Abstract
A polymorphic 68-bp tandem repeat has been identified within the promoter of the human prodynorphin (PDYN) gene. We found that this 68-bp repeat in the PDYN promoter occurs naturally up to five times. We studied the effect of the number of 68-bp repeats, and of a SNP (rs61761346) found within the repeat on PDYN gene promoter activity. Thirteen promoter forms, different naturally occurring combinations of repeats and the internal SNP, were cloned upstream of the luciferase reporter gene, transfected into human SK-N-SH, H69, or HEK293 cells. Cells were then stimulated with TPA or caffeine. We found cell-specific effects of the number of 68-bp repeats on the transcriptional activity of the PDYN promoter. In SK-N-SH and H69 cells, three or four repeats led to lower expression of luciferase than did one or two repeats. The opposite effect was found in HEK293 cells. The SNP also had an effect on PDYN gene expression in both SK-N-SH and H69 cells; promoter forms with the A allele had significantly higher expression than promoter forms with the G allele. These results further our understanding of the complex transcriptional regulation of the PDYN gene promoter.
Keywords: prodynorphin, single nucleotide polymorphism, promoter, 68-bp repeats
INTRODUCTION
Dynorphin peptides bind with high affinity to the kappa opioid receptor and are endogenous ligands for this receptor (Chavkin et al. 1982). By their action on the kappa opioid receptors, dynorphin peptides can reduce the dopaminergic tone in the brain under basal conditions or after intake of some drugs of abuse (Di Chiara, G. et al. 1988, Kreek et al. 2004). Dynorphin peptides are also involved in the regulation of serotonergic and noradrenergic systems. It has been shown that interaction of the dynorphin/kappa receptor system with CRF plays an important role in stress-related behavior (reviewed by Knoll and Carleson, 2010). Through these different properties, dynorphin peptides are involved in a variety of central nervous system functions and disorders such as analgesia, epilepsy, depression and addiction to drugs of abuse (Simonato and Romualdi 1996; Kreek et al. 2004; Solbrig and Koob 2004).
The human prodynorphin (PDYN) gene, located on chromosome 20, consists of four exons. Exons 1 and 2 encode the 5’-untranslated region of the mRNA while most of exon 3 and exon 4 encode the translated region (Horikawa et al. 1983). Besides the canonical four exon-three intron PDYN gene that codes for a full length PDYN mRNA of 2.8 kb, several other transcriptional start sites and splice variants were identified in embryonic human brain and testis, including mRNAs with one, two, or three exons (Telkov et al. 1998; Nikoshkov et al. 2005). In these studies, multiple transcription initiation sites were found upstream of exon 1, exon 2, and within exon 4.
A number of transcription factor binding sites within the PDYN promoter have been shown to play a role in regulating PDYN expression. A polymorphic 68-bp tandem repeat (rs35286281) has been identified within the promoter of the PDYN gene, occurring from one to four times and is located 1250 bp upstream of exon 1 (Horikawa et al. 1983; Zimprich et al. 2000). In one study, it has been reported that the transcription factor activator protein (AP-1) can bind to the 68-bp tandem repeat (Zimprich et al. 2000). In vitro gene reporter assays have shown that reporter constructs containing three or four copies of this 68-bp tandem repeat (compared to one or two) upstream of a herpes simplex virus thymidine kinase (tk) promoter, lead to higher expression of the reporter gene in mouse neuroblastoma cells (NG108-15) when stimulated by the phorbol ester 12-O-tetradecanoylphorbol 13-acetate (TPA) (Zimprich et al. 2000).
A single nucleotide polymorphism (rs61761346) with a G to A transition at the ninth nucleotide of the 68-bp tandem repeat of the PDYN gene promoter has been identified by Rockman et al (2005). It is not known whether the SNP rs61761346 has an impact on the transcriptional activity of the PDYN promoter. Recently, the same group showed complex interaction of five polymorphisms in 5' flanking region, including the polymorphic 68-bp tandem repeat, in regulation of basal expression of PDYN in vitro experiments and two human brain regions, the occipital cortex and temporal cortex (Babbitt et al, 2010).
Expression of the human PDYN gene is downregulated by the calcium sensitive transcription factor downstream regulatory element antagonist modulator (DREAM) that binds to the downstream regulatory element (DRE) located in the 5’-untranslated region within exon 1 (Carrion et al. 1998; Carrion et al. 1999; Jacobson et al. 2006). Expression of the PDYN gene is, in part, regulated by intracellular calcium concentration since calcium induces the release of DREAM from the DRE site (Campos et al. 2003). Caffeine is able to increase the expression of the PDYN gene by inducing the release of calcium from intracellular stores (Campos et al. 2003).
Subtle changes in the transcriptional activity of the PDYN gene may result in substantial effects on phenotypes such as epilepsy, depression, or addiction to drugs of abuse. The aim of this study is to elucidate the functional significance of the 68-bp tandem repeat polymorphism, and the impact of the internal SNP rs61761346 on basal and stimuli-induced transcriptional activity of the PDYN gene promoter in human cell lines using a reporter gene construct. Thirteen forms of the PDYN promoter were cloned upstream of the luciferase reporter gene and were transfected into SK-N-SH, H69, and HEK293 cells. The effect of TPA or caffeine on the expression of the reporter gene was determined in each cell line. We show here that both the number of 68-bp tandem repeats and the internal SNP affect the transcriptional activity of the PDYN promoter and that these effects vary depending on cell type.
MATERIAL AND METHODS
Study subjects and assessments
Consecutively recruited non-related individuals in studies on the genetics of addiction conducted at the Laboratory of the Biology of Addictive Diseases at The Rockefeller University recruited between July 26, 2000 and May 26, 2005 were used to identify subjects meeting our inclusion criteria with carefully defined ethnic cultural background. Caucasian and African American individuals, who met the inclusion criteria, were healthy volunteers with no history of drug use, individuals with cocaine addiction or individuals with cocaine/alcohol co-addiction. In all, 307 subjects were identified meeting these criteria. All subjects gave written informed consent for studies of genetics approved by The Rockefeller University Hospital Institutional Review Board. All subjects were assessed by a physician, a research nurse, or a clinical psychologist with regard to medical and psychiatric history.
Genotyping of the 68-bp tandem region in study subjects
Lymphocytes were prepared from whole blood using a buffy-coat lymphocyte isolation. Genomic DNA was extracted from lymphocytes using a salt-precipitation DNA extraction. One hundred ng of genomic DNA was amplified by PCR using forward (5’-CTG TGT ATG GAG AGG CTG AGT -3’) and reverse (5’- AGG CGG TTA GGT AGA GTT GTC -3’) primers specific for the promoter region of the PDYN gene and located on either side of the 68-bp tandem region. Nucleotides 1923846 – 1924254 on chromosome 20 (NCBI Build 36) were amplified. A step-down PCR was performed for 35 cycles of 30 s at 94°C, 30 s at 66°C, 30 s at 63°C, 30 s at 60°C, 30 s at 57°C, 30 s at 52°C and 30 s at 72°C followed by 6 min at 72°C using an AmpliTaq Gold kit (Applied Biosystems, Foster City, CA). PCR products were electrophoresed on a 2.5% agarose gel, bands were purified, and the amplified DNA was sequenced using the ABI BigDye Terminator (v3)® and an ABI 373 DNA Sequencer (Applied Biosystems).
Cloning PDYN promoter forms in the pGL4.10 luciferase expression vector
A separate ongoing study in our laboratory identified 17 variants of the PDYN promoter (i.e., combination of the number of 68-bp tandem repeats and of the internal SNP rs61761346). Constructs used in this study were generated using human genomic DNA genotyped for the 68-bp tandem repeat region.
We cloned 13 of the 17 forms identified in our laboratory. First, the ~3 kb fragment of the PDYN promoter was amplified from Human Genomic DNA (Clontech Cat#6550-1 Lot N36348-1, Mountain View, CA) using a forward 5’-AGCAATCAGAGGTTGAAGTTGGCAGC-3’ and a reverse 5’-CAGGCAGCATAACTCACCGGCTTG-3’ primers. PCR was performed for 40 cycles of 15 s at 94°C, 15 s at 60°C and 4 min at 72°C followed by 7 min at 72°C using Pfu DNA polymerase (Stratagene, La Jolla, CA) according to manufacturer’s instructions and in a buffer containing 6% DMSO. Taq DNA polymerase (Invitrogen, Carlsbad, CA) was added for 2 min at 72°C and this segment was subcloned into pGEM-T Easy vector (Promega, Madison, WI). A Sal I/Nco I- 3131 bp fragment, from a single clone, containing the PDYN promoter was subcloned into pGL4.10 vector (Promega) digested with Xho I and Nco I. Twenty clones were sequenced in their entirety and contained two copies of the 68-bp tandem repeat with a G at rs61761346 in each repeat. A single clone, which we referred to as pGL4.10-PDYN/2GG, was prepared with Qiafilter Plasmid Maxi Kit (Qiagen, Valencia, CA). We then used this master clone to clone the other twelve variants. Fragments of at least 854 bp from the PDYN promoter containing the 68-bp tandem repeats were amplified from genomic DNA of the study subjects with known genotypes for this polymorphic region using a forward primer containing a Nhe I site (5’-GAGAAAGCTAGCAATCAGAGAGTTGAA-3’) and a reverse primer containing a Mlu I site (5’-GTTGGCTGGTGCACGCGT-3’) (Figure 1B). PCR was performed for 40 cycles of 15 s at 94°C, 15 s at 60°C and 60 s at 72°C followed by 7 min at 72°C using Pfu polymerase. PCR products were digested with Nhe I and Mlu I and cloned into pGL4.10-PDYN/2GG that had been digested with Nhe I and Mlu I (Figure 1B).
