Abstract
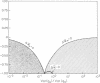
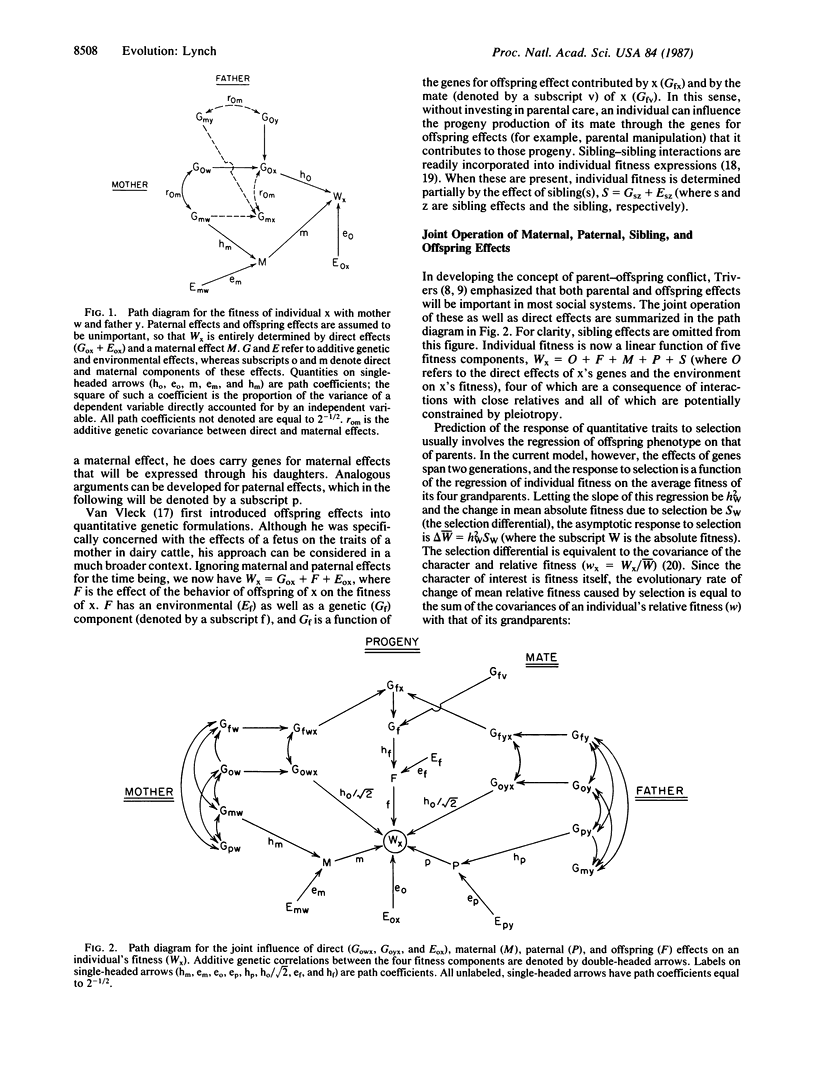
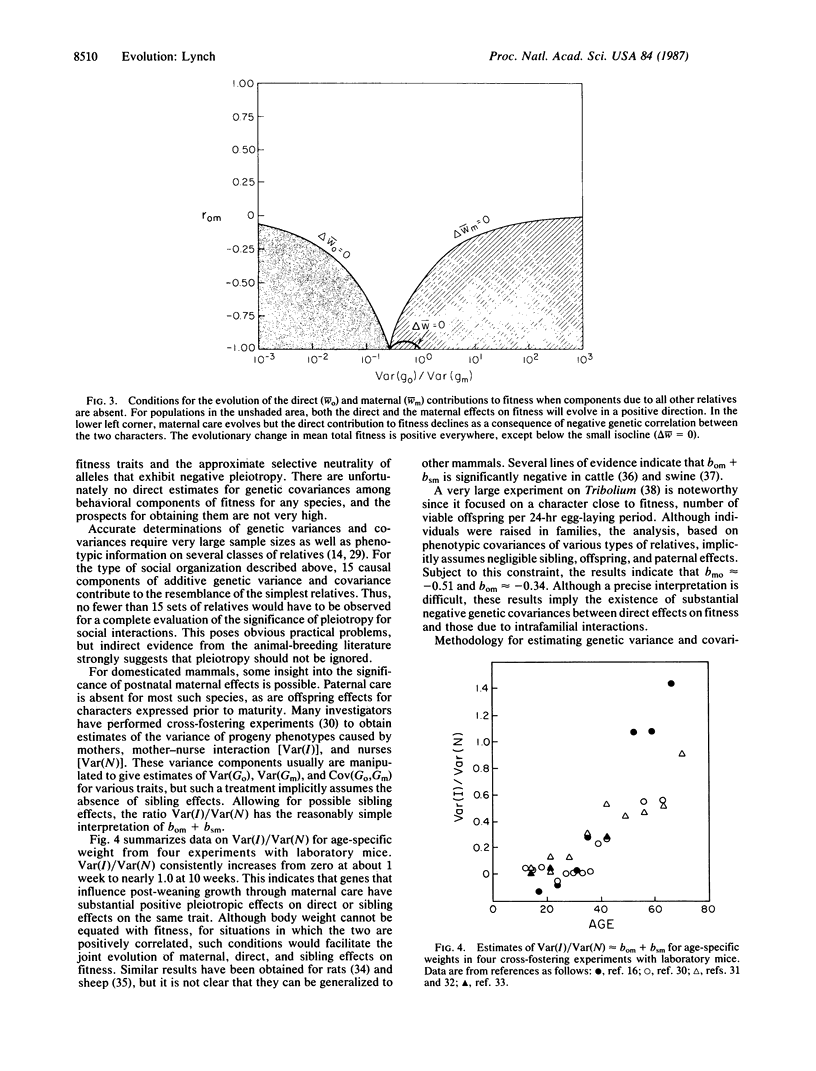
A theory for the evolution of behavioral interactions among relatives is developed that allows for genetic correlations between the types of behavior that are expressed in different social contexts. Both theoretical and empirical considerations indicate that such genetic constraints will almost certainly be common in natural populations. It is shown that when genetic correlations between elements of social behavior exist, Hamilton's rule inaccurately describes the conditions for evolution by way of kin selection. The direction in which social organization evolves is a delicate function of the genetic covariance structure among behaviors expressed as an offspring, sibling, parent, etc. A change in this covariance structure caused by random genetic drift or by a change in environment for a population exhibiting genotype-environment interaction can cause the population to suddenly cross a threshold into a new selective domain. Consequently, radical changes in social organization may arise between closely related species without any major shift in selective pressures external to the population.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blunn C. T. Preweaning growth of cross-transferred rats. J Anim Sci. 1969;28(3):300–304. doi: 10.2527/jas1969.283300x. [DOI] [PubMed] [Google Scholar]
- Cockerham C C. An Extension of the Concept of Partitioning Hereditary Variance for Analysis of Covariances among Relatives When Epistasis Is Present. Genetics. 1954 Nov;39(6):859–882. doi: 10.1093/genetics/39.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow J. F., Aoki K. Group selection for a polygenic behavioral trait: a differential proliferation model. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2628–2631. doi: 10.1073/pnas.79.8.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow J. F., Aoki K. Group selection for a polygenic behavioral trait: estimating the degree of population subdivision. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6073–6077. doi: 10.1073/pnas.81.19.6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen E. J. Mating designs for estimating direct and maternal genetic variances and direct-maternal genetic covariances. Can J Genet Cytol. 1967 Mar;9(1):13–22. doi: 10.1139/g67-002. [DOI] [PubMed] [Google Scholar]
