Abstract
Objective
To assess therapeutic benefits for intervertebral disc matrix repair and regeneration, the potential synergism of IGF-1 and BMP7 on bovine spine discs were evaluated, and molecular/cellular mechanisms were elucidated.
Methods
Bovine nucleus pulposus (NP) cells were treated with BMP7 and IGF-1. The subsequent anabolic effects driven by NP cells were assessed for proteoglycan synthesis by 35S-sulfate incorporation and accumulation by DMMB assays, respectively. Matrix formation was visualized by particle exclusion assay. Key matrix components and transcription factors were analyzed by real-time PCR to determine the signaling pathways by which IGF-1 suppresses noggin, a potent inhibitor of BMP7. Western blot and nuclear translocalization experiments were performed to assess the activation of SMAD proteins.
Results
Stimulation of bovine NP cells by both IGF-1 and BMP7 greatly potentiates anabolism through complementary and synergistic mechanisms on matrix formation when compared to treatment with either growth factor alone. Exogenously added decoy ligand, noggin attenuates the anabolic effects of BMP7, and noggin is substantially increased by BMP7, suggesting a negative feedback regulatory mechanism. On the other hand, IGF-1 significantly suppresses noggin expression via the PI3K/Akt pathways and thus potentiating BMP7 signaling in bovine NP cells. Upon combination treatment, IGF-1 activates SMAD2, while BMP7 activates SMAD1/5/8 and SMAD3, thus inducing all SMAD signaling pathways and mimicking the combinatorial effects of TGFβ plus BMP7.
Conclusion
Combination growth factor therapy using BMP7 and IGF-1 may have considerable promise in the treatment of spine disc degeneration.
Low back pain (LBP) associated with degenerative disc disease (DDD) is a common clinical problem that has major impact on today’s aging population. While the etiology of back pain is likely multi-factorial, it has been associated with intervertebral disc (IVD) degeneration (1–2). Research has shown that progressive breakdown of the extracellular matrix (ECM) is closely associated with disc degeneration (3). Therefore, biological treatments capable of promoting ECM repair and regeneration have been considered, and clinical trials for spine and joint cartilage repair are being conducted (4–5)
Degeneration of the IVD represents a loss of steady state metabolism that likely results from an imbalance between anabolic and catabolic processes (6). Increased expression of pro-inflammatory cytokines such as interleukin-1 (IL-1) (7) and gradual loss of proteoglycan (PG) from the NP have been observed in degenerative discs (8). These changes are linked to increased expression of matrix-degrading enzymes such as matrix metalloproteases (MMPs) and aggrecanases (a disintegrin and metalloprotease with thrombospondin motifs; ADAMTS), both of which are endogenously produced by spine disc cells (8).
One strategy to ameliorate progression of disc degeneration and loss of structural integrity is to shift the metabolic status from catabolic to anabolic by stimulating disc cells with growth factors (9). Anabolic regulators of IVD homeostasis include polypeptide growth factors such as insulin-like growth factor-1 (IGF-1) (10) and the bone morphogenetic proteins (BMPs) (11). BMP7, a member of the transforming growth factor-β (TGF-β) superfamily, is expressed in cartilage and exerts potent anabolic effects by stimulating differentiation and metabolic functions of both osteocytes and chondrocytes (12). BMP7 has similar anabolic effects by stimulating matrix biosynthesis in human adult articular chondrocytes (13), bovine IVD cells (14), rabbit IVD cells (15), and human IVD cells (16).
IGF-1 is a single chain polypeptide that is structurally similar to insulin, a key growth factor that enhances PG synthesis in articular cartilage (17). Osada and colleagues showed that IGF-1 stimulates PG synthesis in bovine NP cells in serum-free conditions in a dose-dependent manner and proposed an autocrine/paracrine mechanism of action (10). Further, Gruber and colleagues found that the addition of IGF-1 increased cell survival upon experimental induction of apoptosis in annulus fibrosus (AF) cells (18), consistent with the anti-catabolic capacity of IGF-1 in the IVD.
Loeser and colleagues noted that neither BMP7 nor IGF-1 alone are mitogenic in human adult articular cartilage, but BMP7 and IGF-1 together may modestly increase chondrocyte number and PG accumulation (19). The key question we addressed here is whether IGF-1 and BMP7 co-treatment can be developed as a combination growth factor therapy for treatment of IVD. Specifically, we assessed the biological and mechanistic effects of co-administering BMP7 and IGF-1 on cartilage homeostasis using bovine IVD as a pre-translational model. Our molecular analyses indicate that the combination of IGF-1 and BMP7 synergizes chondrocytic anabolic responses (i) by IGF-1-mediated inhibition of noggin, rescuing BMP7 from noggin inhibition, and (ii) by the activation of additional SMAD pathways, TGFβ-related Smad2/3, along with BMP-related Smad1/5/8 when these two growth factors are co-administered.