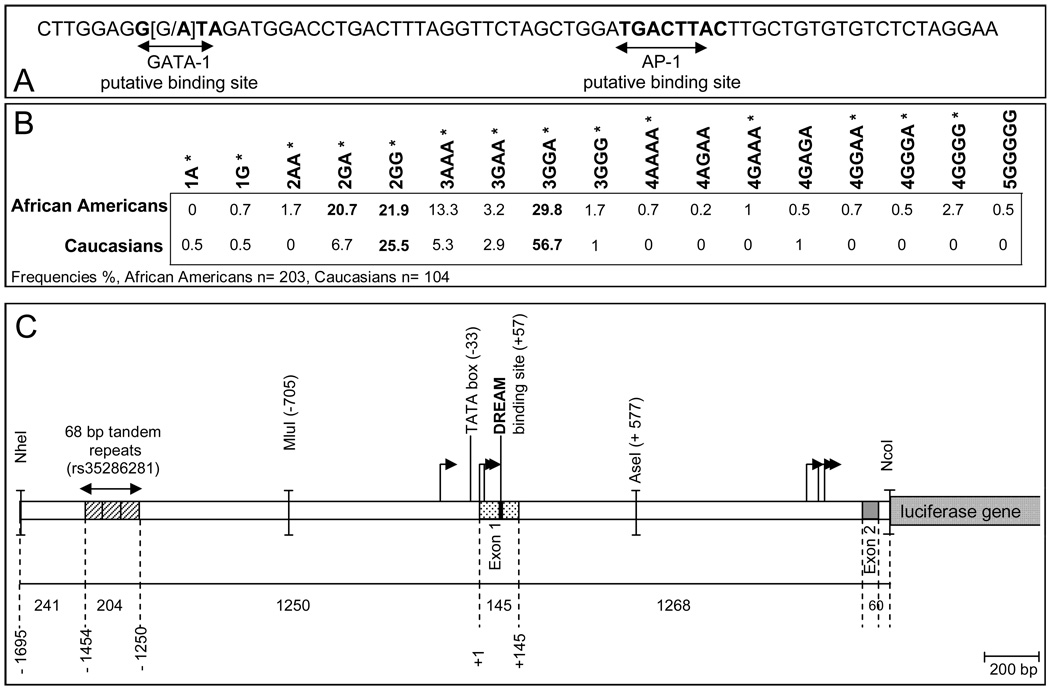
Figure 1.
The 68-bp tandem repeat polymorphism (rs35286281) with the internal SNP (rs61761346) of the PDYN gene promoter, and schematic representation of the PDYN promoter construct used for the luciferase gene reporter assays. A. Sequence of the 68-bp tandem repeat showing the putative AP-1 binding site (bold) and the putative GATA-1 binding site when there is an A allele at the ninth position of the tandem repeat (bold). B. 3.1 kb fragment of the PDYN gene was cloned upstream of the firefly luciferase reporter gene. This fragment includes: 241 bp upstream of the 68-bp tandem repeat region, the 68-bp tandem repeat region (fuschia), 1250 bp upstream of exon 1 including the TATA box located 33 bp upstream of exon 1 and most of the transcription start sites, exon 1 (145 bp, green) containing a binding site for the transcription factor DREAM (red), intron 1 (1268 bp), exon 2 (60 bp) and 17 bp of intron 2. The restriction sites used to generate deletion constructs (Mlu I, Ase I, Nco I) are indicated. The major transcription start sites are indicated by □.
Thirteen different PDYN promoter forms were cloned in the pGL4.10 firefly luciferase expression vector (1A, 1G, 2AA, 2GA, 2GG, 3AAA, 3GAA, 3GGA, 3GGG, 4AAAA, 4GGAA, 4GGGA, 4GGGG). Complete sequencing of the thirteen clones showed that they differed only in the region containing the repeats, and their sequences are identical for the polymorphisms rs11997794 (T) and rs34535593 (9TC).
Nomenclature of pGL4.10-PDYN constructs
We use the following nomenclature to describe the forms of the PDYN promoter: the number of 68-bp tandem repeats followed by the base (either G or A) present in each repeat (e.g., 3GGA refers to three copies of the repeat, the first and the second copies having the G allele at rs61761346 and the third having the A allele).
Deletion constructs of pGL4.10-PDYN/1G construct
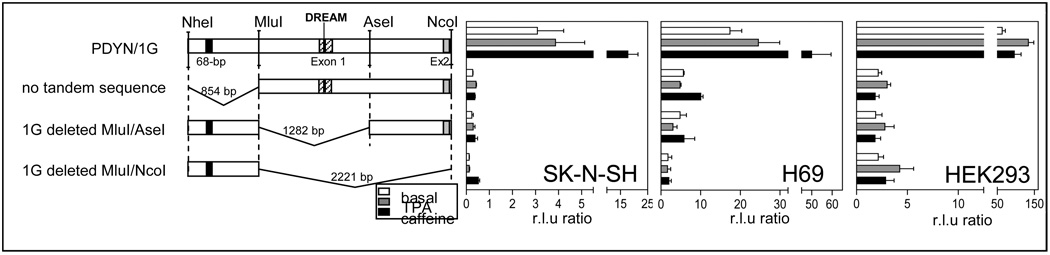
We constructed deletion mutants of the pGL4.10-PDYN/1G construct using the unique restriction sites: Nhe I, Mlu I, Ase I and Nco I (Figure 1C and Figure 2). Deletion mutants were generated using two of these enzymes, Nhe I/Mlu I, Mlu I/Ase I or Mlu I/Nco I. Products were gel purified, extracted, incubated for 30 minutes at 37°C with T4 DNA polymerase to generate blunt ends and ligated using T4 DNA ligase to generate the deletion mutants. These deletion constructs were then sequenced.
Figure 2.
Deletion analysis of the PDYN gene promoter. Luciferase gene reporter assay of full-lenght and deletion constructs of the 1G variant of PDYN gene promoter in SK-N-SH, H69 and HEK293 cells under basal conditions (white bars), stimulated with 100 nM TPA (grey bars) or 10 mM caffeine (black bars). Data are shown as mean + SEM for three different DNA preparations, each performed in triplicate for each construct. Note the different scales in normalized fluorescence levels (r.lu) for three different cells.
Cell culture
Three human cell lines, obtained from American Type Culture Collection (ATCC, Manassas, VA), were cultured at 37° in a 5% CO2 atmosphere. Neuroblastoma SK-N-SH and small cell lung cancer H69 cells were cultured in RPMI (Invitrogen) supplemented with 10% fetal bovine serum (FBS) (Atlas Biologicals, Fort Collins, CO), 100 µg/mL streptomycin and 100 units/mL penicillin. Human embryonic kidney cells, HEK293, were cultured in DMEM (Invitrogen) supplemented with 10% FBS, 100 µg/mL streptomycin and 100 units/mL penicillin.
Luciferase assay
Cells were cotransfected with the pGL4.10 PDYN promoter constructs and with the promotorless vector pGL4.70, which expresses renilla luciferase, using Lipofectamine 2000 (Invitrogen). Plasmid DNA was prepared with Qiafilter Plasmid Maxi Kit. One day before transfection, 2×106/well SK-N-SH cells, 2.5×105 /well HEK293 or H69 cells were plated in twelve-well plates (BD Biosciences, San Jose, CA) in serum-free medium. SK-N-SH cells were cotransfected with 2 µg pGL4.10-PDYN reporter gene construct and 40 ng pGL4.70. For the deletion mutant experiments, HEK293 and H69 cells were cotransfected with 1 µg pGL4.10-PDYN reporter gene construct and 20 ng pGL4.70. For the repeat and allele studies, HEK293 and H69 cells were cotransfected with 250 ng pGL4.10-PDYN reporter gene construct, and 5 ng or 20 ng pGL4.70, respectively. Twenty-four hours later, medium was replaced with serum-free medium with or without 100 nM TPA (#P8139 Sigma-Aldrich, St. Louis, MO) or 10 mM caffeine (#C8960 Sigma-Aldrich) and cells were incubated for a further 24 hours.