- Engels W. R. Evolution of altruistic behavior by kin selection: an alternative approach. Proc Natl Acad Sci U S A. 1983 Jan;80(2):515–518. doi: 10.1073/pnas.80.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W. D. The genetical evolution of social behaviour. I. J Theor Biol. 1964 Jul;7(1):1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- Koch R. M. The role of maternal effects in animal breeding. VI. Maternal effects in beef cattle. J Anim Sci. 1972 Dec;35(6):1316–1323. doi: 10.2527/jas1972.3561316x. [DOI] [PubMed] [Google Scholar]
- Riska B., Atchley W. R., Rutledge J. J. A genetic analysis of targeted growth in mice. Genetics. 1984 May;107(1):79–101. doi: 10.1093/genetics/107.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riska B., Rutledge J. J., Atchley W. R. Covariance between direct and maternal genetic effects in mice, with a model of persistent environmental influences. Genet Res. 1985 Jun;45(3):287–297. doi: 10.1017/s0016672300022278. [DOI] [PubMed] [Google Scholar]
- Robertson P. L. The role of the political usurper: MaCBeth and Boris Godounov. Am Imago. 1966 Summer;23(2):95–108. [PubMed] [Google Scholar]
- Rutledge J. J. Fraternity size and swine reproduction. I. Effect on fecundity of gilts. J Anim Sci. 1980 Oct;51(4):868–870. doi: 10.2527/jas1980.514868x. [DOI] [PubMed] [Google Scholar]
- Rutledge J. J. Fraternity size and swine reproduction. II. Genetical consequences. J Anim Sci. 1980 Oct;51(4):871–874. doi: 10.2527/jas1980.514871x. [DOI] [PubMed] [Google Scholar]
- Rutledge J. J., Robison O. W., Eisen E. J., Legates J. E. Dynamics of genetic and maternal effects in mice. J Anim Sci. 1972 Nov;35(5):911–918. doi: 10.2527/jas1972.355911x. [DOI] [PubMed] [Google Scholar]
- Van Vleck L. D. A genetic model involving fetal effects on traits of the dam. Biometrics. 1978 Mar;34(1):123–127. [PubMed] [Google Scholar]
- Willham R. L. The role of maternal effects in animal breeding. 3. Biometrical aspects of maternal effects in animals. J Anim Sci. 1972 Dec;35(6):1288–1293. doi: 10.2527/jas1972.3561288x. [DOI] [PubMed] [Google Scholar]
- Yokoyama S., Felsenstein J. A model of kin selection for an altruistic trait considered as a quantitative character. Proc Natl Acad Sci U S A. 1978 Jan;75(1):420–422. doi: 10.1073/pnas.75.1.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Oksh H. A., Sutherland T. M., Williams J. S. Prenatal and postnatal maternal influence on growth in mice. Genetics. 1967 Sep;57(1):79–94. doi: 10.1093/genetics/57.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]