Abstract
Channelrhodopsins (ChRs) are light-activated channels from algae that provide these organisms with fast sensors to visible light for phototaxis. Since its discovery, Channelrhodopsin 2 (ChR2) has been utilised as a research tool to depolarise membranes of excitable cells with light. Subsequent chimeragenesis, mutagenesis, and bioinformatic approaches have introduced additional ChR variants such as ChR2/H134R, ChR2/E123T(ChETA), VChR1, VChR2, ChR2/C128X/D156A, ChD, ChEF and ChIEF. Each of these ChR variuants has unique features and limitations, but there are few resources summarising and comparing these ChRs in a systematic manner. In this review, the 7 key properties of ChRs that have significant influences on their effectiveness as research tools are examined: conductance, selectivity, kinetics, desensitisation, light sensitivity, spectral response and membrane trafficking. Using this information, valuable qualities and deficits of each ChR variant are summarised. Optimal uses and potential future improvements of ChRs as optogenetic tools are also discussed.
Channelrhodopsins (ChRs) are the only known ion channels that are directly gated by light. These channels are 7 transmembrane domain proteins that provide algae with visible light ‘perception’. When excited by light, ChR channels open and depolarise the membrane. Since their first ex vivo characterisations (Nagel et al., 2002; Nagel et al., 2003), ChRs have generated much interest as research tools to depolarise neuronal membrane with light (Boyden et al., 2005; Li et al., 2005; Nagel et al., 2005). Although ChRs are applied to neuroscientific research currently, they can be potentially used to activate cardiac myocytes, skeletal muscles and voltage-gated ion channel expressed in cell lines to study other physiological functions. The use of ChRs to control membrane excitabilities of neurons has been extensively reviewed previously (Zhang et al., 2006), and will not be the main focus of this review. Instead, this review aims to provide an overview of the unique biophysical properties and limitations of the different ChR variants and highlight the specific experiments for which each variant is best suited.
Factors influence the effectiveness of ChR-induced membrane depolarisation
Ideally when using ChRs to manipulate membrane potential, the change should be instantaneous and predictable. However, the intrinsic properties of the membrane (resistance and capacitance) limit the rate and level of achievable depolarisation. Assuming instantaneous opening of the ChR and the absence of voltage-gated ion channels in the membrane, the membrane charges or discharges exponentially with the time constant of the membrane, which is dependent on the membrane capacitance and number of open channels. The level of depolarisation is determined by the membrane resistance and membrane current (Ohm’s law). The unique attributes of each ChR have significant impact on the ability of the experimenter to control depolarisation with light. Seven properties of ChRs can affect the accuracy of manipulating membrane potentials with this technology:
Channel conductance: The estimation of single channel conductance of ChR2 is below 1 pS (Nagel et al., 2003; Bamann et al., 2008; Feldbauer et al., 2009; Lin et al., 2009b), which is less than the conductance of the common membrane channels. Conductance directly determines the effectiveness of light-induced depolarisation. In a cell of 50 MΩ membrane resistance, ~500,000 functional ChRs need to open simultaneously to depolarise the membrane 15 mV (assuming 100 fS single-channel conductance, reversal potential of 0 mV, membrane potential −60 mV).
Ion selectivity: All ChRs are cation permeant, non-selective towards H+, Na+, K+ and Ca2+, and have a reversal potential near 0 mV at physiological pH (Nagel et al., 2003; Berthold et al., 2008; Zhang et al., 2008; Lin et al., 2009b; Tsunoda and Hegemann, 2009; Gunaydin et al., 2010). The maximal change in membrane potential that can be achieved with ChRs is near its reversal potential of 0 mV if the cell has minimal basal ‘leak’ current.
Kinetics: Opening and closing rates of ChRs are critical factors in achieving temporally precise manipulation of membrane potentials. The speed of membrane potential change induced by ChR is associated both with the channel kinetics and the intrinsic membrane properties of the cell. Although the ideal kinetics of a ChR should be as fast as is physically allowed, very rapid kinetics must be balanced with sacrifices in light sensitivity.