MATERIALS AND METHODS
IVD Cell Isolation and Culture
Tails from young adult bovine animals (15–18 months old) were purchased from a local slaughterhouse. Coccygeal discs were opened en bloc, and the NP of each disc was separated. The cells were released by enzymatic digestion in DMEM/Ham’s F-12 (1:1) culture medium with sequential treatments of 0.2% pronase and 0.025% collagenase P, as previously described (12). Three dimensional alginate bead culture that maintains chondrocytic phenotype and monolayers were prepared for long-term (for 21 days) and short-term (1–2 days) studies, respectively as we previously performed (3, 5, 12, 14). Triplicates were performed for each condition for alginate cultures and for monolayers with at least five independent experiments for each condition. For alginate bead culture, isolated disc cells were resuspended in 1.2% alginate, and beads were formed by dropwise addition into a CaCl2 solution as previously described (20, 21). Cells were treated with BMP7 100 ng/ml (Stryker Biotech, Hopkinton, MA, USA), IGF-1 100 ng/ml (R&D system, Minneapolis, MN, USA) and IL-1 1 ng/ml (Amgen, Thousand Oaks, CA). Triplicate wells were used for each condition. Media was changed every other day for a 21 day period before dimethylmethylene blue (DMMB) assay. For monolayer cultures, isolated NP cells were counted and plated onto 12-well plates at 8×105 cells/cm2 as previously described (12). The cells were treated with IGF-1 (1–100 ng/ml), BMP7 (100 ng/ml), noggin (100 ng/ml; PerproTech Inc., Rocky Hill, NJ, USA), a well-known BMP antagonist, Erk/MAPK pathway-specific inhibitor (PD98059, 25 µM; Calbiochem, Gibbstown, NJ, USA), PI3K/Akt pathway-specific inhibitor (LY294002, 50 nM; Calbiochem, Gibbstown, NJ, USA), and combinations of the above factors. Supernatants were collected 24 hrs after the initiation of each treatment and subjected to western blotting, as described below. Cells were treated for 24 hrs before total RNA harvesting.
Western blotting
Cell and tissue lysates were prepared using modified RIPA buffer as previously described (22). Total protein concentrations of cell lysates were determined by BCA protein assays (Pierce, Rockford, IL, USA). Equal amounts of protein were resolved by 10% SDS-PAGE and transferred to nitrocellulose membrane for western blot analyses as described previously (23). Immunoreactivity was visualized using the ECL system (Amersham Biosciences, Piscataway, NJ, USA).
Reverse Transcription and Real-Time Polymerase Chain Reaction
Total RNA was isolated using the Trizol reagent (Invitrogen, Carlsbad, CA) following the instructions provided by the manufacturer. Reverse transcription (RT) was carried out with 1 µg total RNA using ThermoScript ™ RT-PCR system (Invitrogen, Carlsbad, CA) for first strand cDNA synthesis. For real-time PCR, cDNA was amplified using MyiQ Real-Time PCR Detection System (Bio-Rad Hercules, CA). A threshold cycle (Ct value) was obtained from each amplification curve using iQ5 Optical System Software provided by the manufacturer (Bio-Rad). Relative mRNA expression was determined using the ΔΔCT method, as detailed by manufacturer (Bio-Rad). The deviations in samples represent three different donors in three separate experiments. The primer sequences and their conditions for use are summarized in Table 1.
Table 1.
Real-Time PCR Primer sequence.
| Gene | Primer Seq. | Annealing Tm | NCBI Accession No. |
|---|---|---|---|
| Aggrecan | Forward: 5’-TGAAACCACCTCCACCTTCCATGA-3’ Reverse: 5’-TCAAAGGCAGTGGTTGACTCTCCA-3’ |
55°C | NM_173981.2 |
| Collagen Type II | Forward: 5’-TCATAAGGATGTGTGGAAGCCCGA-3’ Reverse: 5’-GGCTGAGGCAGTCTTTCATGTCTT-3’ |
55°C | AY743675.1 |
| SOX-9 | Forward: 5’-ACCATGTCCGAGGACTCTGC-3’ Reverse: 5’-AACTTGTCCTCCTCGCTCTCCTTCTT-3’ |
55°C | AF278703 |
| Noggin | Forward: 5’-AGCGAGATCAAAGCGCTGGAGTT-3’ Reverse: 5’-TCTGTAACTTCCTCCGCAGCTTCT-3’ |
55°C | XM_582573.4 |
| β-actin | Forward: 5’-AAGAGATCAATGACCTGGCACCCA-3’ Reverse: 5’-ACTCCTGCTTGCTGATCCATCT-3’ |
55°C | BC142413.1 |
DMMB assay for Proteoglycan Production and DNA Assay for Cell Numbers
At day 21 of culture, the alginate beads were collected and processed for PG assays using the DMMB assay, as previously described (21). The PG levels measured in the cell-associated matrix (24) were quantified in relation to cell number (as measured by DNA content) to determine the relative cellular production and retention of PGs in alginate bead cultures (12). Cell number based on total DNA content was determined using PicoGreen (Molecular Probes, Carlsbad, CA) as previously described (12).
35S-sulfate Incorporation into Newly-Synthesized Proteoglycans
At Day 7 of culture in alginate, the medium was removed and replaced by fresh medium. One hour later, this medium was replaced with fresh medium containing [35S]-sulfate at 20 µCi/ml (Amersham Corp, Arlington Heights, IL). After incubation for 4 hrs, the labeling medium was removed and beads were rinsed twice in cold 1.5 mM sulfate-containing washing media. Beads were dissolved to separate out the CM and digested with papain (20 µg/ml in 0.1 M sodium acetate, 0.05 M EDTA, pH 5.53) at 60°C for 16 hrs. Sulfate incorporation into PGs was measured using the Alcian blue precipitation method (25). All samples were analyzed in duplicate and normalized for DNA content using Hoechst 33258 as previously described (25).
