Abstract
Objective
Infiltrating immune cells play a central role in degenerative joint disease associated with osteoarthritis (OA) and peri-prosthetic particle-mediated osteolysis. The goal of this study was to characterize a newly identified population of synovial tissue infiltrating NK cells in OA and peri-prosthetic joint inflammation.
Methods
Synovial and interfacial tissues were collected from OA patients undergoing primary and revision total joint replacement (TJR). Histological features of primary OA synovium and revision interfacial tissues were determined by immunohistochemistry and immunofluorescence. Synovial tissue infiltrating NK cells were evaluated for expression of surface receptors by flow cytometry. Chemoattractant and cytokine protein and RNA levels in synovial/interfacial tissues/fluids were assessed by Luminex and RT-QPCR. Cytokine production and degranulation by stimulated synovial tissue vs. normal blood NK cells was evaluated by intracellular cytokine staining.
Results
NK cells comprised nearly 30% of the CD45+ mononuclear cell infiltrate in synovial tissues from both primary and revision TJR. NK cells from both groups expressed CXCR3, CCR5, L-selectin, α4 integrins, and CLA. Synovial fluid (SF) from revision patients contained elevated concentrations of NK cell attractants CCL4, CCL5, CXCL9, CXCL10, and chemerin, all higher than in primary SF. Cytokine-stimulated IFN-γ production was significantly impaired in primary and revision NK cells compared to blood NK cells.
Conclusions
NK cells are a principal tissue infiltrating lymphocyte subset in OA and peri-prosthetic inflammation, and display a quiescent phenotype consistent with post-activation exhaustion.
Patients with degenerative joint disease (most commonly OA) that is refractory to conservative management are candidates for TJR, which alleviates pain and restores limb function in >80% of cases (1, 2). Aseptic loosening of joint prostheses is the most significant long-term complication, occurring in 7–18% of patients at 25-year follow up (1, 2), and requires many to undergo revision surgery (3). The loss of bony fixation is due to inflammatory responses triggered by wear particles generated at the bearing surfaces and along the implant interface (4).
Although NK cells are classically known for their role in killing virally-infected cells or tumor cells, NK cell infiltrates have been identified and characterized in a variety of other pathologic settings, such as psoriatic skin and RA SF (5, 6). In one study, CD56+ CD16− NK cells comprised 16% of the infiltrating lymphocytes in RA SF (7), and most expressed the activation marker CD69 and the cytotoxic receptor NKp44, and lacked the inhibitory killer cell Ig-like receptors (KIRs) CD158a and CD158b (6, 7), and 25% produced IFN-γ upon stimulation in vitro, suggesting that the cells are active participants in joint inflammation.
The recruitment of NK cells to peripheral inflammatory sites is dependent on cell-expressed chemoattractant receptors and adhesion molecules, as well as chemoattractants present at the site. In peripheral blood, immunoregulatory CD16− NK cells express CXCR3, CCR5, and CCR7 and migrate to CXCL9–11, CCL3–5, CCL19, and CCL21 in vitro (8). NK cells in inflamed joint SF were reported to express CCR5 and CXCR3, and chemokine ligands for these receptors have been detected in RA SF and synovial tissue (7, 9), suggesting a role for CCR5 and CXCR3 in NK cell recruitment to the joint.
Here we show that CD45+CD56+CD3− NK cells are a principal leukocyte infiltrate in synovial tissue from OA patients undergoing primary and revision TJR. We propose that the infiltrating CD56+ cells are recruited immunoregulatory NK cells, and that chronic activation in the joint environment leads to exhaustion of their capacity to secrete IFN-γ.
MATERIALS AND METHODS
Human Sample Collection
Synovial tissues and fluid normally discarded during TJR surgery were obtained from 18 OA patients and 22 revision joint replacement patients (See Supplementary Table 1 for patient demographics and additional information). PBMC were obtained from healthy blood donors (Stanford Blood Center). The Institutional Review Board at Stanford University School of Medicine (Stanford, CA) approved all human subject protocols.
Histological Analysis
Synovial tissue specimens from 4 patients undergoing primary joint replacement and 9 patients undergoing revision joint replacement were fixed, in 10% formalin, and paraffin blocks were prepared, sectioned, and stained with H&E by Histo-Tec laboratory (Hayward, CA). For immunofluorescence staining, 8-μm cryostat sections were stained with directly conjugated antibodies as previously described (10).