Desensitisation and recovery from the desensitised state: In the presence of pcontinuous, strong illumination or repetetive, pulsed stimulation, the response of ChR2 decays 80% from a peak response to a steady-state level response (Nagel et al., 2003). This ‘desensitisation’ requires the expression of 5-fold more functional ChR2 to achieve depolarisation above threshold after desensitisation. All known ChRs desensitise and some variants do not recover fully after initial stimulation (Nagel et al., 2002; Lin et al., 2009b; Lin et al., 2009a; Schoenenberger et al., 2009). .
Light sensitivity: ChRs vary in light sensitivity. Mutations that increase light sensitivity of ChRs often have negative impacts on the kinetics of the channel (Berndt et al., 2009; Lin et al., 2009b). The transition rate from the open to the closed state of the channel reflects the energy difference between the two states of the channel, which directly reflects on the energy required to sustain the channel in the opening state.
Spectral response: The maximal and steady-state components of the photocurrents may have different spectral excitation peaks (Lin et al., 2009b; Lin et al., 2009a). Many ChRs can be ‘preconditioned’ with a second light pulse of a different wavelength to speed up the recovery of the desensitised component (Lin et al., 2009b; Lin et al., 2009a).
Membrane trafficking and expression: Low expression and/or intracellular aggregation of ChRs reduce their effectiveness of membrane depolarisation. (Lin et al., 2009a; Tsunoda and Hegemann, 2009). Overexpression of an exogenous membrane protein can be toxic and/or adversely alter on the membrane properties.
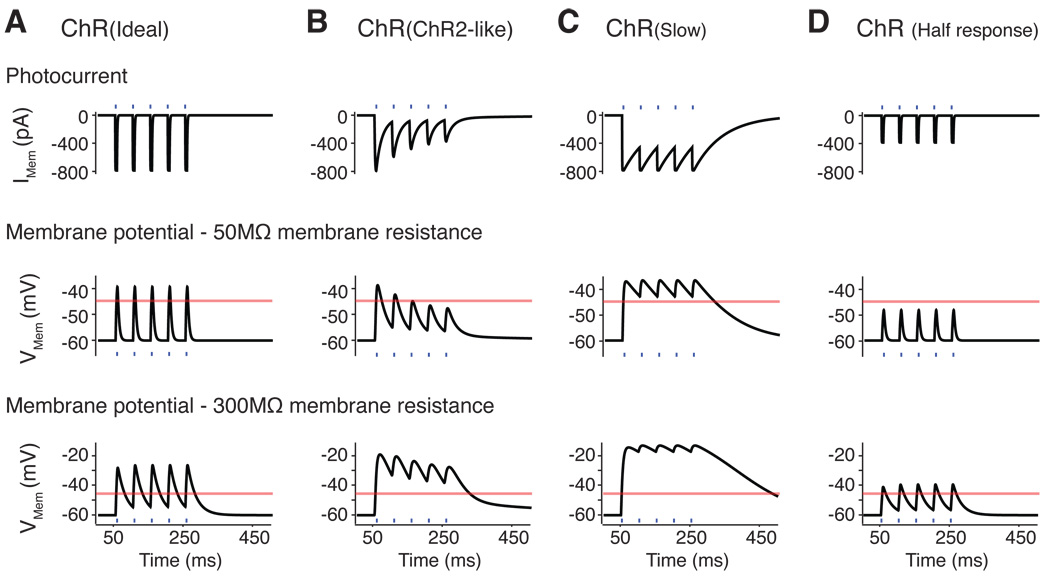
Figure 1 and Supplementary Figure 1 demonstrate the effects of changes in ChR conductance, expression, desensitisation and kinetics on achieving temporally precise membrane depolarisation by light pulses on passive membrane. Increased desensitisation reduces the consistency of depolarisation, whereas the changes in kinetics alter the repolarisation rate. Improved membrane trafficking and/or channel conductance can increase the amplitudes of membrane depolarisation, but also reduce effective repolarisation. Some shortcomings of ChRs (low conductance, desensitisation) can be partially compensated by overexpression. High expression is not ideal because this can change the native membrane environment. The level of membrane depolarisation and rate of repolarisation are also heavily influenced by the intrinsic properties of the membrane, in addition to the expression of voltage-gated channels in the membrane.