Particle Exclusion Assay for Matrix Assessment
The cells with their pericellular matrix were visualized using the particle exclusion assay, as previously described (26). Briefly, after day 21 of culture in alginate, the beads were solubilized with sodium citrate. The cells were pelleted by centrifugation, resuspended in DMEM, and then placed in the bottom of a multi-well plate. The cells were allowed to settle and attach to the plates for 6 to 12 hrs, and formalin-fixed erythrocytes were then added and allowed to settle for 10 to 15 min. Cells were then observed and photographed with an inverted phase-contrast microscope (Nikon, Melville, NY).
Nuclear Translocation
Phospho-R-Smad nuclear translocation was tested as previously described (27). Briefly, after treatment, the cells were fixed with 1% paraformaldehyde, permeabilized in 0.2% Triton X-100, extensively washed with PBS, and nonspecific signals were blocked with normal goat serum. After multiple washes, chondrocytes were incubated overnight at 4°C with rabbit anti-phospho-R-Smad polyclonal antibodies (anti-phospho-Smad 1/5/8 & 2 (Millipore, Billerica, MA, USA), anti-phospho-Smad 3 antibody (Invitrogen, Carlsbad, CA) followed by incubation with Oregon green®488–conjugated goat anti-rabbit IgG (Molecular Probes, Eugene, OR). In addition, as a nuclear counterstain, all cells were incubated with 4,6-diamidino-2-phenylindole (Vectastain, Burlingame, CA). Nuclear images were visualized with an ultraviolet filter; the cellular fluorescent stain was visualized with a green filter using a Nikon Eclipse E600 microscope connected to a personal computer, with the results analyzed on MetaMorph Imaging software (series 6.1; Universal Imaging, West Chester, PA). Both images were overlaid with Universal Imaging MetaView imaging software.
Statistical Analysis
Analysis of variance was performed using StatView 5.0 software (SAS Institute, Cary, NC). P-values lower than 0.05 were considered significant.
RESULTS
Combination of IGF-1 and BMP7 synergizes matrix formation by NP cells
Bovine NP cells were cultured in alginate beads for 21 days in the presence or absence of IGF-1 (100 ng/ml) and/or BMP7 (100 ng/ml). The resulting PG accumulation by NP cells was analyzed by DMMB assay (Fig. 1A). Incubation of cells with IGF-1 alone does not significantly modulate the PG production. Treatment of cells with BMP7 alone, however, significantly increases PG accumulation per cell by > 200% compared to control (p<0.01), as we previously reported (3). Cells treated with a combination of IGF-1 and BMP7 synergistically increased PG up to 400% and 200% per cell compared to control (p<0.001) and individual treatment with BMP7 alone (p<0.01), respectively, suggesting a potent anabolic enhancement on PG production by the combination of IGF-1 and BMP7 in NP cells.
Figure 1.
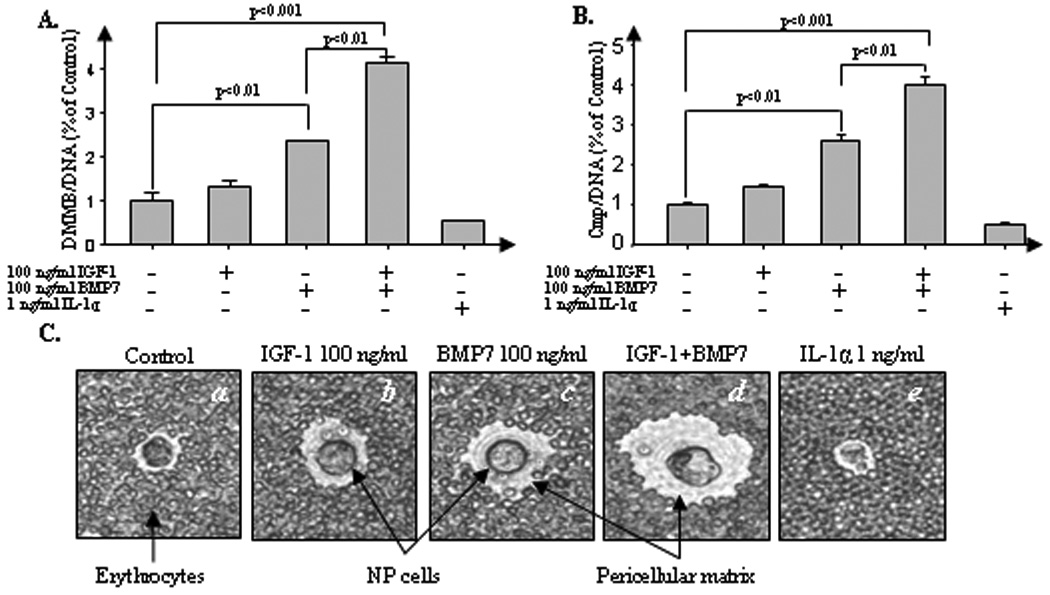
Combination of IGF-1 and BMP7 synergizes matrix formation by NP cells. NP cells were cultured in 1.2% alginate in serum-free medium with mini-ITS (control) or the control medium plus 100 ng/ml IGF-1, 100 ng/ml of BMP7, cocktail of IGF-1 and BMP7, and 1 ng/ml IL-1α. A, NP cells were stimulated for 21 days in indicated treatment condition. At the end of the culture period, the amount of PG in the cell-associated matrix were measured by DMMB assay and normalized to cell numbers using DNA measurement. Samples were measured in triplicate and presented as a percentage of the day 21 control cultures. B, NP cells were stimulated for 7 days by indicated treatment condition. At the end of the culture period, PG synthesis was measured during the last 4 hrs of culture using 35S-sulfate incorporation and was normalized to cell numbers by DNA assay. C, NP cell pericellular matrix production after alginate culture for 21 days by indicated treatment condition was measured in an exclusion assay as described in Materials and methods. A representative cell was photographed using an inverted phase-contrast microscope. The CM can be seen excluding the erythrocytes from the cell plasma membrane (original magnification ×400). Four independent experiments in triplicates were performed.