Isolation and Stimulation of Untouched NK Cells
Minced synovial tissues were cultured in 60 U/mL rhIL-2 (eBioscience) for 4 days, and untouched NK cells from synovial tissues or freshly isolated PBMC were isolated via negative depletion with magnetic beads (Miltenyi Biotec), with a purity of >95% CD3−CD56+. Purified NK cells were then cultured with 20 U/mL rhIL-2 for 48 hours prior to addition of one of the following cytokine combinations: IL-12 (10 ng/mL) and IL-15 (10 ng/mL), IL-12 and IL-18 (100 ng/mL), or IL-15 and IL-18 (eBioscience). After 18 hours, cells were incubated for 1 hour at 37°C with LAMP-1-PE antibody (20uL/mL, BD Biosciences). Monensin (50 mM, eBioscience) and Brefeldin A (10 μg/mL, Sigma) were added to cultures for an additional 4 hours. NK cells were then stained for surface markers, washed, fixed and permeabilized with Cytofix/Cytoperm (BD PharMingen), and stained for intracellular cytokines.
Luminex Assay
SF samples were diluted 1:4 in PBS and analyzed, in duplicate, by the Stanford Human Immune Monitoring Center using a 50-plex analyte platform. The coefficient of variation was below 15% in >95% of replicates.
RESULTS
Natural killer cells infiltrate inflamed synovial tissue
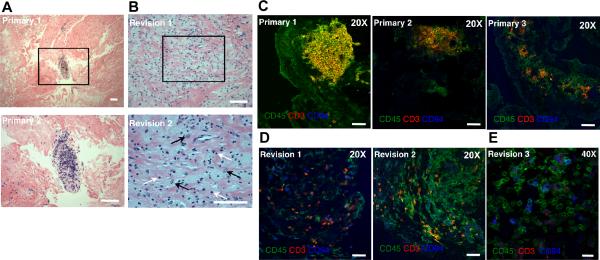
Inflamed synovial tissue from OA patients undergoing either primary or revision TJR contained dense bands of fibrous tissue interspersed with aggregates of macrophages and lymphocytes (Fig.1A,B). CD45+CD3+CD94− T lymphocytes were organized in discrete patches in primary synovium, with CD45+CD3−CD94+ NK cells located on the periphery of these clusters (Fig.1C). In contrast, in revision interfacial tissues, lymphocytes were instead scattered throughout the specimens and were primarily CD3+CD94− T cells or CD3−CD94+ NK cells (Fig.1D,E).
FIGURE 1.
Histological features of primary OA synovium and revision interfacial tissues. Paraffin sections of synovial tissues from primary (A) or revision (B) TJR were stained with H&E to evaluate the synovial tissue architecture. (A) Lymphocytes in primary synovial tissues congregated in clusters among masses of largely acellular fibrous tissue. An enlargement of the boxed region is shown in the lower panel (white bar =100μm). (B) In contrast, lymphocytes in revision interfacial samples were scattered throughout the tissue (2 different patients shown). An enlargement of the boxed region from revision sample 2 is displayed in the bottom panel (white bar =100μm). White arrows indicate some of the infiltrating lymphocytes; black arrows indicate some of the macrophages. Cryostat sections (8 mm) of joint tissues from primary (C) or revision (D, E) patients were stained for CD45 (FITC), CD3 (PE), and CD94 (APC). (C) CD3+ lymphocytes were organized in lymphocyte clusters in primary synovial samples (3 different patients). (D) CD3+ T cells and CD3−CD94+ NK cells were scattered throughout revision interfacial tissues (2 different patients); white bar = 10μm. An enlargement of a stained revision section is shown in (E) (white bar = 20μm).