Figure 1.
Theoretical changes in the membrane potential induced by ChRs with 20 Hz pulsed light stimulation (blue lines) on a passive membrane model. (A) The ideal ChR with no desensitisation and rapid kinetics (labelled ChR (Ideal)), responds well to pulsed stimulation with consistent responses and the repolarisation is primarily determined by the membrane properties. (B) The photocurrents from ChR with ChR2-like channel (ChR(ChR2-like)) desensitise strongly to light pulses, lead to reduced membrane depolarisation with later light pulses in a membrane with low resistance. In a membrane with high resistance, the cell enters ‘depolarisation block’ and repetitive stimulation does not allow repolarisation. (C) Photocurrents from a ChR with slow kinetics (ChR (Slow)) does not allow sufficient repolarisation of the membrane between pulses leading to ‘depolarisation block’. (D) Reduction of ChR conductance and/or level of membrane expression (ChR(Half response)) can result in insufficient depolarisation with light. For modelling parameters see Supplementary Figure 1. Orange lines indicate threshold of −45 mV.
Unique properties and limitations of ChR variants
ChR2
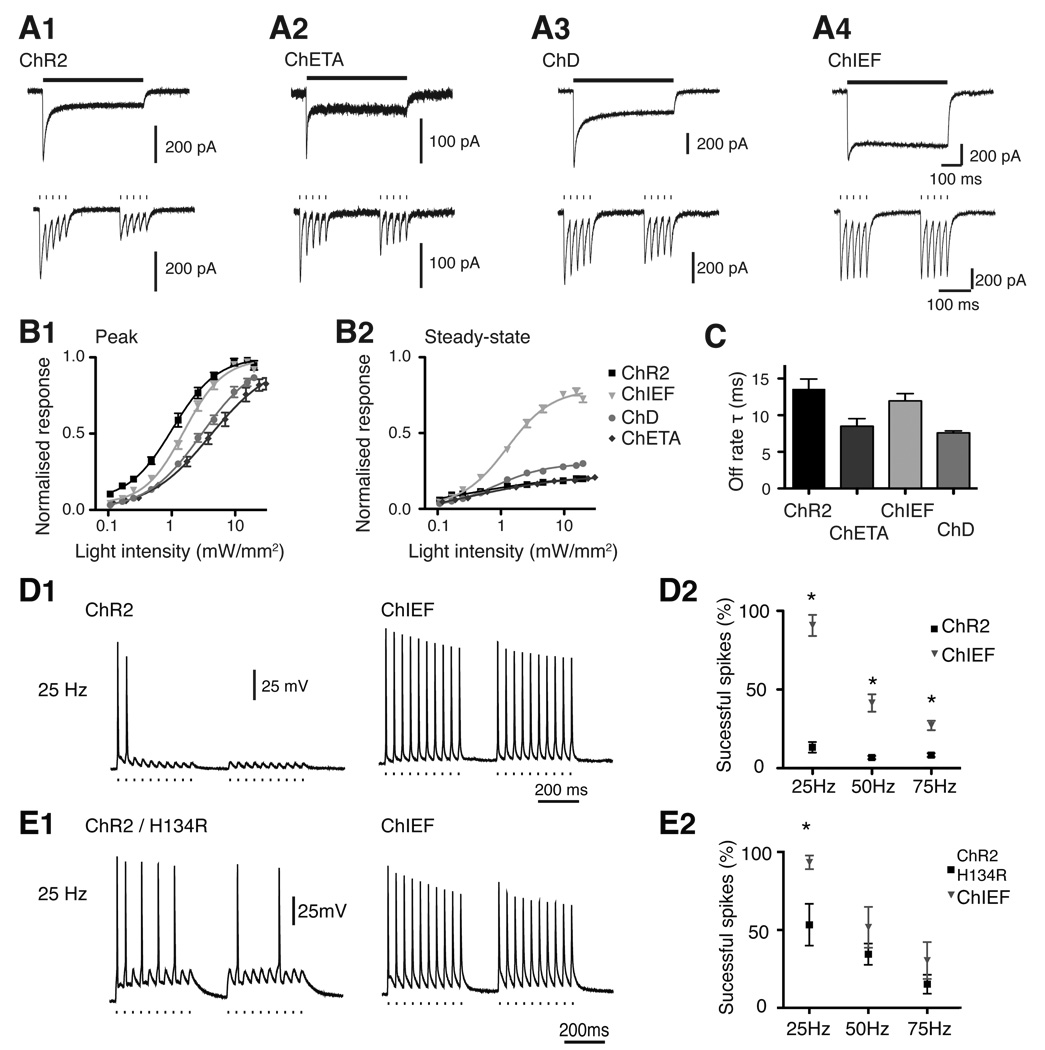
ChR2 was the first channelrhodopsin used to excite neurons with light (Boyden et al., 2005; Li et al., 2005; Nagel et al., 2005). When stimulated with high intensity light, ChR2 has a rapid on-rate and moderate channel closing rate (Figure 2) (Nagel et al., 2003; Ishizuka et al., 2006; Lin et al., 2009b). ChR2 is maximally excited by 470nm light (Figure 3)((Nagel et al., 2003; Ishizuka et al., 2006; Lin et al., 2009b). The main deficiency of ChR2 is the high