To determine whether increased PG accumulation by IGF-1 and BMP7 is due to stimulation of PG synthesis, we quantified newly synthesized PG based on incorporation of 35S-sulfate in bovine NP cells. Parallel experiments were performed with the well-known catabolic cytokine IL-1 which represents a negative control. IGF-1 alone does not significantly stimulate PG synthesis, but BMP7 alone stimulates PG synthesis > 200% compared to control (p<0.01). These findings are consistent with our previous results using bovine NP cells (3). More importantly, co-administration of both IGF-1 and BMP7 reveals that acceleration of PG accumulation is, at least in part, due to increased PG synthesis (Fig. 1B).
The above findings were further corroborated by particle exclusion assays in which the pericellular matrix formed by NP cells was visualized (Fig. 1C). After 21 days in culture, alginate beads were solubilized and exclusion of erythrocytes by the pericellular matrix formed by the NP cells was examined using an inverted phase-contrast microscope. Stimulation of bovine NP cells with a combination of IGF-1 plus BMP7 significantly increased matrix production (Fig. 1C, d ) compared to stimulation with either growth factor alone (b, c). As above, the catabolic inflammatory cytokine IL-1α (1 ng/ml) was used as a negative control (e). These results clearly demonstrate the significant anabolic capacity of both IGF-1 plus BMP7 in bovine NP cells.
IGF-1 and BMP7 have complementary effects on anabolic gene expression
To understand how the combination of IGF-1 and BMP7 exerts synergistic anabolic responses, we examined the effects of these ligands on expression of target genes for key components of the ECM in bovine NP cells. Real-time PCR results demonstrate that stimulation of cells cultured in monolayer with IGF-1 and BMP7 induces 1.9-fold (p<0.05, Fig. 2A, b ) and 4-fold (p<0.01, c) increase in aggrecan gene expression, respectively. The expression of collagen type II, the most abundant collagen found in the IVD and articular cartilage (28), is induced by approximately 2-fold by IGF-1 (p<0.05, Fig. 2B, g ), but not by BMP7 (h). Although BMP7 has no effect on collagen type-II gene expression (Fig. 2B), the combination of IGF-1 with BMP7 further increases collagen type II gene expression above levels induced by IGF-1 alone (p<0.05, i). These results suggest that the two growth factors mediate anabolic effects by complementary stimulation of aggrecan and collagen type-II production in bovine NP cells.
Figure 2.
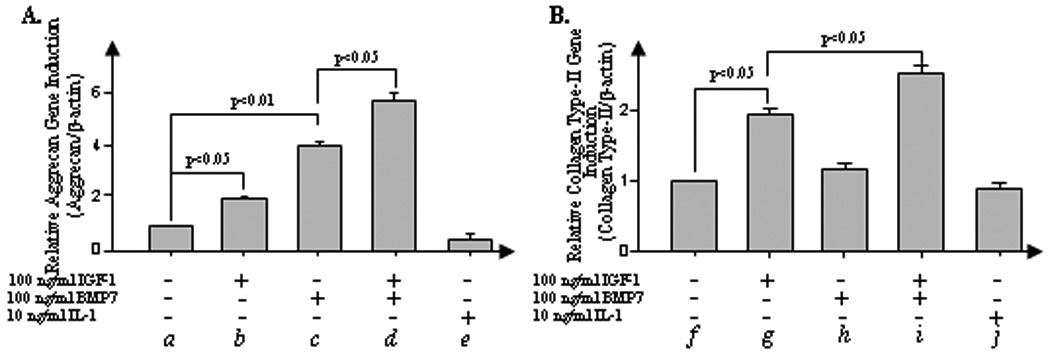
Combination of IGF-1 and BMP7 exerts a compensatory anabolic gene expression effect. NP cells isolated from bovine IVD were cultured in a monolayer in 12-well plates at 8×105 cells/cm2. NP cells were stimulated in serum-free medium (control) or the control medium plus 100 ng/ml IGF-1, 100 ng/ml BMP7, cocktail of IGF-1 and BMP7, and 10 ng/ml IL-1α for 24 hrs. After stimulation, cells were harvested and the total RNA was extracted to perform real-time PCR of aggrecan (A) and collagen type-II genes (B). Error bars represent three different donors in three separate experiments. Analysis of variance was performed using StatView 5.0 software (SAS Institute, Cary, NC). P-values lower than 0.05 were considered significant. Six independent experiments in triplicates were performed.