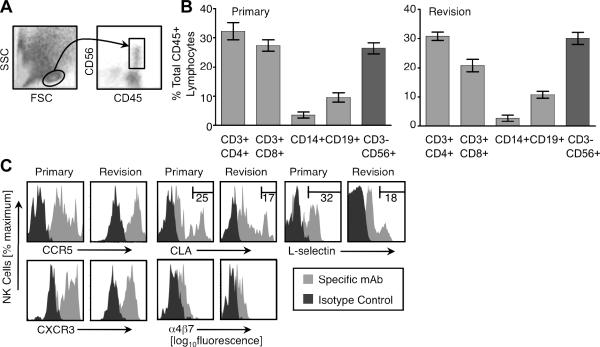
Synovial tissue specimens were mechanically dissociated and single cell suspensions were analyzed by flow cytometry. Although we cannot formally exclude effects of differential recovery during isolation from tissues, we were surprised to find that CD56+CD3− NK cells comprised nearly 30% of the CD45+ lymphocytes (light scatter gated) in both primary and revision tissue samples (Fig. 2A,B). Approximately 30% of infiltrating synovial tissue lymphocytes were CD3+CD4+ T cells, 20–30% were CD3+CD8+ T cells, 2–5% were CD14+ monocytes, and ~10% were CD19+ B cells with no significant differences in leukocyte subset percentages between primary and revision infiltrates (Fig. 2B).
FIGURE 2.
NK cells infiltrate primary and revision synovial joint tissue. (A) Freshly isolated synovial tissue leukocytes were harvested and analyzed by flow cytometry. A lymphocyte gate was established based on forward and side light scatter (FSC and SSC, respectively), and CD45 was used to identify leukocytes; CD56 co-staining is shown. (B) The percentage of the indicated CD-marker defined lymphocyte subset (of total CD45+ cells in the lymphocyte-light scatter gate) in primary (top panel) and revision patient (bottom panel) synovial samples is shown. The mean ± SD is displayed, n= 6 for each group. (C) Joint tissue-infiltrating NK cells (CD45+CD3−CD56+) were stained with antibodies for the indicated chemoattractant or homing receptors. Histograms are representative of n= 3 patients per group.
We next investigated the expression of chemoattractant and homing receptors that might contribute to the recruitment of NK cells to inflamed joints. Infiltrating synovial tissue NK cells from both patient groups expressed chemoattractant receptors CCR5 and CXCR3 (Fig. 2C), and were negative for CCR7 and CXCR1 (data not shown), receptors previously shown to be expressed by blood NK cells (11). Most synovial tissue NK cells also expressed low levels of cutaneous lymphocyte antigen (CLA), L-selectin (CD62L), and α4β7, while subsets in both patient groups expressed high levels of CLA (25% vs. 17%, primary vs. revision) and L-selectin (32% vs. 18%, primary vs. revision) (Fig. 2C).
NK cell chemoattractants in inflamed synovial tissue and fluid
We evaluated the protein levels and RNA expression of known NK cell chemoattractants. Synovial fluid from both patient groups contained detectable protein levels of CCL3 (35±5 vs. 73±13 pg/ml; mean ± SEM, primary vs. revision SF, respectively), CCL4 (223±36 vs. 1021±353 pg/ml), and CCL5 (541±233 vs. 2940±1507 pg/ml) (CCR5 ligands); CXCL9 (54±13 vs. 524±266 pg/ml) and CXCL10 (537±120 vs. 4697±1990 pg/ml) (CXCR3 ligands); and chemerin (12± 2 vs. 9± 1 pg/ml) (CMKLR1 ligand) (Suppl. Fig. 1A). SF from revision patients contained significantly higher concentrations of CCL3, CCL4, and CXCL10 (Suppl. Fig. 1A). Furthermore, SF from revision joints contained significantly more bioactive chemerin than primary SF, as measured by CMKLR1+ cell migration (Suppl. Fig. 1B).
We also investigated the RNA expression of NK cell chemoattractants in synovial tissue by RT-QPCR. Chemerin had the highest relative RNA expression level, followed by CCL4, and there were no significant differences in expression between primary and revision for these attractants, or for CXCL9, CXCL10, or CXCL11 (Suppl. Fig. 1C). In contrast, CCL3 and CCL5 were significantly elevated in the revision samples (Suppl. Fig. 1C).