Cells were lysed for 5 minutes incubation in 300 µL Passive Lysis Buffer (Promega). Firefly and renilla luciferase activities were quantified on a Monolight 2010 Luminometer (BD Biosciences) using the Dual-Luciferase Reporter Assay System (Promega). For each of the three independent DNA preparations of each form of the PDYN promoter construct, independent experiments were conducted once or twice as indicated in the legends of Figures 3, 4 and 5. Three triplicate transfections were performed separately for each DNA preparation. Luminescence was measured twice and expressed as a ratio of firefly to renilla luciferase activity relative light units (r.l.u).
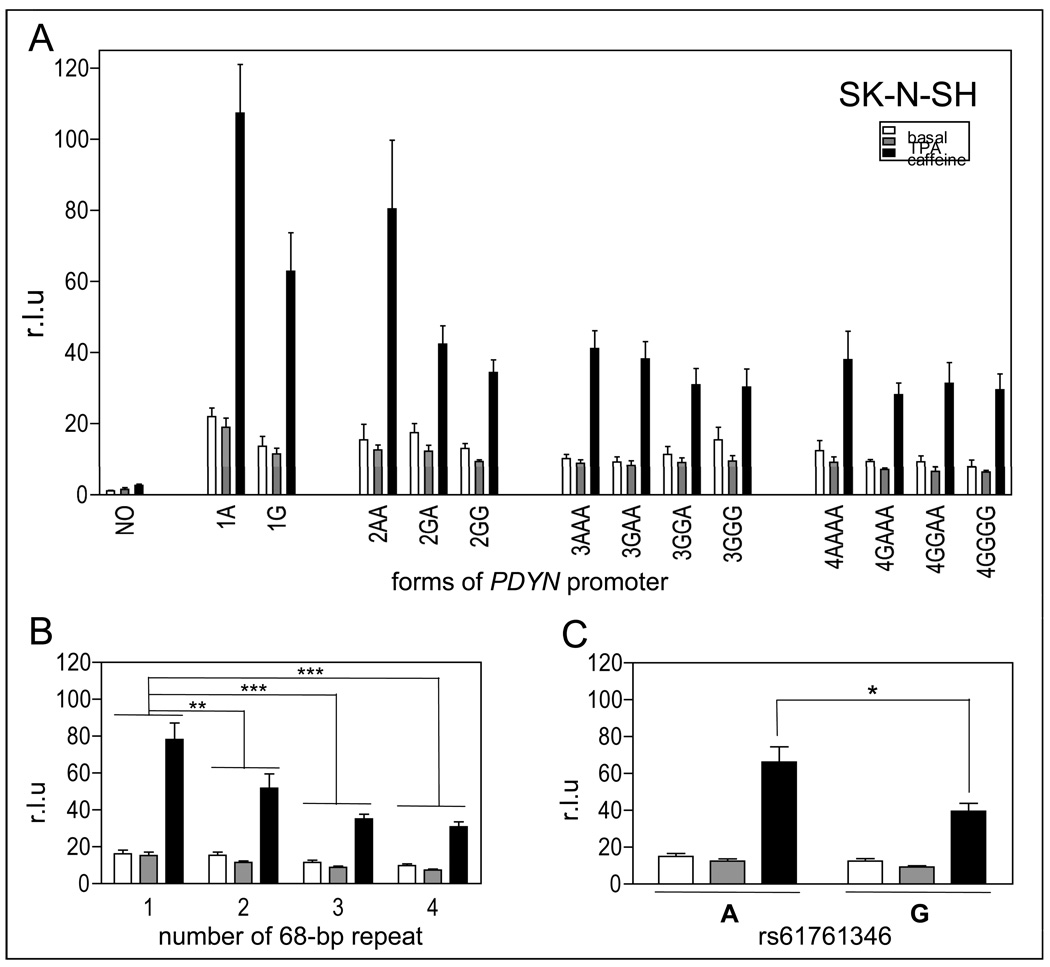
Figure 3.
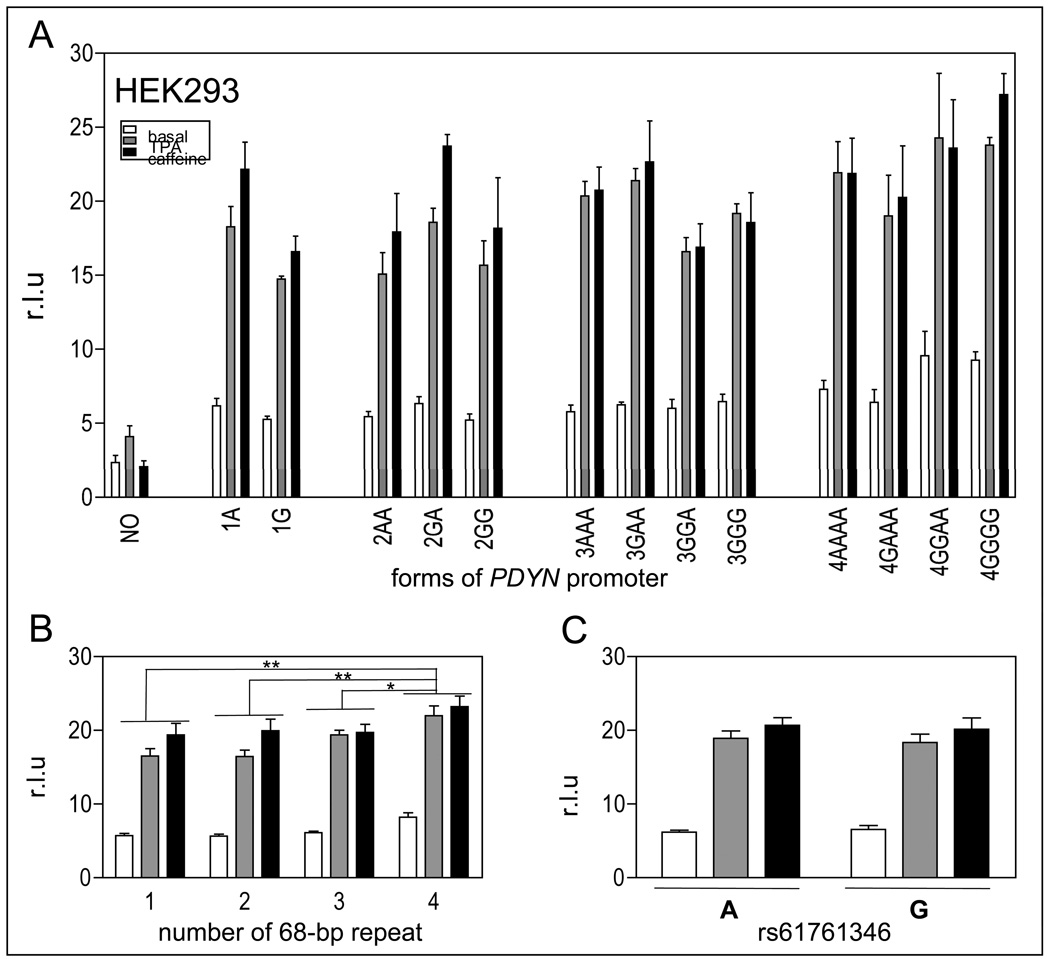
Effect of the number of 68-bp tandem repeats (rs35286281) and of the internal SNP (rs61761346) on PDYN gene promoter activity in SK-N-SH cells. A. Luciferase gene reporter assay of 13 different naturally-occurring variants of PDYN gene promoter and a control promoter construct, with deletion of a ~1 kb fragment containing the repeats (NO), in SK-N-SH cells under basal conditions (white bars), stimulated with 100 nM TPA (grey bars) or 10 mM caffeine (black bars). Data are shown as mean + SEM of two experiments with three different DNA preparations, each performed in triplicate for each construct. B. Data have been grouped by number of tandem repeats, 1 represents 1A and 1G, 2 represents 2AA, 2GA and 2GG, 3 represents 3AAA, 3GAA, 3GGA and 3GGG, and 4 represents 4AAAA, 4GAAA, 4GGAA and 4GGGG. In SK-N-SH cells, constructs with 1 tandem repeat showed higher expression of luciferase reporter gene than constructs with 2, 3 or 4 tandem repeats. Constructs with 1 tandem repeat showed higher expression of luciferase reporter gene than constructs with 2, 3 or 4 tandem repeats. C. In SK-N-SH cells under caffeine stimulation, cells containing the A allele showed higher luciferase expression than those with the G allele (* p < 0.05, ** p < 0.005, *** p < 0.0005).
