a control period. In 2006, without academic detailing, the use of GFR+ decreased 66%. This reflects the strong influence of academic detailing on the uptake of GFR+ during the study. In 2008, when academic detailing for GFR+ was routinely introduced into clinical practice by the pharmacy department, the uptake of GFR+ returned to identical levels seen when academic detailing was used during the study period. This indicates that the process created in the study was readily transferrable into clinical practice, with an identical impact. The rates of uptake of GFR+ with and without academic detailing in this study indicate it should be strongly considered as an adjunct to the implementation of any CDSS that is unable to be incorporated directly into the workflow.
While academic detailing improved the uptake of GFR+, and was accessed 64% of the time for decision-making around enoxaparin and gentamicin dosing, it was difficult to attribute the changes in other outcomes directly to one component or the other. For example, the improvement in holding renally cleared drugs during episodes of ARF was likely to be partly driven by the new clinical knowledge gained through academic detailing, and partly by the alerts on GFR+. The academic detailing certainly created awareness among prescribers of GFR+ and the clinical reasoning behind its implementation, and better facilitated the interaction between people, technology and organizational workflow required for successful implementation. The academic detailing also allowed us to focus primarily on junior medical staff, as this is the group primarily responsible for calculating drug doses, in most need of clinical assistance in this area, and most likely to use GFR+. Formal feedback from users indicated a broad and enthusiastic acceptance of both the GFR+ module and the academic detailing process.
Conclusion
Clinical decision support aimed at improving the prescribing of key renally cleared drugs in the hospital setting, but existing outside the direct clinician workflow, was successfully implemented, with a positive impact on drug management. The use of academic detailing dramatically increased the uptake of GFR+, and should be strongly considered as a valuable adjunct for the introduction of computerized CDSS outside any primary workflows.
Footnotes
Competing interests: None.
Ethics approval: The study was approved by the local hospital ethics committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Rowe JW, Andres R, Tobin JD, et al. The effect of age on creatinine clearance in men: a cross-sectional and longitudinal study. J Gerontol 1976;31:155–63 [DOI] [PubMed] [Google Scholar]
- 2.Akbari A, Swedko PJ, Clark HD, et al. Detection of kidney disease with laboratory reporting of estimated glomerular filtration rate and an educational program. Arch Intern Med 2004;164:1788–92 [DOI] [PubMed] [Google Scholar]
- 3.Corsenello A, Pedone C, Corica F, et al. Concealed renal insufficiency and adverse drug reactions in elderly hospitalised patients. Arch Intern Med 2005;165:790–5 [DOI] [PubMed] [Google Scholar]
- 4.Kirtane AJ, Piazza G, Murphy SA, et al. Correlates of bleeding events among moderate to high-risk patients undergoing percutaneous coronary intervention and treated with ebtifibatide. J Am Coll Cardiol 2006;47:2374–9 [DOI] [PubMed] [Google Scholar]
- 5.Lesar TS, Briceland L, Stein DS. Factors related to errors in medication prescribing. JAMA 1997;277:312–17 [PubMed] [Google Scholar]
- 6.Long CL, Raebel MA, Price DW, et al. Compliance with dosing guidelines in patients with chronic kidney disease. Ann Pharmacother 2004;38:853–8 [DOI] [PubMed] [Google Scholar]
- 7.Vidal L, Shavit M, Fraser A, et al. Systematic comparison of four sources of drug information regarding adjustment of dose for renal function. BMJ 2005;331:263–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chertow GM, Lee J, Kuperman GJ, et al. Guided medication dosing for inpatients with renal insufficiency. JAMA 2001;286:2839–44 [DOI] [PubMed] [Google Scholar]
- 9.O'Brien MA, Rogers S, Jamtvedt S, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2007:(4);CD000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avorn J, Soumerai SB. Improving drug-therapy decisions through educational outreach. A randomised controlled trial of academically based “detailing”. N Engl J Med 1983;308:1457–63 [DOI] [PubMed] [Google Scholar]
- 11.Green B, Duffull SB. Development of a dosing strategy for enoxaparin in obese patients. Br J Clin Pharmacol 2003;56:96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barras MA, Duffull SB, Atherton JJ, et al. Individualized compared with conventional dosing of enoxaparin. Clin Pharmacol Ther 2008;83:882–8 [DOI] [PubMed] [Google Scholar]
- 13.Kirkpatrick C, Duffull SB, Begg EJ. Pharmacokinetics of gentamicin in 957 patients with varying renal function. Br J Clin Pharmacol 1999;47:637–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen NM, Chen AY, Alexander KP, et al. Enoxaparin dosing and associated risk of in-hospital bleeding and death in patients with non-ST segment elevation acute coronary syndromes. Arch Intern Med 2007;167:1539–44 [DOI] [PubMed] [Google Scholar]
- 15.Montalescot G, Collet JP, Tanguy ML, et al. Anti-Xa activity relates to survival and efficacy in unselected acute coronary syndrome patients treated with enoxaparin. Circulation 2004;110:392–8 [DOI] [PubMed] [Google Scholar]
- 16.Waers RL, Berg M. Computer technology and clinical work. Still waiting for Godot. JAMA 2005;293:1261–3 [DOI] [PubMed] [Google Scholar]
- 17.Kawamoto K, Houlihan CA, Balas EA, et al. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ 2005;330:765–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jha AK, DesRoches CM, Campbell EG, et al. Use of electronic health records in U.S. hospitals. N Engl J Med 2009;360:1628–38 [DOI] [PubMed] [Google Scholar]