level of desensitisation (Figure 2), which reduces the current by ~80% at physiological pH (Nagel et al., 2003; Ishizuka et al., 2006; Lin et al., 2009b). The desensitised response fully recovers after 25s in the dark (Figure 3)(Lin et al., 2009b). ChR2 trafficks to the membrane well when expressed at low levels but forms intracellular aggregates at high levels (Lin et al., 2009b; Lin et al., 2009a). The conductance of ChR2 is estimated to be 50–250 fS (Nagel et al., 2003; Bamann et al., 2008; Feldbauer et al., 2009; Lin et al., 2009b).
Figure 2.
Comparisons of the ‘fast’ ChRs with ChR2. (A) Voltage-clamp recordings of ChR2 (A1), ChETA (A2), ChD (A3), and ChIEF (A4) transfected HEK293 cells to 500 ms continuous light and pulsed light stimulation (50 Hz). ChIEF show the most consistent photocurrents for both stimulation protocols. ChETA and ChD have faster kinetics than ChR2 after the termination of light pulses. All recordings were made in small HEK293 cells to ensure accurate voltage-clamping. (B) The light-intensity response curve for ChR variants for their peak (B1) and the steady-state response (B2) normalised to the projected peak response. The sensitivity to light is ChR2 > ChIEF > ChD > ChETA. (C) Comparison of the off-rate time constant for ChR2, ChETA, ChD and ChIEF. (D1) Examples of ChR2 and ChIEF expressed at low levels in cultured neurons and their light-induced action potential trains at 25 Hz. ChIEF shows superior performance relative to ChR2. (D2) Summary of D1 in multiple neurons. (E1) Similar comparison as in D1, with ChR2/H134R and ChIEF, but at a higher expression level. (E2) Summary of E1 in multiple neurons. * indicates p < 0.01%.