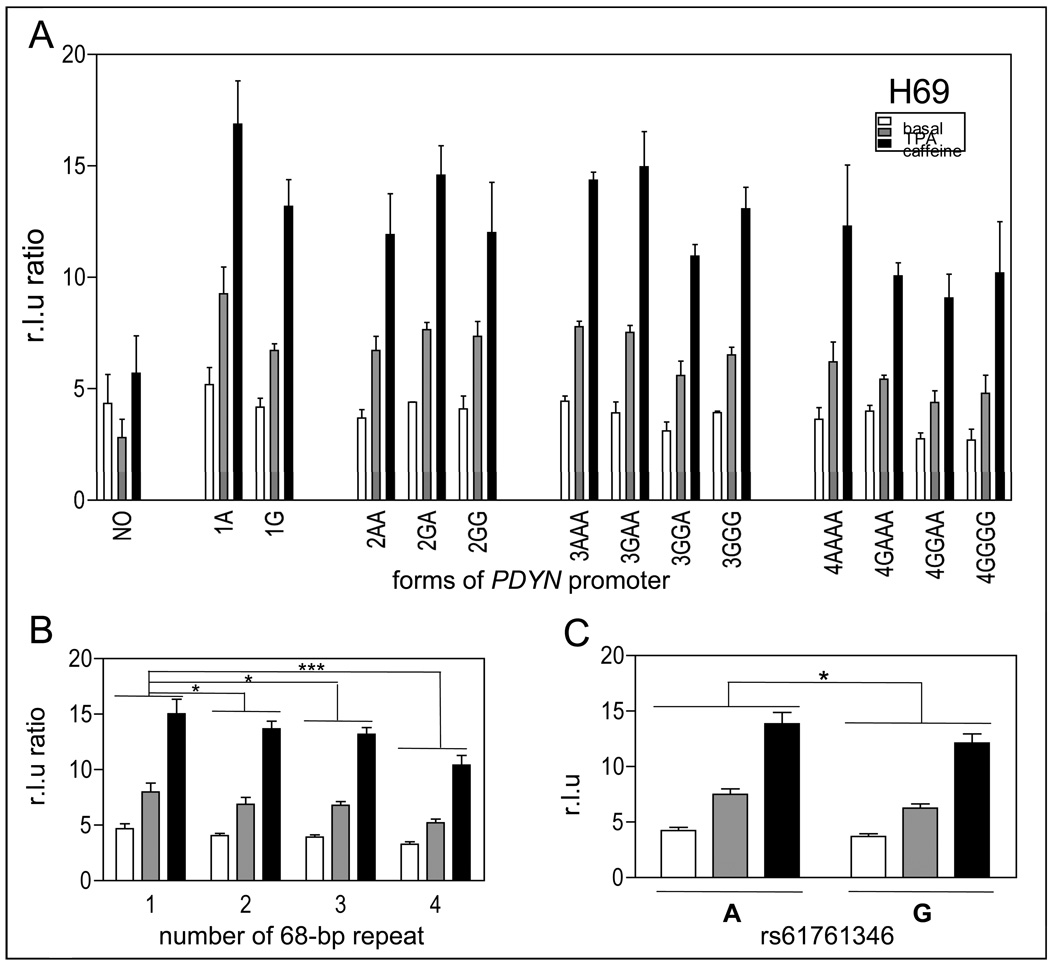
Figure 4.
Effect of the number of 68-bp tandem repeats (rs35286281) and of the internal SNP (rs61761346) on PDYN gene promoter activity in H69 cells. A. Luciferase gene reporter assay of 13 different naturally-occurring variants of the PDYN gene promoter and a control promoter construct, with deletion of a ~1 kb fragment containing the repeats (NO), in H69 cells under basal conditions (white bars), stimulated with 100 nM TPA (grey bars) or 10 mM caffeine (black bars). Data are shown as mean + SEM of one experiment with three different DNA preparations, each performed in triplicate for each construct. B. Data have been grouped by number of tandem repeats, as in Figure 4. In H69 cells, constructs with 1 tandem repeat showed higher expression of luciferase reporter gene than constructs with 2, 3 or 4 tandem repeats. Constructs with 1 tandem repeat showed higher expression of luciferase reporter gene than constructs with 2, 3 or 4 tandem repeats. C. H69 cells containing only the A allele at rs61761346 showed higher expression of the luciferase reporter gene than those with the G allele (* p < 0.05, ** p < 0.005, *** p < 0.0005).
Figure 5.
Effect of the number of 68-bp tandem repeats (rs35286281) and of the internal SNP (rs61761346) on PDYN gene promoter activity in HEK293 cells. A. Luciferase gene reporter assay of 13 different naturally-occurring variants of PDYN gene promoter and a control promoter construct, with deletion of a ~1 kb fragment containing the repeats (NO), in H69 cells under basal conditions (white bars), stimulated with 100 nM TPA (grey bars) or 10 mM caffeine (black bars). Data are shown as mean + SEM of one experiment with three different DNA preparations, each performed in triplicate for each construct. B. Data have been grouped by number of tandem repeats, as in Figure 4. Cells transfected with constructs containing 1, 2 or 3 copies of the 68-bp tandem repeat showed lower expression of the luciferase reporter gene than constructs containing 4 copies. C. In HEK293 cells, there was no difference of luciferase expression between cells containing only the A allele at rs61761346 and those with the G allele.
5’ RLM-RACE PCR analysis of PDYN construct
One day before transfection, 2×106 SK-N-SH cells per well, 5×105 HEK293 or H69 cells per well were plated in six-well plates. SK-N-SH cells were transfected with 4 µg of pGL4.10-PDYN/3GGA. HEK293 and H69 cells were transfected with 500 ng of pGL4.10-PDYN/3GGA. Twenty-four hours after transfection, medium was replaced with serum-free medium with or without 100 nM TPA or 10 mM caffeine and cells were incubated for a further 24 hours. Cells were lysed and RNA was purified using RNeasy kit (Qiagen). Five µg of total RNA were used for 5’ RLM RACE PCR using GeneRacer™ Kit (Invitrogen) according to manufacturer’s protocol. RACE-PCR was performed using forward GeneRacer™ 5’-Nested primer and a reverse primer (5’-TGGCGCTGGGCCCTTCTTAA-3’) matching the sequence of the luciferase reporter gene for 40 cycles of 30 s at 94°C, 3 min at 60°C and 30 sec at 72°C followed by 7 min at 72°C. Products were then amplified in a second round of PCR for 40 cycles of 30 s at 94°C, 30 s at 62°C and 2 min at 72°C followed by 7 min at 72°C using a forward nested primer matching the GeneRacer™ Nested primer (5’-TGACATGGACTGAAGGAGTA-3’) and a reverse nested primer matching the sequence of the luciferase reporter gene (5’-CTTAATGTTTTTGGCATCTT-3’). Products from the two successive rounds of PCR were gel purified and cloned into pCR4-TOPO vector (Invitrogen). Clones were sequenced and analyzed using VectorNTI10 software (Invitrogen).
RESULTS
Previous studies have shown that the PDYN promoter contains one to four 68-bp tandem repeats, 1250 bp upstream of exon 1; we identified one individual with five 68-bp tandem repeats (Figure 1B). Furthermore, sequencing of the 68-bp tandem repeat region led to the identification of a SNP G/A (rs61761346) at the ninth nucleotide of each repeat (Figure 1A) (Rockman et al. 2005).
The 68-bp tandem repeat polymorphism was reported to play a potential role in the transcriptional activity of the PDYN gene due to the presence of a putative AP-1 transcription factor binding site (Zimprich et al. 2000). Since the transcription factor AP-1 has been shown to bind to the 68-bp tandem repeat, this transcription factor has been considered to play a role in the transcriptional activity of the PDYN gene promoter.
We therefore studied the effect of the SNP G/A rs61761346 and of the number of 68-bp tandem repeats on transcriptional activity of the PDYN gene promoter.
Diversity of the combination of the SNP rs61761346 and the number of 68-bp tandem repeats
In order to identify the different forms of the 68-bp tandem repeat region, genomic DNA of 307 study subjects of two ethnicities (African American and Caucasian) have been sequenced for the region of interest. In 203 African American individuals, the most common forms (>20%) of the PDYN gene promoter are 2GA (20.7%), 2GG (21.9%) and 3GGA (29.8%) (Figure 1B). In 104 Caucasian individuals, the most common forms of the PDYN gene promoter are 2GG (25.5%) and 3GGA (56.7%). The frequencies of most of the alleles varied between ethnicities. Eight forms that were found in African Americans, were not found in Caucasians (Figure 1B).
We identified 17 different forms of the 68-bp tandem repeat region of the PDYN gene promoter. We cloned 13 of these naturally occurring forms, both the most common and some of the rare: 1A, 1G, 2AA, 2GA, 2GG, 3AAA, 3GAA, 3GGA, 3GGG, 4AAAA, 4GAAA, 4GGAA and 4GGGG (Figure 1B). Four of the other rare forms of the 68-bp tandem region were not cloned: 4AGAA, 4GAGA, 4GGGA and 5GGGGG.
The pattern of distribution of the SNP rs61761346 G/A by number of 68-bp tandem repeat varied between ethnicities and reflects the higher diversity of genotypes among African Americans (Figure 1B).