IGF-1 enhances the anabolic response of BMP7 by blocking expression of the BMP-inhibitor noggin
We investigated whether IGF-1-mediated enhancement of BMP7 anabolism proceeds by suppression of noggin, an endogenous inhibitor of the BMP pathways. BMP7 significantly induces noggin at both mRNA (Fig. 3A, upper panel) and protein levels (lower panel) in NP cells in a dose-dependent manner. Our real-time PCR results demonstrate that addition of exogenous noggin significantly suppresses the BMP7-mediated upregulation of anabolic genes such as aggrecan and transcription factor SOX-9 in bovine NP cells (p<0.01; Fig. 3B). We observed that IGF-1 markedly suppresses noggin gene expression in a dose-dependent manner (0 to 100ng/ml) (Fig. 3C). Maximal inhibition (90%) of noggin at both the mRNA (p<0.001; upper panel) and protein levels is observed at a concentration of 50ng/ml IGF-1 (lower panel).
Figure 3.
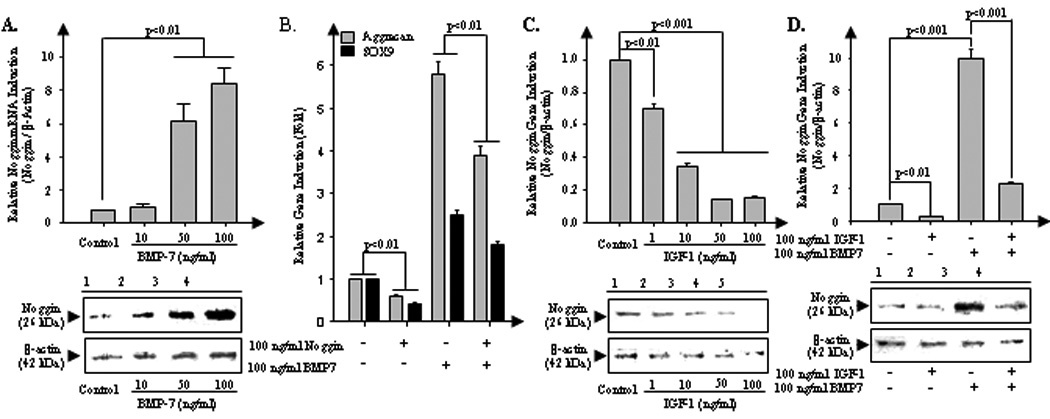
IGF-1 enhances BMP7-mediated anabolism by a potent inhibitory effect on noggin expression. A, To measure the BMP7 mediated noggin upregulation in bovine NP cells, serum-starved NP cells were stimulated by 10, 50, and 100 ng/ml BMP7 for 24 hrs. And then expression level of noggin, natural BMP receptor antagonist, was measured by real-time PCR (upper) and western blotting (bottom). B, To examine whether noggin inhibits BMP7-meidated anabolic effect, serum-starved NP cells were stimulated by endogenous noggin (100 ng/ml), BMP7 (100 ng/ml), and cocktail of noggin and BMP7 for 24 hrs. And then mRNA induction level of aggrecan and transcriptional factor SOX9 were measured by real-time PCR. C, To measure the IGF-1 mediated noggin downregulation, serum-starved NP cells were cultivated with presence of 1, 10, 50, and 100 ng/ml IGF-1 for 24 hrs. And then expression of noggin was measured by real-time PCR (upper) and western blotting (bottom). D, To observe whether IGF-1 inhibits BMP7-mediated noggin induction in bovine disc cells, serum-starved NP cells were stimulated by IGF-1 (100 ng/ml), BMP7 (100 ng/ml), and cocktail of IGF-1 and BMP7 for 24 hrs. Analysis of variance was performed using StatView 5.0 software. P-values lower than 0.05 were considered significant. For independent experiments in triplicates were performed.
Next, we examined whether IGF-1 inhibits BMP7-mediated noggin induction in bovine disc cells. Stimulation of NP cells with BMP7 significantly (p<0.001) induces noggin at both mRNA (Fig. 3D, upper panel, lane 3) and protein levels (lower panel, lane 3). Co-stimulation with IGF-1 dramatically suppresses BMP7-induced noggin at both mRNA and protein levels in NP cells (Fig. 3D, upper panel for mRNA (p<0.001), lower panel for protein level, lane 4). Taken together, we propose that IGF-1 blocks negative feedback regulation of BMP7 by noggin in bovine NP cells thus establishing a mechanism for the anabolic synergism between IGF-1 and BMP7.
IGF-1-mediated alterations of noggin and aggrecan gene expression are via the PI3K/Akt pathway
To assess which IGF-1-dependent signaling pathways modulate noggin and aggrecan expression in bovine NP cells, we examined activation of PI3K/Akt and ERK MAP kinase signaling pathways that have previously been linked to IGF-1 effects in human articular chondrocytes (29). Western blot results show that IGF-1 (100 ng/ml) activates both the ERK and PI3K/Akt pathways reflected by phosphorylation within 5 min and sustains activation for >1 hr after stimulation with IGF-1 (Fig. 4A). There was no significant activation of other MAP kinase subgroups (i.e., JNK, p38) or NFκB by IGF-1 (data not shown), suggesting that IGF-1-mediated biological effects are mainly via the PI3K/Akt and ERK pathways in bovine NP cells which are similar to those in human articular chondrocytes.