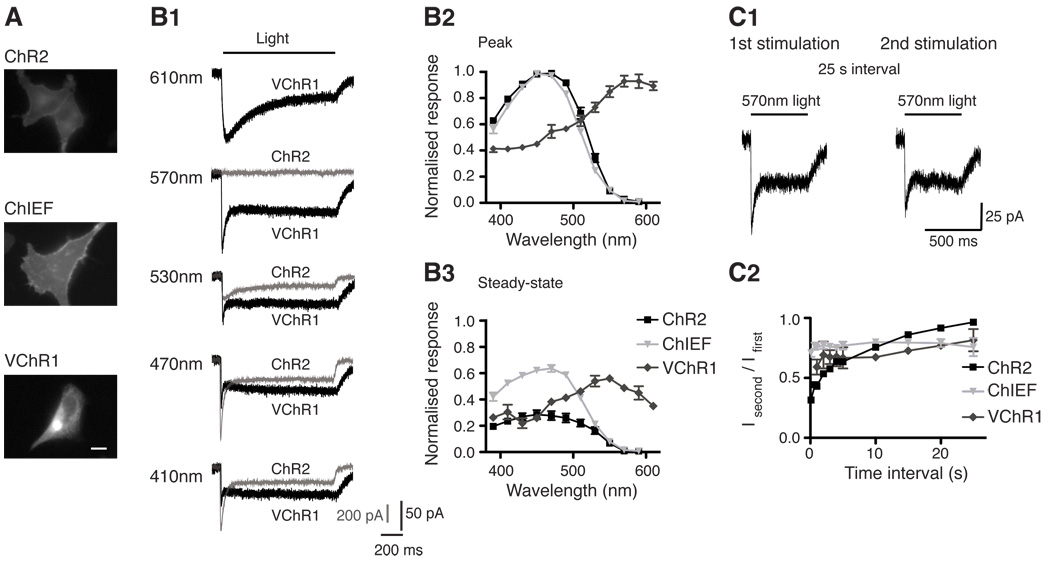
Figure 3.
Comparisons of VChR1, ChR2 and ChIEF. (A) Expression of ChR2, ChIEF, and VChR1 in HEK293 cells showing membrane trafficking of the variants. (B1) Typical responses of ChR2 (gray) and VChR1 (black) to different colour lights (same photon flux). (B2) The response spectra of the peak and steady-state responses of the VChR1, ChR2 and ChIEF, normalised to their peak response. (C1) The response of VChR1 to 570 nm light does not completely recover in the dark. (C2) Recovery of ChR2, VChR1 and ChIEF responses at various time points after the initial stimulation. Scale bar in A is 10 µm.
ChR2/H134R
ChR/H134R has a modest reduction in desensitisation, a slight increase in light sensitivity, and slower channel closing compared to ChR2 (Nagel et al., 2005; Lin et al., 2009b; Lin et al., 2009a). These changes increase photocurrents, but the slower kinetics makes ChR2/H134R less temporally precise than ChR2. Although not tested, ChR2/H134R is assumed to have the same ion permeability and recovery from desensitisation as ChR2. ChR2/H134R has no advantages over the newer ChEF and ChIEF variants (Lin et al., 2009b).
ChR2/C128X(X = T/A/S), and ChR2/D156A
The introduction of C128X (Berndt et al., 2009) or D156A mutation (Bamann et al., 2010) to ChR2 increases light sensitivity, but slows kinetics (off-rate 2–100 s) and reduces photocurrent. The channel closure rate can be modestly sped up with orange light. The first published study described ChR2/C128X as ‘step-function’ or ‘bistable’ opsins, and these variants were used to induce prolonged subthreshold depolarisation (Berndt et al., 2009). A recent study reveals ChR2/C128X recovers little from the strong desensitisation in the dark, which is not ‘bistable’ as initially described (Schoenenberger et al., 2009). Despite these complications, ChR2/C128X or ChR2/D156A are useful for inducing prolonged depolarisation when temporal precision and depolarisation level are not critical (Schoenenberger et al., 2009).
ChR2/E123T (ChETA)
The E123T mutation in ChR2 (ChETA) creates faster kinetics but reduces photocurrent amplitude (Gunaydin et al., 2010). ChETA has strong desensitisation in our hands and much reduced light sensitivity (Figure 2 and Table 1). The reduced photocurrent might be beneficial, as it decreases the incidence of depolarisation block associated with overexpression of ChR2. The first published result with ChETA in neurons incorporates the H134R mutation to increase the amplitudes of photocurrent, but the H134R mutation also negates some increases in kinetics. The first publication described ChETA as useful for high frequency stimulation, but whether ChETA provides any advantages over ChD or ChIEF is unclear. The reduced photocurrent, red-shifted spectral response and reduced sensitivity of ChETA may be ideal for performing simultaneous calcium imaging experiments with Fura-2 (Zhang et al., 2006).