Strategy of study of the PDYN promoter gene transcriptional activity
We inserted, upstream of the luciferase reporter gene, a 3 kb fragment of the PDYN gene which includes: 241 bp upstream of the 68-bp tandem repeat region, the 68-bp tandem repeat region, 1250 bp upstream of exon 1 including the TATA box located 33 bp upstream of exon 1 and most of the transcription start sites, exon 1 (145 bp), intron 1 (1268 bp), exon 2 (60 bp) and 17 bp of intron 2 (see Figure 1C). Working with the 68-bp tandem repeats in their natural location allowed us to study the effect of the number of repeats and of the internal rs61761346 SNP on the activity of the PDYN gene. To study specific mechanisms of PDYN gene activity, we did not use a heterologous promoter as an earlier study did (Zimprich et al. 2000). For the gene reporter assay experiments, we used two human cell lines, SK-N-SH and H69, that were reported to express the PDYN gene (Geijer et al. 1991), and one human cell line, HEK293, not reported to express components of the opioid system.
Transcriptional activity of the PDYN promoter construct deletions
To determine the influence of the region containing the 68-bp repeats on the transcriptional activity of the PDYN gene, we made three deletions of the PDYN promoter construct (Figure 2). As our full-length construct, we used the construct containing one 68-bp tandem repeat with the G allele at rs61761346 (PDYN/1G). We first deleted an 854-bp Nhe I/Mlu I fragment, removing the tandem repeat (‘no tandem sequence’). We also generated two other deletion constructs. One (1G-deleted Mlu I/Ase I) conserves the region containing the 68-bp tandem repeat, but deletes a Mlu I/Ase I 1282 bp fragment, removing the promoter basal elements (e.g., the TATA box and the transcription start sites). The other construct (1G-deleted Mlu I/Nco I) was generated by deleting the Mlu I/Nco I 2221 bp fragment, removing the basal promoter elements, the transcription start sites and intron 1, and potential transcription start sites upstream of exon 2, and therefore has only the 854-bp region with the 68-bp tandem repeat.
In SK-N-SH cells under basal conditions, we observed 11- to 25-fold lower expression of the reporter gene in the three deletion constructs compared to the PDYN/1G full-length construct (Figure 2). Of the three deletion constructs, 1G-deleted Mlu I/Nco I expressed the lowest levels of luciferase. Under TPA stimulation, the deletion constructs showed 3- to 30-fold lower expression of the deletion constructs and the weakest expression was observed with the mutant 1G-deleted Mlu I/Nco I. In SK-N-SH cells stimulated with caffeine, the three deletion mutants had 27- to 39-fold lower expression; the construct with the 68-bp tandem repeat region deleted (‘no tandem sequence’) had the lowest level of luciferase expression.
In H69 cells under basal conditions, we observed 3- to 10-fold lower expression of the reporter gene in the three deletion constructs compared to the PDYN/1G full-length construct; the deletion construct 1G-deleted Mlu I/Nco I expressed the lowest levels of luciferase. When stimulated with TPA, we observed 5- to 15-fold lower expression; the lowest expression was found with the construct 1G-deleted Mlu I/Nco I. After caffeine stimulation, the three deletion constructs had 5- to 25-fold lower expression; the construct with only the region containing the 68-bp tandem repeat (1G-deleted Mlu I/Nco I) had the lowest expression.
In HEK293 cells under basal conditions, we observed 18- to 36-fold lower expression of the reporter gene in the three deletion constructs; the deletion construct 1G-deleted Mlu I/Nco I expressed the lowest level of luciferase. After stimulation with TPA, we found 44- to 81-fold lower expression of the deletion constructs with the lowest levels of reporter gene expression observed with the construct 1G-deleted Mlu I/Nco I. When stimulated with caffeine, the three deletion constructs had 35- to 52-fold lower expression of the reporter gene and the construct ‘no tandem sequence’ had the lowest expression.
Comparison of deletion constructs with the full-length PDYN promoter 1G construct suggests that the 1282 bp region containing the TATA box and the 854-bp region containing the 68-bp tandem repeat are necessary for transcriptional activity of the PDYN gene promoter in vitro.
To determine the impact of the number of 68-bp tandem repeats and of the internal SNP rs61761346 on the activity of the PDYN gene, we performed gene reporter assays using 13 constructs containing different forms of the PDYN promoter and a control construct that had a ~1kb region containing the 68-bp tandem repeat region deleted (NO for ‘no tandem sequence’), each transfected into the three human cell lines (Figures 3A, 4A and 5A). Two approaches to statistical analysis were taken. First, the effect of the number of repeats was examined by a two-way ANOVA, condition by number of repeats without regard to the G or A variant, expressed as the mean of the gene reporter assay by number of tandem repeats (Figure 3B, 4B and 5B). One 68-bp tandem repeat represents the forms 1A and 1G, two represents the forms 2AA, 2GA and 2GG, three represents the forms 3AAA, 3GAA, 3GGA and 3GGG, and four represents the forms 4AAAA, 4GAAA, 4GGAA and 4GGGG.
Effect of the number of 68-bp tandem repeats (rs35286281) and of the internal SNP (rs61761346) on PDYN gene promoter activity in SK-N-SH cells
We first chose to work with the SK-N-SH cells because they are neuroblastoma cells, that endogenously express PDYN. SK-N-SH cells could therefore serve as a model of the effect of the PDYN polymorphisms in some brain tissues.
In SK-N-SH cells, we found a significant effect of repeat number, F(3,27) = 9.34, p < 0.0005 (Figure 3B). Constructs with one copy of the tandem repeat had a higher expression of the luciferase reporter gene than constructs with two, three or four copies (p < 0.005, 0.0005, and 0.0005 respectively, Newman-Keuls post hoc test; Figure 3B). To examine the effect of a repeat number in a manner similar to earlier reports, we then compared “short” promoters (one or two repeats) to “long” promoters (three or four repeats), using the same ANOVA, but with planned comparison (Zimprich et al. 2000; Williams et al. 2007). Constructs with one or two repeats had higher expression of the reporter gene than constructs with three or four copies only after stimulation with caffeine p < 0.000002. We also found a main effect of condition (basal, TPA, caffeine) F(2,27) = 67.04, p < 0.000001. Cells treated with caffeine expressed significantly higher levels of luciferase than cells under basal conditions or those treated with TPA (p < 0.0002 in each case, Newman-Keuls post-hoc test).
To study the effect of the SNP rs61761346 in SK-N-SH cells, we analyzed the results presented in Figure 3A by grouping them depending on the rs61761346 allele. Forms that were not homogeneous regarding the SNP were excluded from this analysis. Results presented in Figure 3C are the mean + SEM of the luciferase gene reporter assay by variant. “A” represents the forms 1A, 2AA, 3AAA and 4AAAA, and “G” represents the forms 1G, 2GG, 3GGG and 4GGGG. Thus, in our second statistical approach, we used a two-way ANOVA, allele by condition.
In SK-N-SH cells, we found no significant main effect of variant, p = 0.090, but in cells treated with caffeine, which showed the highest expression of luciferase, constructs with the A allele had higher expression than those with the G allele, p < 0.05, planned comparison (Figure 3C).
Effect of the number of 68-bp tandem repeats (rs35286281) and of the internal SNP (rs61761346) on PDYN gene promoter activity in H69 cells
H69 cells are small cell lung cancer cells endogenously expressing PDYN. They therefore provide a model of the effect of PDYN polymorphisms in peripheral tissues. Results were analyzed as described above in SK-N-SH cells.
In H69 cells, we found a main effect of repeat number, F(3,27) = 8.16, p < 0.001 (Figure 4B). Constructs containing one copy of the repeat had higher expression of luciferase than constructs containing two (p < 0.05), three (p < 0.02) or four (p < 0.0005) copies (Figure 4B). Constructs with one or two copies of the repeat had higher expression of the reporter gene than constructs with three or four copies after stimulation with TPA (p < 0.05) or caffeine (p < 0.01), but not under basal conditions. We found a main effect of condition, F(2,27)=166.08, p < 0.000001. H69 cells treated with TPA or caffeine expressed significantly higher levels of luciferase than cells under basal conditions (p < 0.0002 in each case). Furthermore, H69 cells treated with caffeine had higher expression of luciferase than cells treated with TPA, p < 0.002.
In H69 cells, there was a significant main effect of allele, F(1,18) = 5.99, p < 0.05 (Figure 4C). Constructs containing only the A allele at rs61761346 had higher luciferase expression than constructs with the G allele.
Effect of the number of 68-bp tandem repeats (rs35286281) and of the internal SNP (rs61761346) on PDYN gene promoter activity in HEK293 cells
In HEK293 cells, we found a main effect of repeat number, F(3,27) = 7.04, p < 0.002, but the effect was the opposite to that in the other two cell lines (Figure 5B). Constructs containing one (p < 0.005), two (p < 0.005) or three (p < 0.02) copies of the repeat had lower expression of luciferase than constructs containing four copies. Constructs with one or two copies of the repeat had significantly lower expression of luciferase than constructs with three or four copies only after stimulation with TPA (p < 0.005). We also found a main effect of condition, F(2,27) = 142.23, p < 0.000001. Cells treated with TPA or caffeine expressed significantly higher levels of luciferase than cells under basal conditions, p < 0.002 (Figure 5B). As in the other two cell lines, HEK293 cells treated with caffeine expressed higher levels of luciferase than those treated with TPA, p < 0.05.