Figure 4.
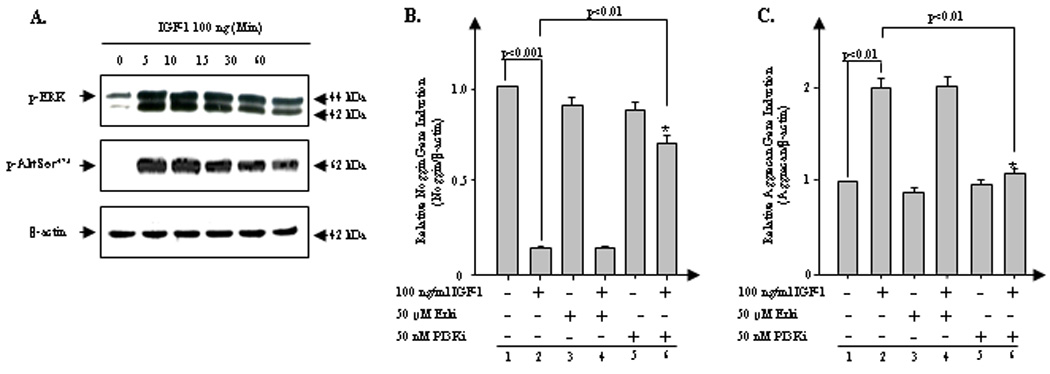
IGF-1-mediated suppression of noggin and stimulation of aggrecan are via the PI3K/Akt pathway. A. Serum-starved NP cells were stimulated by IGF-1 (100 ng/ml) for the indicated periods of time. Cell lysates were then prepared and analyzed by western blotting with specific anti-phospho-Erk and anti-phosph-Akt Ser473 antibody. β-actin was used as a control for normalization. B and C. The cells were treated with IGF-1 (100 ng/ml) in the presence or absence of Erk/MAPK pathway-specific inhibitor (PD98059, 25 µM), PI3K/Akt pathway-specific inhibitor (LY294002, 50 nM). The cells were harvested 24 hrs after the initiation of each treatment to perform real-time PCR (B, aggreccan; C, noggin). β-actin was used as a control for normalization. The data represent three different donors measured in triplicate for each experiment. Analysis of variance was performed using StatView 5.0 software (SAS Institute, Cary, NC). P-values lower than 0.05 were considered significant. Three independent experiments in triplicates were performed.
We determined which IGF-1 induced pathways suppress noggin expression by performing real-time PCR in the presence or absence of pathway-specific inhibitors of either PI3K/Akt (PI3Ki) or ERK (Erki). The presence of PI3Ki, but not Erki, rescues the IGF-1-mediated suppression of noggin expression, (p<0.01; Fig. 4B, lane 6). Similarly, co-incubation of IGF-1 with PI3Ki (p<0.01; Fig. 4C, lane 6), but not with Erki (lane 4), completely prevents the IGF-1-stimualtion of aggrecan gene expression. Collectively, these findings demonstrate that IGF-1 suppresses noggin and enhances aggrecan gene expression by activation of the PI3K, but not ERK/MAPK pathway, in bovine NP cells.
IGF-1 and BMP7 co-activate both BMP-related SMAD1/5/8 and TGFβ-related SMAD2/3 response limbs
To further delineate the molecular mechanisms by which IGF-1 synergizes with BMP7 to induce anabolic responses in bovine NP cells, we investigated whether these ligands activate SMAD proteins as the principal transducers of TGFβ/BMP signaling pathways (30). Western blot results reveal that BMP7 activates the SMAD1/5/8 in bovine NP cells (Fig. 5A; 1st panel) as expected (31). Surprisingly, BMP7 also potently activates SMAD3, a SMAD component for the TGFβ pathway (2nd panel). However, SMAD2, another SMAD component of the TGFβ pathway, is not significantly modulated by BMP7 (3rd panel). IGF-1, on the other hand, activates the SMAD2 (Fig. 5B, 2nd panel), but not the SMAD3 pathway (3rd panel). Thus, the combination of IGF-1 and BMP7 mimics the mechanistic effects of combining BMP7 and TGFβ (SMAD2/3) (32), and is clearly reminiscent of the potent anabolic effects of combining BMP7 and IGF-1.
Figure 5.