Immunophenotypic characterization of infiltrating synovial NK cells
Although the relative expression of CD56 (i.e. CD56bright vs. CD56dim) can define functional blood NK cell subsets, synovial tissue NK staining for CD56 was unimodal. CD45+CD3−CD56+ NK cells from both patient groups uniformly expressed CD27, CD94, low levels of NKp30 and NKp46, and were negative for NKp44, NKG2A, NKG2C, NKG2D, CD25, CD69, CD158a (KIR2DL1), and CD158b (KIR2DL2) (Suppl Fig. 2 and data not shown). Freshly isolated synovial tissue NK cells also failed to express intracellular perforin or granzymes, unlike CD16+CD56dim blood NK cells (data not shown). Thus, the majority of synovial tissue-infiltrating NK cells express a combination of surface receptors consistent with a non-cytotoxic, immunoregulatory phenotype (similar to blood CD16–CD56bright CD27+ NK cells)(summarized in Suppl. Table 2).
Synovial tissue NK cells show reduced IFN-γ production in response to activating cytokines
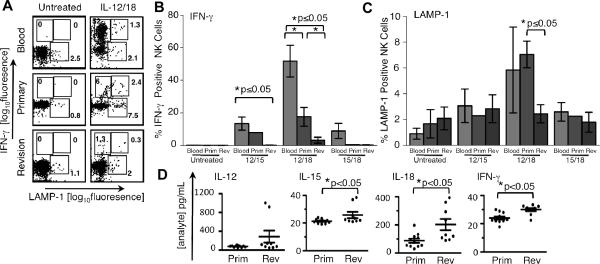
NK cells purified from synovial tissue were expanded with IL-2 and stimulated with cytokine combinations known to trigger IFN-γ production and degranulation. Synovial tissue and blood NK cells were left untreated or incubated with IL-12/IL-15, IL-12/IL-18, or IL-15/IL-18, and stained for intracellular IFN-γ and for surface LAMP-1, a marker of degranulation. In general, NK cells from revision and primary synovial tissue were less responsive to cytokine activation than blood NK cells. IL-12/IL-18 was the most potent cytokine combination tested, triggering IFN-γ production in 52% of blood NK cells, while only 15% of primary synovial tissue NK cells and 3% of revision synovial tissue NK cells were IFN-γ+ (Fig. 3A,B). Treatment with IL-12/IL-15 showed a similar trend, with 13% of blood NK cells IFN-γ+ after treatment, vs. 8% of primary synovial NK cells and <1% of revision synovial NK cells. Only blood NK cells responded to treatment with IL-15/IL-18 (9% IFN-γ+)(Fig. 3B).
FIGURE 3.
Synovial joint NK cells do not efficiently produce IFN-γ in response to stimulating cytokines. (A–C) Purified NK cells from blood and synovial tissues were stimulated as indicated. (A) Flow cytometric analysis of intracellular IFN-γ vs. surface expression of the degranulation marker, LAMP-1. Dot plots are representative of n=3 surgical patients or blood samples, except for primary synovial NK samples treated with IL-12/15 and IL-15/18, where n=2. (B and C) Percent of IFN-γ+ NK cells (B) or LAMP-1+ NK cells (C). Blood: peripheral blood NK Cells; Prim: primary synovial tissue NK cells; Rev: revision synovial tissue NK cells. For (B and C), error bars represent SEM where n=3 samples or more, and range where n=2. (D) The concentration of IL-12p40, IL-15, IL-18, and IFN-γ was assessed in primary SF (Prim, n=11) and revision SF (Rev, n=9) by Luminex. The circle symbols correspond to individual patient samples (mean of duplicate wells); the overall mean ± SEM within patient groups is shown. *p<0.05 by Student's t-test or Mann-Whitney rank sum test. For comparison, the reported ranges for IFN-γ in OA, RA, and spondyloarthropathy SF are 10–100 pg/ml, 20–900 pg/ml, and 1,000–5,000 pg/ml, respectively (15).
In contrast to the robust induction of IFN-γ in blood NK cells by IL-12/IL-18, LAMP-1 expression was only modestly increased by treatment with this cytokine combination (6% vs. 1% in the untreated controls) (Fig. 3A,C). IL-12/IL-18 induced significantly more degranulation in primary NK cells vs. revision NK cells (7% vs. 2%, respectively; untreated controls, 2% vs. 2%) (Fig. 3A,C). Treatment with IL-12/IL-15 or IL-15/IL-18 had little effect on LAMP-1 staining on any of the NK cells tested (Fig. 3C).