In HEK293 cells, there was no effect of the allele, F(1,18) < 1.0 (Figure 5C).
Transcription start sites used by the PDYN promoter/reporter gene
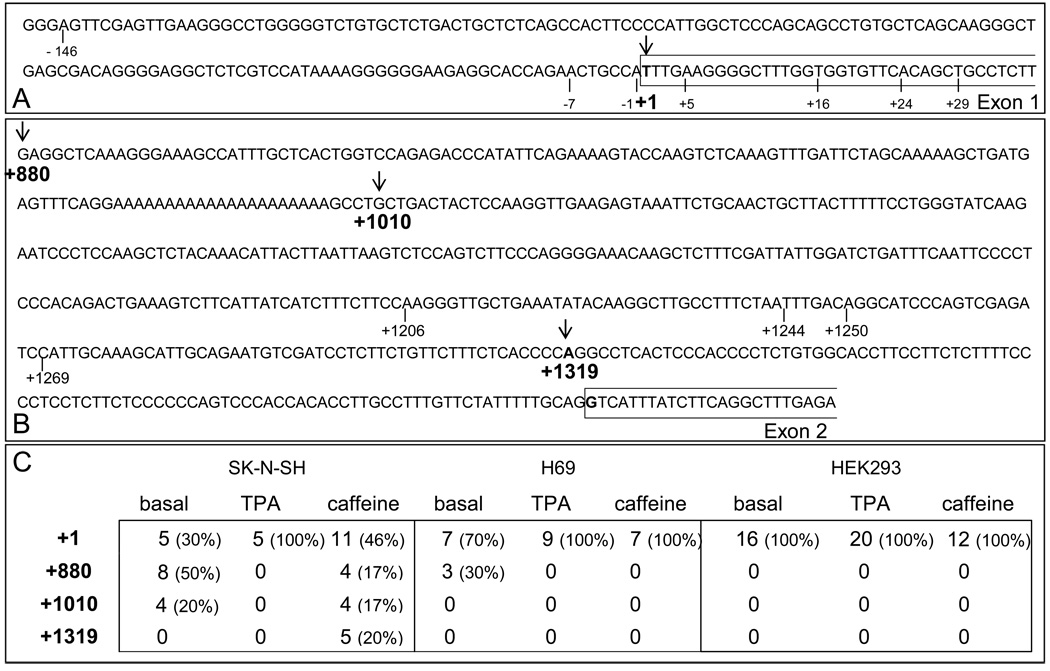
We performed 5’ RLM-RACE PCR to confirm that the transcription start sites used by the PDYN promoter construct are similar to those used by the PDYN gene in human cells and tissues, and to verify that correct splicing of the transcripts occurs in the three cell lines. We avoided amplifying mRNA of the endogenous PDYN by using a reverse primer matching the sequence of the luciferase gene. We identified four transcription start sites (Figure 6A and 6B) used differentially in the three cell lines. Constructs, when transfected into SK-N-SH, H69 and HEK293 cells, utilized the main transcription start site of the PDYN promoter/reporter gene, located at the first base of the exon 1 (Figure 6A and 6C). However, although there were several additional transcription start sites in the SK-N-SH cells, only two were used in the H69 cells (and only under basal conditions) and the first base of exon 1 was the only transcription start site of the PDYN promoter/reporter gene construct in HEK293 cells (Figure 6C). All transcripts that started at the first base of the exon 1 were correctly spliced across intron 1.
Figure 6.
5’-RLM-RACE PCR analysis of transcription start sites of the PDYN gene promoter construct in SK-N-SH, H69 and HEK293 cell lines. A. Sequence of the PDYN gene promoter from −143 bp to +39 bp showing the previously identified transcription start sites located at positions −146, −7, −1, +1, +5, +16, +24 and +29 (bars below sequence). An ↓ indicates the transcription start site identified by 5’-RLM-RACE PCR. B. Sequence of the PDYN gene promoter from +880 to +1437 showing the previously identified transcription start located at positions +1206, +1244, +1250 and +1269 (bars below sequence). An ↓ indicate the transcription start site identified by 5’-RLM-RACE PCR in this study. C. Frequencies of used transcription start sites in SK-N-SH, H69 and HEK293 cells under basal conditions or after stimulation with either 100 nM TPA or 10 mM caffeine.
Additional transcription start sites have been identified for the PDYN gene in brain tissues and they are located upstream of exon 2, at positions +1206, +1244, +1250 and +1269 (Nikoshkov et al. 2005). We found that alternative transcription start sites located in intron 1, upstream of exon 2, are also present in certain conditions in the cell lines studied. We identified three different transcription start sites located at positions +880, +1010 and +1319 (Figure 6B and 6C). The transcription start site located at position +880 was only used by SK-N-SH cells under basal conditions and after stimulation of cells with caffeine, and by H69 cells under basal conditions (Figure 6C). The transcription start site located at position +1010 was used by SK-N-SH cells under basal condition and after stimulation with caffeine (Figure 6C). The transcription start site located at position +1319 was used by SK-N-SH cells when stimulated with caffeine (Figure 6C). Multiple clones were found that mapped to each transcription start site.
DISCUSSION
Dynorphin peptides, including α-neoendorphin, β-neoendorphin, dynorphin A and dynorphin B, are generated after post-translational processing and enzymatic cleavage of the preprodynorphin protein. The PDYN gene is expressed in several regions of the CNS, including the hypothalamus, striatum, hippocampus, cerebral cortex and spinal cord, and it is also expressed in peripheral and endocrine tissues (Khachaturian et al. 1986; Hurd and Herkenham 1995). Expression of the PDYN gene is increased on an acute and persistent basis by drugs of abuse such as cocaine (Sivam 1989; Hurd et al. 1992; Spangler et al. 1993; Zhou et al. 2002; Schlussman et al. 2003; Schlussman et al. 2005). It has been hypothesized that dynorphin peptides would reduce the dopaminergic tone in the brain under basal conditions or after intake of a drug of abuse (Simonato and Romualdi 1996; Kreek et al. 2004; Zhang et al. 2004).
In this study, deletion analysis of a PDYN promoter construct has shown the importance of several promoter regions in the transcriptional activity of the PDYN gene. We found, in the three cell lines, that deleting the 854-bp region containing the 68-bp tandem repeats dramatically reduced basal and inducible expression of the reporter gene, demonstrating that the region containing the 68-bp tandem repeat is necessary for transcriptional activity. In contrast, in another study, deletion analysis of the PDYN gene fused with a CAT reporter gene has shown that, in NB69 and SK-N-MC cells, deletion of a 644-bp region that contains the 68-bp tandem repeats, increased expression under basal conditions or after forskolin stimulation (Carrion et al. 1998). In light of the Babbitt et al. (2010) study, showing a strong effect of sequence polymorphisms on the basal PDYN expression, differences in the results of these two studies may be due, in part, to differences in expression constructs with different repressing or activating sequences surrounding the 68-bp tandem repeats.
Two compounds, TPA and caffeine, were used to induce the expression of the reporter gene. TPA enhanced the expression of the reporter gene in H69 and HEK293 cells, but not in SK-N-SH cells. Caffeine increases the expression of the PDYN gene by removing the inhibitory effect of DREAM (Campos et al. 2003). In each cell line, we observed a strong and cell-specific effect of caffeine on luciferase expression. In SK-N-SH cells, while TPA did not affect expression, caffeine strongly induced luciferase expression. In H69 cells, we observed a larger effect of caffeine than of TPA on expression. However in HEK293 cells, induction of expression was similar with TPA and caffeine.
We found cell-specific effects of the number of 68-bp tandem repeats and of the SNP rs61761346 on the induction of transcriptional activity in the three different human cell lines studied. Cell-specific effects of variable number tandem repeat (VNTR) have already been described for the serotonin transporter 5-HTT (Klenova et al. 2004); moreover several studies have described cell-specific effects of a SNP located within promoters on transcriptional activity (e.g., Kim et al. 2006; Wang et al. 2006).
Interestingly, Babbitt et al. study found no consistent relationship between repeat number and PDYN basal expression, and showed that 68-bp repeat number had different consequences for expression, depending on their association with other polymorphic sites (Babbitt et al. 2010).
Another explanation of the effect of the 68-bp tandem repeats on the transcriptional activity of the PDYN gene is that an alteration in distance between two hypothetical regulatory elements such as binding site for transcription factors located one upstream and the other downstream of the 68-bp tandem repeat region may influence the efficiency of transcription. The distance between two important transcription elements could therefore contribute to the regulation of transcription. The impact of the distance between two regulatory elements of a promoter has already been described (Sugiyama et al. 1998; Vardhanabhuti et al. 2007).
However, a cell-specific effect of the 68-bp tandem repeat on the transcriptional activity of the PDYN promoter has not been described before. In mouse neuroblastoma cells stimulated with TPA, it has been shown that three or four copies of the 68-bp tandem repeats fused upstream of the heterologous tk promoter in front of a CAT reporter gene had higher expression than constructs with one or two copies (Zimprich et al. 2000). In contrast, we found different and cell-specific results using three human cell lines and a 3 kb fragment of the PDYN gene promoter.