Table 1.
Channel and kinetic properties of ChR2, ChR2/H134R, ChETA, VChR1, ChD, ChEF and ChIEF.
| Response Spectra Peak |
Level of Desensitisatio n |
Light Sensitivity / EC50 | Opening Rate τ (ms) |
Closing Rate τ (ms) |
|||
|---|---|---|---|---|---|---|---|
| Peak Response |
Steady- state Response |
Isteady-state / IPeak |
Peak Response |
Steady-state Response |
19.8mW/mm2 Light Intensity |
||
| ChR2 | ~470 nm | ~450 nm | ~ 0.22 (470 nm) |
~ 1.10 mW/mm2 |
~ 1.05 mW/mm2 |
~ 1.21 ms | ~13.5 ms |
| ChR2/H134R | ~450 nm | ~450 nm | ~ 0.39 (470 nm) |
~ 1.07 mW/mm2 |
~ 0.98 mW/mm2 |
~ 1.92 ms | ~ 17.9 ms |
| ChETA | ~490 nm # | ~ 0.24 (470nm) + |
~ 5.02 mW/mm2 + |
~ 0.62 mW/mm2 + |
~ 0.86 ms + | ~7.9–8.5 ms #+ |
|
| VChR1 | ~570 nm | ~550 nm | ~ 0.48 (570nm) |
Not tested | Not tested | ~ 2.8 ms (15mW/mm2**) |
> 90 ms ** |
| ChD | ~450 nm | ~450 nm | ~ 0.31 (470nm) |
~ 3.23 mW/mm2 |
~ 1.02 mW/mm2 |
~ 1.49 ms | ~ 7.82 ms |
| ChEF | ~470 nm | ~490 nm | ~ 0.70 (470 nm) |
~ 0.72 mW/mm2 |
~ 0.46 mW/mm2 |
~ 1.56 ms | ~ 24.9 ms |
| ChIEF | ~450 nm | ~450 nm | ~ 0.80 (470 nm) |
~ 1.65 mW/mm2 |
~ 1.38 mW/mm2 |
~ 1.62 ms | ~ 12.0 ms |
Most values are from Lin et al. 2009 and 2009b, except for # from Gunaydin et al. 2010, ** from Zhang et al. 2008 and + unpublished results.
VChR1
This Volvox cateri ChR was initially characterised as a red-shifted ChR variant with peak response of ~530nm (Zhang et al., 2008). We found with same photon flux for stimulation and 410nm preconditioning light to induce full recovery, the peak response occurs at ~570 nm and the steady-state response at ~550 nm (Figure 3)(Lin et al., 2009a). Despite the red-shift, VChR1 is excited strongly by 400 nm light and simultaneous use with a second ChR or calcium imaging is not possible without cross-excitation. VChR1 also has the following limitations: 1) Slow channel kinetics, leading to temporal imprecision with photostimulation (Zhang et al., 2008); 2) Incomplete recovery from the desensitised state (Lin et al., 2009a); and 3) Poor membrane trafficking and expression (Figure 3)(Lin et al., 2009a; Tsunoda and Hegemann, 2009). Introducing the E123T (ChETA mutation) in VChR1 modestly improves the kinetics of VChR1 (~35 ms off-rate, unpublished observation). Currently, VChR1 has limited utility, but is an useful template to engineer improved red-shifted ChR variants.