We next asked if NK cell-activating cytokines were present in vivo. Synovial fluid from both patient groups contained detectable levels of IL-12p40 (79± 6 vs. 288±128 pg/ml; mean ± SEM, primary vs. revision, respectively), IL-15 (21±1 vs. 26±2 pg/ml) and IL-18 (88±15 vs. 203±40 pg/ml) (Fig. 3D). SF from revision patients contained significantly higher concentrations of IL-15 and IL-18 compared to primary SF (Fig. 3D). Finally, we found that revision SF contained significantly more IFN-γ than primary SF (24±1 vs. 30±1 pg/ml), although the levels were low in both cases (Fig. 3D).
DISCUSSION
At least four notable observations have emerged from our analyses of surgically excised synovial tissues and SF samples in patients undergoing primary and revision arthroplasty: 1) NK cells are a principal synovial tissue infiltrating lymphocyte subset in patients with OA and particle-mediated peri-prosthetic inflammation; 2) the infiltrating tissue NK cells are functionally distinct from blood NK cells, and respond poorly to stimulation as measured by IFN-γ production; 3) SF from the patients contains NK cell chemoattractants and NK cell-activating cytokines; and 4) synovial tissue NK cells express CCR5 and CXCR3.
Chemoattractant receptors, attractant ligands, and adhesion molecules contribute to the accumulation of NK cell accumulation at peripheral sites. In human psoriatic skin, production of CCL5 and CXCL10 secreted by activated keratinocytes leads to recruitment of CCR5+ CXCR3+ NK cells (5). Similar to psoriatic skin infiltrating NK cells, we show that synovial tissue NK cells are CCR5+ CXCR3+. High levels of CXCR3 and CCR5 ligands present in revision SF, as well as evidence of particle-induced chemokine production by macrophages (12), suggest a mechanism for NK recruitment during peri-prosthetic osteolysis.
We propose that synovial tissue infiltrating NK cells in both primary OA and osteolytic revision TJR patients are recruited immunoregulatory-type NK cells. Synovial tissue NK cells from both primary and revision TJR patient groups were positive for CD27 and CD94, surface markers expressed on immunoregulatory blood NK cells, and were negative for intracellular perforin, granzyme B, and KIRs normally expressed by cytotoxic effector blood NK cells (summarized in Suppl. Table 2).
Recruited NK cells can lose their ability to express IFN-γ as a consequence of chronic stimulation by (and desensitization to) activating ligands and pro-inflammatory mediators, and enter a state of “post-activation exhaustion” (13). For example, in studies of a mouse model of diabetes, NK-cell-activating cytokines IL-12, IL-15, and IL-18 were found at high levels in mouse pancreatic islets, but a majority of islet-infiltrating NK cells displayed poor IFN-γ production and expressed significantly lower levels of LAMP-1 following in vitro stimulation (14). Similar to pancreatic islet inflammation, we report that synovial fluid contains NK cell activating cytokines, and that synovial tissue infiltrating NK cells displayed impaired IFN-γ production when stimulated in vitro (Fig. 3). This characteristic NK cell quiescence is consistent with post-activation exhaustion, and may relate to their role or history in the pathophysiology of joint inflammation. The similarity in NK cell phenotype between our two patients groups may reflect a common background of chronic OA, with the more severe joint inflammation in the revision patients tracking with increased NK cell exhaustion in the joint. Given the central role of IFN-γ in osteoclastogenesis and bone resorption in the joint, additional characterization of NK cells in degenerative joint disease may provide insight into the pathophysiology of severe joint inflammation, and offer new avenues for therapeutic intervention.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Sheri Krams for helpful discussions on the topic of NK cells.
Grant Supporters: This work is supported by NIH (AI-59635, AI-47822, and GM-37734); Specialized Center of Research Grant HL-67674; Digestive Disease Center Grant DK-56339; and a Merit Award from the Veterans Administration to E.C.B. B.A.Z. is supported by NIH grant AI-079320; a pilot award from the Digestive Disease Center; and the SPARK Program (Stanford University). B.A.Z. has received salary and stock options from ChemoCentryx, Inc. S.B.G. is supported by NIH grant R01AR55650 and the Ellenburg Chair in Surgery from Stanford University. R.S.H. was supported by the HHMI Medical Fellows program and the Stanford University Medical Scholars Program.