This study reveals the complexity due to differences between cell lines and yields information that is potentially important for integrated human neurobiology. In a study by another laboratory, the impact of the number of 68-bp tandem repeats was studied in relation to the mRNA levels in specific regions of 56 adult postmortem brain specimens (Nikoshkov et al. 2008). In specific brain regions, the levels of PDYN mRNA were found to be higher in subjects with inducible “I/I” alleles (three or four 68-bp repeats) as compared with subjects with non-inducible “N/N” alleles (one or two repeats). This study emphasizes the functionality of the 68-bp tandem repeats, but does not exclude a different effect of the number of 68-bp repeats on PDYN gene expression in different brain regions, different tissues and different cell types. Since SK-N-SH cells are neuroblastoma derived, of the three cell lines studied here, they may be closest in nature to brain neurons. If these cells are similar to neurons, we would expect more repeats or the presence of the G allele to reduce dynorphin expression in brain. The fact that PDYN did not respond to TPA but did to caffeine in the SK-N-SH cells, while the two other cells lines responded to both TPA and caffeine, may be due to a difference in the trans-acting factors and signal transduction pathways specific to the cell lines involved in the regulation of PDYN transcription.
The transcription start sites used by the PDYN promoter construct were similar to those used by the PDYN gene in human cells and tissues (Telkov et al. 1998; Nikoshkov et al. 2005), and correct splicing of the construct occurred in the three different cell lines. We also showed that alternative transcription start sites located in intron 1, upstream of exon 2, were utilized under specific conditions and were cell specific. Such differences in the utilization of transcription start sites between cell lines may partly explain the differences we observed in the gene reporter assays.
The 68-bp tandem repeat polymorphism within the promoter of the PDYN gene has been found to be associated with dependence on several drugs of abuse. However, studies have shown contradictory results. The first association study of the 68-bp repeat polymorphism with cocaine dependence showed that individuals of diverse ethnicities with three or four copies of the 68-bp repeat have a lower risk for vulnerability to develop cocaine abuse and/or cocaine dependence (Chen et al. 2002). Another study in African Americans showed a similar trend for association of genotype and allelic frequencies with opioid dependence, but no significant association was found in European Americans (Ray et al. 2005). In contrast, two independent studies with stringent phenotyping reported a statistically significant association between the 68-bp tandem repeat and cocaine/alcohol dependence in African Americans (Dahl et al. 2005; Williams et al. 2007). In both of these studies, individuals with three or four copies of the 68-bp tandem repeat showed an increased vulnerability to develop cocaine dependence or cocaine/alcohol codependence. A significant association between the number of 68-bp tandem repeats and methamphetamine dependence has also been reported; alleles with three or four copies of the 68-bp repeat were found more frequently in individuals with methamphetamine dependence (Nomura et al. 2006).
We identified 17 naturally-occurring forms of the PDYN promoter and their frequencies differed between ethnicities. Interestingly, eight forms of PDYN promoter were found only in African Americans, not in Caucasians. Such striking differences between ethnicities may account for the differing results of association studies of the PDYN promoter polymorphisms with vulnerability to develop (or protection from developing) dependence on drugs of abuse. Similarly divergent results have also been found in association studies of the 68-bp tandem repeats with temporal lobe epilepsy (Stogmann et al. 2002; Bovo et al. 2008; Kauffman et al. 2008), the repeats considered to play a role in the transcriptional activity of the PDYN gene promoter. Our analysis of putative transcription factor binding sites within the 68-bp tandem repeat region suggests that the substitution of the G allele by the A allele at the ninth nucleotide of the repeat creates a putative GATA-1 transcription factor binding site. Since we found that PDYN promoters containing the A allele at rs61761346 have greater transcriptional activity than promoters containing the G allele, future association studies should now consider this new SNP within the 68-bp tandem repeat.
In conclusion, studying the functionality of the 68-bp tandem repeat polymorphism and of the SNP rs61761346 within the 68-bp tandem repeat region showed cell-specific effects of these two polymorphisms on transcription of the PDYN gene. Studies of the association of the 68-bp tandem repeat polymorphism with dependence on different drugs of abuse or with other neurological diseases such as temporal lobe epilepsy, should now take into consideration the complex effects of the 68-bp tandem repeats and of the SNP rs61761346. It would be interesting to study interactions of the 68-bp tandem repeat polymorphism and of the SNP rs61761346 with other polymorphisms in the PDYN gene in responses to drugs of abuse and a variety of stressors. This study shows, for the first time, cell-specific effects of the 68-bp tandem repeat and of the internal SNP rs61761346 of the PDYN gene on transcriptional activity. It demonstrates the complex transcriptional regulation of the PDYN gene, emphasizes the functionality of the 68-bp tandem repeat polymorphism, and further documents the potential importance of an internal SNP such as rs61761346 within the 68-bp tandem repeat.
Acknowledgments
The authors would like to acknowledge Dr. Thomas Tuschl for allowing us to use some specialized equipment in his laboratory, Matthew Randesi and Caitlin Smith for their technical assistance and Susan Russo for her editorial contribution. This work was supported by grants from NIH-NIDA P60-05130 and NIDA NIMH MH79880(MJK) and the Philippe Foundation (MR).
References
- Babbitt CC, Silverman JS, Haygood R, Reininga JM, Rockman MV, Wray GA. Multiple functional variants in cis modulate PDYN expression. Mol Biol Evol. 2010;27:465–479. doi: 10.1093/molbev/msp276. [DOI] [PubMed] [Google Scholar]
- Bovo G, Diani E, Bisulli F, Di Bonaventura C, Striano P, Gambardella A, Ferlazzo E, Egeo G, Mecarelli O, Elia M, Bianchi A, Bortoluzzi S, Vettori A, Aguglia U, Binelli S, De Falco A, Coppola G, Gobbi G, Sofia V, Striano S, Tinuper P, Giallonardo AT, Michelucci R, Nobile C. Analysis of LGI1 promoter sequence, PDYN and GABBR1 polymorphisms in sporadic and familial lateral temporal lobe epilepsy. Neurosci. Lett. 2008;436:23–26. doi: 10.1016/j.neulet.2008.02.045. [DOI] [PubMed] [Google Scholar]
- Campos D, Jimenez-Diaz L, Carrion AM. Ca(2+)-dependent prodynorphin transcriptional derepression in neuroblastoma cells is exerted through DREAM protein activity in a kinase-independent manner. Mol. Cell Neurosci. 2003;22:135–145. doi: 10.1016/s1044-7431(03)00040-x. [DOI] [PubMed] [Google Scholar]
- Carrion AM, Mellstrom B, Naranjo JR. Protein kinase A-dependent derepression of the human prodynorphin gene via differential binding to an intragenic silencer element. Mol. Cell Biol. 1998;18:6921–6929. doi: 10.1128/mcb.18.12.6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion AM, Link WA, Ledo F, Mellstrom B, Naranjo JR. DREAM is a Ca2+-regulated transcriptional repressor. Nature. 1999;398:80–84. doi: 10.1038/18044. [DOI] [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Chen AC, LaForge KS, Ho A, McHugh PF, Kellogg S, Bell K, Schluger RP, Leal SM, Kreek MJ. Potentially functional polymorphism in the promoter region of prodynorphin gene may be associated with protection against cocaine dependence or abuse. Am. J. Med. Genet. 2002;114:429–435. doi: 10.1002/ajmg.10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl JP, Weller AE, Kampman KM, Oslin DW, Lohoff FW, Ferraro TN, O'Brien CP, Berrettini WH. Confirmation of the association between a polymorphism in the promoter region of the prodynorphin gene and cocaine dependence. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005;139:106–108. doi: 10.1002/ajmg.b.30238. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J. Pharmacol. Exp. Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- Geijer T, Bergh J, Terenius L. Expression of preprodynorphin in human small cell lung carcinoma cell lines. Regul. Pept. 1991;34:181–188. doi: 10.1016/0167-0115(91)90177-i. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Aldrich JV, Anderson KJ, Bakalkin G, Christie MJ, Hall ED, Knapp PE, Scheff SW, Singh IN, Vissel B, Woods AS, Yakovleva T, Shippenberg TS. Pathobiology of dynorphins in trauma and disease. Front. Biosci. 2005;10:216–235. doi: 10.2741/1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa S, Takai T, Toyosato M, Takahashi H, Noda M, Kakidani H, Kubo T, Hirose T, Inayama S, Hayashida H, et al. Isolation and structural organization of the human preproenkephalin B gene. Nature. 1983;306:611–614. doi: 10.1038/306611a0. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Herkenham M. The human neostriatum shows compartmentalization of neuropeptide gene expression in dorsal and ventral regions: an in situ hybridization histochemical analysis. Neuroscience. 1995;64:571–586. doi: 10.1016/0306-4522(94)00417-4. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Brown EE, Finlay JM, Fibiger HC, Gerfen CR. Cocaine self-administration differentially alters mRNA expression of striatal peptides. Brain Res. Mol. Brain Res. 1992;13:165–170. doi: 10.1016/0169-328x(92)90058-j. [DOI] [PubMed] [Google Scholar]