ChEF and ChIEF
ChEF and ChIEF variants were engineered by chimeragenesis of ChR1 and ChR2 (Lin et al., 2009b). Both ChEF and ChIEF have increased steady-state phase responses without reduction of the peak photocurrent. ChEF and ChIEF have the most consistent photocurrent responses when stimulated with continuous light or high frequency pulsed light (Figure 2)(Lin et al., 2009b). The kinetics of ChIEF is faster than ChEF, but comparable to ChR2 (Figure 2). ChIEF has slightly reduced light sensitivity relative to ChR2 and should not interfere with its use. The desensitised response of ChEF and ChIEF do not fully recover in the dark, but recovery of the photo-response can be induced by 570 nm light (Lin et al., 2009b). The greater steady-state responses of CHEF and ChIEF compensate for the desensitised peak response despite incomplete recovery. ChEF and ChIEF express better than ChR2 in mammalian cells and traffick efficiently to the membrane (Lin et al., 2009a; Wang et al., 2009). Efficient membrane trafficking may result in toxicity when ChEF and ChIEF are expressed at high levels. ChIEF is the best ChR for conducting experiments with high frequency, repetitive stimulation or continuous illumination, because the response amplitudes are most consistent with millisecond time-scale kinetics (Figure 2).
Other ChR variants: ChR1, VChR2 and ChD
ChR1 was the first ChR characterised ex vivo (Nagel et al., 2002) (Berthold et al., 2008; Tsunoda and Hegemann, 2009). The low conductance of ChR1 at pH7 limits its use as a scientific tool.
Volvox carteri VChR2 (Ernst et al., 2008) has similar properties as ChR2, but has slower kinetics (Lin et al., 2009a). VChR2 has no obvious advantages over other ChRs.
The ChD variant was generated by the chimeragenesis of ChR1 and ChR2 (Wang et al., 2009; Lin et al., 2009b). ChD is comparable to ChETA in kinetics, but has better membrane trafficking/expression (Wang et al., 2009; Lin et al., 2009b). ChD may be used similarly to ChETA (Figure 2), but does not require the H134R mutation to increase the photocurrent.
The properties of the ChR variants are summarised in Table 1.
Future developments of ChR variants as research tools
The following improvements should enhance the utility of the ChR technology:
Higher conductance: An increased conductance would enhance efficiency and reduce the need for overexpression.
Change ion selectivity: ChRs selectively permeate chloride or potassium ions would theoretically hyperpolarise the membrane when activated. The theoretical efficiency of these ChRs would be higher than the current microbial opsin pumps (Zhang et al., 2007). Calcium-selective and calcium-impermeable ChRs would be useful in signal transduction and imaging experiments, respectively.
Improving kinetics without sacrificing light sensitivity: The ideal ChR would have a rectangular response with rapid kinetics, a true ‘step function opsin’, and these mutations would not reduce light sensitivity.
Narrowing VChR1 spectral responses: Reducing the response of VChR1 to blue light (~470 nm) will allow for the simultaneous use with calcium imaging dyes and other ChRs in the same preparation without cross-excitation.
Red-shift the spectral response above 600 nm: Peak and steady-state responses above 600nm would allow for stimulation in deep tissue with minimal light absorption and scattering. VChR1 can respond to light above 600 nm, but its kinetics and properties at this wavelength greatly limit its usefulness (Figure 3).
The use of ChRs allows for the manipulation of membrane potential in genetically-defined cells with light. ChRs can be invaluable research tools of the future as indicated by the increased literatures utilising this technology. While many ChR variants have been developed, many characteristics still need to be optimised to generate better ChRs. Care should be taken to characterise new variants and compare to known variants to ensure adequate information for the users of these tools.
Supplementary Material
Acknowledgments
Acknowledgements and additional notes
J.Y. Lin received funding from the Foundation of Research, Science and Technology of New Zealand. Research is supported by grants to Roger Y. Tsien from NIH (NS027177) and Howard Hughes Medical Institute. I thank Drs. S.B. Sann and E.A. Rodriguez for editorial assistance.
Table 1, Figures 2 and 3 were modified with permission from Lin et al. 2009a.