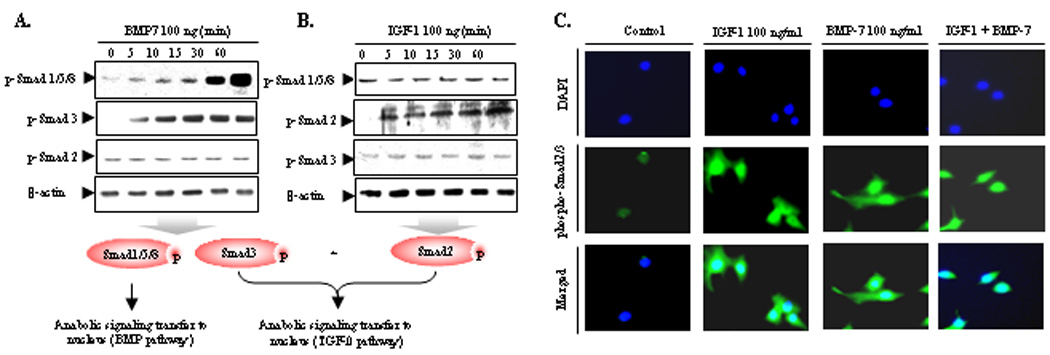
Combination of IGF-1 and BMP7 activates not only SMAD1/5/8 but also SMAD2/3 mimicking TGF effect. A and B, Serum-starved NP cells were stimulated by IGF-1 (100 ng/ml) and BMP7 (100 ng/ml) for the indicated periods of time. Cell lysates were then prepared and analyzed by western blotting with specific anti-phospho-Smad 1/5/8, anti-phospho-Smad2, and anti-phospho-Smad3 antibody. C, Serum-starved NP cells were cultured in monolayer in 4-well chamber slide for 24 hrs before treatment. Cells were treated with IGF-1 (100 ng/ml), BMP7 (100 ng/ml), and cocktail of IGF-1 and BMP7. 3 hrs later, the cells were fixed with 1% paraformaldehyde, permeabilized in 0.2% Triton X-100. Nonspecific signals were blocked with normal goat serum. NP cells were incubated overnight at 4°C with rabbit anti-phospho-R-Smad polyclonal antibodies followed by incubation with Oregon green®488–conjugated goat anti-rabbit IgG. As a nuclear counterstain, all cells were incubated with 4,6-diamidino-2-phenylindole. The nuclear image was visualized with an ultraviolet filter; the cellular fluorescent stain was visualized with a green filter using a Nikon Eclipse E600 microscope connected to a personal computer, with the results analyzed on MetaMorph Imaging software (series 6.1; Universal Imaging, West Chester, PA). Both images were overlaid with Universal Imaging MetaView imaging software. Three independent experiments in triplicates were performed.
SMAD complexes serve as transcription factors in gene regulation (33). Specifically, phosphorylated R-SMADs translocate from the cytoplasm into the nucleus upon stimulation of gene transcription (27). Initial time-course experiments revealed that the highest level of nuclear translocalization of activated SMADs induced by growth factors occur 3 hrs after stimulation of NP cells (data not shown). Individual treatment with IGF-1 or BMP7, results in activation of p-SMAD2/3 primarily within the cytoplasmic compartments, with moderate increases in nuclear translocalization, presumably due to activation of SMAD2 and SMAD3 by IGF-1 and BMP7, respectively. The combination of IGF-1 and BMP7 results in robust nuclear co-localization of p-SMAD2/3 with no sign of cytoplasmic localization (Fig. 5C). We conclude that co-treatment with IGF-1 and BMP7 results in the phosphorylation and nuclear translocation of both TGFβ and BMP responsive SMAD proteins that modulate TGFβ–SMAD2/3-, and BMP-SMAD1/5/8-dependent anabolic target genes in bovine NP cells.
DISCUSSION
This study illustrates the potent anabolic effects of combination growth factor therapy using IGF-1 and BMP7 on bovine IVD homeostasis. Our findings reveal the significant potential of combined growth factor therapy for disc degeneration. We found that IGF-1 powerfully reverses negative feedback regulation of BMP7 by inhibiting decoy ligand noggin in IVD tissue, providing a key mechanistic explanation for the biological synergy of IGF-1 and BMP7. We note that IGF-1 inhibits noggin expression in human NP tissue as well as in human articular cartilage (unpublished data), suggesting that IGF-1-mediated suppression of noggin may be a common mechanism in spine discs and articular cartilage. These findings are quite promising as they may apply to a better understanding of the pathophysiology of different degenerative joint diseases. We also established that the PI3K/Akt, but not the ERK/MAPK pathways, is responsible for IGF-1-mediated suppression of noggin and induction of aggrecan, as well as co-activation of multiple SMAD pathways by BMP7 and IGF-1, mimicking the combination of BMP7 and TGFβ effect in the IVD.
Accumulating evidence has demonstrated the anabolic potential of individual growth factor treatments to slow or reverse cartilage degeneration. Results from our laboratory and other groups have previously demonstrated the anabolic potential of BMP7 to stimulate PG and collagen synthesis in human adult articular chondrocytes (13), rabbit IVD cells (15,34), bovine NP (3) and human NP and AF cells (16) in vitro. In vivo, BMP7 administration has tremendous potential for disc regeneration (34). A single injection of recombinant BMP7 restores the biochemical, histological, and biomechanical properties of rabbit IVDs after induction of degeneration by annular puncture (35), chondroitinase-ABC chemonucleolysis (15–16, 36), or IL-1 administration (36). Similarly, the anabolic capacity of IGF-1 has been studied in IVD tissues, although not much in vivo studies have yet been reported. In vitro, IGF-1 has been found to inhibit apoptosis (18) and prevent premature senescence in human AF cells (37), as well as increase PG synthesis (10) and inhibit MMP-2 production in bovine NP cells (38).