- Jacobson DA, Cho J, Landa LR, Jr, Tamarina NA, Roe MW, Buxbaum JD, Philipson LH. Downstream regulatory element antagonistic modulator regulates islet prodynorphin expression. Am. J. Physiol. Endocrinol. Metab. 2006;291:E587–E595. doi: 10.1152/ajpendo.00612.2005. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman MA, Consalvo D, Gonzalez MD, Kochen S. Transcriptionally less active prodynorphin promoter alleles are associated with Temporal Lobe Epilepsy: A case-control study and meta-analysis. Dis. Markers. 2008;24:135–140. doi: 10.1155/2008/723723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachaturian H, Sherman TG, Lloyd RV, Civelli O, Douglass J, Herbert E, Akil H, Watson SJ. Pro-dynorphin is endogenous to the anterior pituitary and is co-localized with LH and FSH in the gonadotrophs. Endocrinology. 1986;119:1409–1411. doi: 10.1210/endo-119-3-1409. [DOI] [PubMed] [Google Scholar]
- Kim SH, Bae JS, Holloway JW, Lee JT, Suh CH, Nahm DH, Park HS. A polymorphism of MS4A2 (− 109T > C) encoding the beta-chain of the high-affinity immunoglobulin E receptor (FcepsilonR1beta) is associated with a susceptibility to aspirin-intolerant asthma. Clin. Exp. Allergy. 2006;36:877–883. doi: 10.1111/j.1365-2222.2006.02443.x. [DOI] [PubMed] [Google Scholar]
- Klenova E, Scott AC, Roberts J, Shamsuddin S, Lovejoy EA, Bergmann S, Bubb VJ, Royer HD, Quinn JP. YB-1 and CTCF differentially regulate the 5-HTT polymorphic intron 2 enhancer which predisposes to a variety of neurological disorders. J. Neurosci. 2004;24:5966–5973. doi: 10.1523/JNEUROSCI.1150-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, LaForge KS. Genes associated with addiction: alcoholism, opiate, and cocaine addiction. Neuromolecular Med. 2004;5:85–108. doi: 10.1385/NMM:5:1:085. [DOI] [PubMed] [Google Scholar]
- Nikoshkov A, Drakenberg K, Wang X, Horvath MC, Keller E, Hurd YL. Opioid neuropeptide genotypes in relation to heroin abuse: dopamine tone contributes to reversed mesolimbic proenkephalin expression. Proc. Natl. Acad. Sci. U. S. A. 2008;105:786–791. doi: 10.1073/pnas.0710902105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoshkov A, Hurd YL, Yakovleva T, Bazov I, Marinova Z, Cebers G, Pasikova N, Gharibyan A, Terenius L, Bakalkin G. Prodynorphin transcripts and proteins differentially expressed and regulated in the adult human brain. FASEB J. 2005;19:1543–1545. doi: 10.1096/fj.05-3743fje. [DOI] [PubMed] [Google Scholar]
- Nomura A, Ujike H, Tanaka Y, Otani K, Morita Y, Kishimoto M, Morio A, Harano M, Inada T, Yamada M, Komiyama T, Sekine Y, Iwata N, Sora I, Iyo M, Ozaki N, Kuroda S. Genetic variant of prodynorphin gene is risk factor for methamphetamine dependence. Neurosci. Lett. 2006;400:158–162. doi: 10.1016/j.neulet.2006.02.038. [DOI] [PubMed] [Google Scholar]
- Ray R, Doyle GA, Crowley JJ, Buono RJ, Oslin DW, Patkar AA, Mannelli P, DeMaria PA, Jr, O'Brien CP, Berrettini WH. A functional prodynorphin promoter polymorphism and opioid dependence. Psychiatr. Genet. 2005;15:295–298. doi: 10.1097/00041444-200512000-00013. [DOI] [PubMed] [Google Scholar]
- Rockman MV, Hahn MW, Soranzo N, Zimprich F, Goldstein DB, Wray GA. Ancient and recent positive selection transformed opioid cis-regulation in humans. PLoS Biol. 2005;3:e387. doi: 10.1371/journal.pbio.0030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlussman SD, Zhang Y, Yuferov V, LaForge KS, Ho A, Kreek MJ. Acute 'binge' cocaine administration elevates dynorphin mRNA in the caudate putamen of C57BL/6J but not 129/J mice. Brain Res. 2003;974:249–253. doi: 10.1016/s0006-8993(03)02561-7. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Zhou Y, Bailey A, Ho A, Kreek MJ. Steady-dose and escalating-dose "binge" administration of cocaine alter expression of behavioral stereotypy and striatal preprodynorphin mRNA levels in rats. Brain Res. Bull. 2005;67:169–175. doi: 10.1016/j.brainresbull.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Schug J. Using TESS to predict transcription factor binding sites in DNA sequence. Chapter 2. Curr Protoc Bioinformatics. 2008 doi: 10.1002/0471250953.bi0206s21. Unit 2.6. [DOI] [PubMed] [Google Scholar]
- Simonato M, Romualdi P. Dynorphin and epilepsy. Prog. Neurobiol. 1996;50:557–583. doi: 10.1016/s0301-0082(96)00045-7. [DOI] [PubMed] [Google Scholar]
- Sivam SP. Cocaine selectively increases striatonigral dynorphin levels by a dopaminergic mechanism. J. Pharmacol. Exp. Ther. 1989;250:818–824. [PubMed] [Google Scholar]
- Solbrig MV, Koob GF. Epilepsy, CNS viral injury and dynorphin. Trends Pharmacol. Sci. 2004;25:98–104. doi: 10.1016/j.tips.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Spangler R, Unterwald EM, Kreek MJ. 'Binge' cocaine administration induces a sustained increase of prodynorphin mRNA in rat caudate-putamen. Brain Res. Mol. Brain Res. 1993;19:323–327. doi: 10.1016/0169-328x(93)90133-a. [DOI] [PubMed] [Google Scholar]
- Stogmann E, Zimprich A, Baumgartner C, Aull-Watschinger S, Hollt V, Zimprich F. A functional polymorphism in the prodynorphin gene promotor is associated with temporal lobe epilepsy. Ann. Neurol. 2002;51:260–263. doi: 10.1002/ana.10108. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Scott DK, Wang JC, Granner DK. Structural requirements of the glucocorticoid and retinoic acid response units in the phosphoenolpyruvate carboxykinase gene promoter. Mol. Endocrinol. 1998;12:1487–1498. doi: 10.1210/mend.12.10.0187. [DOI] [PubMed] [Google Scholar]
- Telkov M, Geijer T, Terenius L. Human prodynorphin gene generates several tissue-specific transcripts. Brain Res. 1998;804:284–295. doi: 10.1016/s0006-8993(98)00706-9. [DOI] [PubMed] [Google Scholar]
- Vardhanabhuti S, Wang J, Hannenhalli S. Position and distance specificity are important determinants of cis-regulatory motifs in addition to evolutionary conservation. Nucleic Acids Res. 2007;35:3203–3213. doi: 10.1093/nar/gkm201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Ngoi S, Wang J, Chong SS, Lee CG. The promoter region of the MDR1 gene is largely invariant, but different single nucleotide polymorphism haplotypes affect MDR1 promoter activity differently in different cell lines. Mol. Pharmacol. 2006;70:267–276. doi: 10.1124/mol.105.019810. [DOI] [PubMed] [Google Scholar]
- Williams TJ, LaForge KS, Gordon D, Bart G, Kellogg S, Ott J, Kreek MJ. Prodynorphin gene promoter repeat associated with cocaine/alcohol codependence. Addict. Biol. 2007;12:496–502. doi: 10.1111/j.1369-1600.2007.00069.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effect of the endogenous kappa opioid agonist dynorphin A(1–17) on cocaine-evoked increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology (Berl.) 2004;172:422–429. doi: 10.1007/s00213-003-1688-3. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Schlussman SD, Yuferov VP, Sora I, Ho A, Uhl GR, Kreek MJ. Effects of acute "binge" cocaine on preprodynorphin, preproenkephalin, proopiomelanocortin, and corticotropin-releasing hormone receptor mRNA levels in the striatum and hypothalamic-pituitary-adrenal axis of mu-opioid receptor knockout mice. Synapse. 2002;45:220–229. doi: 10.1002/syn.10101. [DOI] [PubMed] [Google Scholar]
- Zimprich A, Kraus J, Woltje M, Mayer P, Rauch E, Hollt V. An allelic variation in the human prodynorphin gene promoter alters stimulus-induced expression. J. Neurochem. 2000;74:472–477. doi: 10.1046/j.1471-4159.2000.740472.x. [DOI] [PubMed] [Google Scholar]