ChD, ChEF and ChIEF Constructs can be requested from http://www.tsienlab.ucsd.edu. or requested from John Y. Lin (j8lin@ucsd.edu).
References
- Bamann C, Kirsch T, Nagel G, Bamberg E. Spectral Characteristics of the Photocycle of Channelrhodopsin-2 and Its Implication for Channel Function. Journal of molecular biology. 2008 doi: 10.1016/j.jmb.2007.10.072. [DOI] [PubMed] [Google Scholar]
- Bamann C, Gueta R, Kleinlogel S, Nagel G, Bamberg E. Structural guidance of the photocycle of channelrhodopsin-2 by an interhelical hydrogen bond. Biochemistry. 2010;49:267–278. doi: 10.1021/bi901634p. [DOI] [PubMed] [Google Scholar]
- Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K. Bi-stable neural state switches. Nat Neurosci. 2009;12:229–234. doi: 10.1038/nn.2247. [DOI] [PubMed] [Google Scholar]
- Berthold P, Tsunoda SP, Ernst OP, Mages W, Gradmann D, Hegemann P. Channelrhodopsin-1 initiates phototaxis and photophobic responses in chlamydomonas by immediate light-induced depolarization. Plant Cell. 2008;20:1665–1677. doi: 10.1105/tpc.108.057919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Ernst OP, Sanchez Murcia PA, Daldrop P, Tsunoda SP, Kateriya S, Hegemann P. Photoactivation of channelrhodopsin. J Biol Chem. 2008;283:1637–1643. doi: 10.1074/jbc.M708039200. [DOI] [PubMed] [Google Scholar]
- Feldbauer K, Zimmermann D, Pintschovius V, Spitz J, Bamann C, Bamberg E. Channelrhodopsin-2 is a leaky proton pump. Proc Natl Acad Sci U S A. 2009;106:12317–12322. doi: 10.1073/pnas.0905852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin LA, Yizhar O, Berndt A, Sohal VS, Deisseroth K, Hegemann P. Ultrafast optogenetic control. Nat Neurosci. 2010;13:387–392. doi: 10.1038/nn.2495. [DOI] [PubMed] [Google Scholar]
- Ishizuka T, Kakuda M, Araki R, Yawo H. Kinetic evaluation of photosensitivity in genetically engineered neurons expressing green algae light-gated channels. Neurosci Res. 2006;54:85–94. doi: 10.1016/j.neures.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, Hegemann P, Landmesser LT, Herlitze S. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc Natl Acad Sci U S A. 2005;102:17816–17821. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Yang J, Tsien RY. Society for Neuroscience. Chicago: 2009a. Development and characterization of channelrhodopsin variants for improved optical control of neuronal excitability. [Google Scholar]
- Lin JY, Lin MZ, Steinbach P, Tsien RY. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys J. 2009b doi: 10.1016/j.bpj.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol. 2005;15:2279–2284. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AM, Bamberg E, Hegemann P. Channelrhodopsin-1: a light-gated proton channel in green algae. Science. 2002;296:2395–2398. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenenberger P, Gerosa D, Oertner TG. Temporal control of immediate early gene induction by light. PLoS ONE. 2009;4:e8185. doi: 10.1371/journal.pone.0008185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda SP, Hegemann P. Glu 87 of channelrhodopsin-1 causes pH-dependent color tuning and fast photocurrent inactivation. Photochem Photobiol. 2009;85:564–569. doi: 10.1111/j.1751-1097.2008.00519.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Sugiyama Y, Hikima T, Sugano E, Tomita H, Takahashi T, Ishizuka T, Yawo H. Molecular determinants differentiating photocurrent properties of two channelrhodopsins from chlamydomonas. J Biol Chem. 2009;284:5685–5696. doi: 10.1074/jbc.M807632200. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat Methods. 2006;3:785–792. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- Zhang F, Prigge M, Beyriere F, Tsunoda SP, Mattis J, Yizhar O, Hegemann P, Deisseroth K. Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri. Nat Neurosci. 2008 doi: 10.1038/nn.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.