REFERENCES
- 1.Schulte KR, Callaghan JJ, Kelley SS, Johnston RC. The outcome of Charnley total hip arthroplasty with cement after a minimum twenty-year follow-up. The results of one surgeon. J Bone Joint Surg Am. 1993;75(7):961–75. doi: 10.2106/00004623-199307000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Callaghan JJ, Bracha P, Liu SS, Piyaworakhun S, Goetz DD, Johnston RC. Survivorship of a Charnley total hip arthroplasty. A concise follow-up, at a minimum of thirty-five years, of previous reports. J Bone Joint Surg Am. 2009;91(11):2617–21. doi: 10.2106/JBJS.H.01201. [DOI] [PubMed] [Google Scholar]
- 3.Huddleston JI, Maloney WJ, Wang Y, Verzier N, Hunt DR, Herndon JH. Adverse events after total knee arthroplasty: a national Medicare study. J Arthroplasty. 2009;24(6 Suppl):95–100. doi: 10.1016/j.arth.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Purdue PE, Koulouvaris P, Nestor BJ, Sculco TP. The central role of wear debris in periprosthetic osteolysis. HSS J. 2006;2(2):102–13. doi: 10.1007/s11420-006-9003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ottaviani C, Nasorri F, Bedini C, de Pita O, Girolomoni G, Cavani A. CD56brightCD16(−) NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. Eur J Immunol. 2006;36(1):118–28. doi: 10.1002/eji.200535243. [DOI] [PubMed] [Google Scholar]
- 6.Pridgeon C, Lennon GP, Pazmany L, Thompson RN, Christmas SE, Moots RJ. Natural killer cells in the synovial fluid of rheumatoid arthritis patients exhibit a CD56bright, CD94bright, CD158negative phenotype. Rheumatology (Oxford) 2003;42(7):870–8. doi: 10.1093/rheumatology/keg240. [DOI] [PubMed] [Google Scholar]
- 7.Dalbeth N, Callan MF. A subset of natural killer cells is greatly expanded within inflamed joints. Arthritis Rheum. 2002;46(7):1763–72. doi: 10.1002/art.10410. [DOI] [PubMed] [Google Scholar]
- 8.Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR, et al. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001;166(11):6477–82. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- 9.Iwamoto T, Okamoto H, Toyama Y, Momohara S. Molecular aspects of rheumatoid arthritis: chemokines in the joints of patients. FEBS J. 2008;275(18):4448–55. doi: 10.1111/j.1742-4658.2008.06580.x. [DOI] [PubMed] [Google Scholar]
- 10.Spanogle JP, Miyanishi K, Ma T, Epstein NJ, Smith RL, Goodman SB. Comparison of VEGF-producing cells in periprosthetic osteolysis. Biomaterials. 2006;27(21):3882–7. doi: 10.1016/j.biomaterials.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 11.Parolini S, Santoro A, Marcenaro E, Luini W, Massardi L, Facchetti F, et al. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood. 2007;109(9):3625–32. doi: 10.1182/blood-2006-08-038844. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima Y, Sun DH, Trindade MC, Chun LE, Song Y, Goodman SB, et al. Induction of macrophage C-C chemokine expression by titanium alloy and bone cement particles. J Bone Joint Surg Br. 1999;81(1):155–62. doi: 10.1302/0301-620x.81b1.8884. [DOI] [PubMed] [Google Scholar]
- 13.Oppenheim DE, Roberts SJ, Clarke SL, Filler R, Lewis JM, Tigelaar RE, et al. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6(9):928–37. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 14.Brauner H, Elemans M, Lemos S, Broberger C, Holmberg D, Flodstrom-Tullberg M, et al. Distinct phenotype and function of NK cells in the pancreas of nonobese diabetic mice. J Immunol. 2010;184(5):2272–80. doi: 10.4049/jimmunol.0804358. [DOI] [PubMed] [Google Scholar]
- 15.Schlaak JF, Pfers I, Meyer Zum Buschenfelde KH, Marker-Hermann E. Different cytokine profiles in the synovial fluid of patients with osteoarthritis, rheumatoid arthritis and seronegative spondylarthropathies. Clin Exp Rheumatol. 1996;14(2):155–62. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.