BMP7 plus IGF-1 cocktails have both modest mitogenic and potent anabolic effects in both normal and OA human articular chondrocytes (19, 39). The innovative aspect of this study is the mechanistic elucidation of the anabolic effects of combining IGF-1 and BMP which provides the pre-translational basis for clinical approaches to treat IVD. Biological synergism of BMP7 and IGF-1 is also evident in our previous report on human articular chondrocytes (12). We demonstrated that IGF-1 plus BMP7 suppresses expression of matrix-degrading enzymes (e.g., MMP-13) by downregulating pro-inflammatory cytokine pathways and their downstream mediators such as NFκB and AP-1 (12). BMP7 significantly attenuates the expression levels MMP-1, -13 and aggrecanses (ADAMTS) in bovine NP cells which resemble the cellular response in human articular chondrocytes (unpublished data). Given the similarities in cellular responses between human articular chondrocytes and IVD NP cells, it is likely that BMP7 enhancement of the mRNA levels for IGF-1 and IGF-1 receptor, as observed in human articular chondrocytes (12), may also extend to IVD cells and could contribute to combined effects of IGF-1 and BMP7.
Our results also unveil the specific downstream pathways mediated by IGF-1 to suppress noggin and to stimulate aggrecan expression in bovine NP cells. IGF-1 activates both the PI3K/Akt and ERK/MAPK signaling pathways in a number of cell types (29, 40). Recently, IGF-1 was found to utilize both the ERK and PI3K/Akt pathways to exert mitogenic effects in bovine NP and AF cells (41). Similarly, our results show that IGF-1 activates the ERK and PI3K/Akt pathways in bovine NP cells. Our findings suggest that upregulation of aggrecan and suppression of noggin by IGF-1 is primarily via activation of PI3K/Akt, but not the ERK pathway. These findings corroborate those results from other studies using human articular chondrocytes (29).
Another novel finding of the current study is that the combination of IGF-1 and BMP7 mimics the TGFβ pathway in bovine IVD cells. Although TGFβ/BMP superfamily members share co-SMAD4 for their biological influences, TGFβ and BMP transduce their signals via distinctive regulatory SMAD (R-SMAD) complexes, i.e., SMAD2/3 by TGFβ, and SMAD1/5/8 by BMP, respectively (30). This unique utilization of R-SMAD complexes by TGFβ/BMP family members is quite universal for various tissue/cell types, including mammary epithelial cells (42), human articular cartilage (43), and human IVD tissues (44). Our results demonstrate that IGF-1 activates SMAD2, but not SMAD3, while BMP7 activates SMAD3 but not SMAD2. Therefore, the combination of IGF-1 and BMP7 generates the TGFβ-like activation of SMAD2/3 pathway leading to the stimulation of matrix formation. Importantly, the biological synergism mediated by IGF-1 plus BMP7 is much greater than TGFβ plus BMP7. Perhaps, it is due to, in part, the significant induction of noggin by TGFβ as a negative regulatory mechanism (data not shown).
Clinically, there are recognized limitations of this study that must be taken into account. First, our findings are restricted to a cell culture model system, which does not recapitulate the complex variety of factors that influences onset and progression of disc degeneration in vivo. Second, Price and colleagues noted that sustained delivery of IGF-1 in a rat degenerative disc model induced histomorphometric tissue changes in the proximal tubules of the kidneys after 28 days, illustrating the potentially harmful systemic effects of exogenous growth factor therapy for DDD. Therefore, future studies are warranted to elucidate the long-term effects of direct injections of IGF-1 plus BMP7 on the IVD and other organ systems, as well as to clarify the optimal dose and concentration of the two growth factors in vivo. Our findings are obtained using bovine NP cells. Hence ramifications for clinical setting may not be possible until the effects of IGF-1 and BMP7 in tandem are studied using human IVD tissue, and animal studies in vivo.
ACKNOWLEDEGEMENTS
The current studies were supported by the NIH R01 AR053220 (H-J Im), NIH training grant 2T-AR-007590, Arthritis Foundation (H-J Im), Arthritis National Research Foundation (H-J Im), and NIH AR48152 (HS An). We would like to thank Dr. Ranjith Udayakumar for his technical support and Dr. Xin Li for her kind help throughout the submission process of the manuscript. We also thank to Stryker Biotech for providing BMP7 and NCI for providing IL-1α for the study.
LIST OF ABBREVIATIONS
- LBP
Low back pain
- IVD
Intervertebral disc
- DDD
Degenerative disc disease
- ECM
Extracellular matrix
- AF
Annulus fibrosis
- NP
Nucleus pulposus
- PGs
Proteoglycans
- IL-1
Interleukin-1
- MMPs
Metalloproteases
- ADAMTS
A disintegrin and metalloprotease with thrombospondin motifs
- IGF-1
Insulin-like growth factor-1
- BMPs
Bone morphogenetic proteins
- TGF-β
Transforming growth factor-β
- DMEM
Dulbecco’s modified Eagle’s medium
- DMMB
Dimethylmethylene blue
- MAPK
Mitogen activated protein kinase
- SDS
Sodium dodecyl sulfate
- RT
Reverse Transcription
- PCR
Polymerase chain reaction
- CM
Cell-associated matrix
- EDTA
Ethylenediaminetetraacetic acid
REFERENCES
- 1.Mooney V. The classification of low back pain. Ann Med. 1989;21:321–325. doi: 10.3109/07853898909149215. [DOI] [PubMed] [Google Scholar]
- 2.Freemont TJ, LeMaitre C, Watkins A, Hoyland JA. Degeneration of intervertebral discs: current understanding of cellular and molecular events, and implications for novel therapies. Expert Rev Mol Med. 2001;2001:1–10. doi: 10.1017/S1462399401002885. [DOI] [PubMed] [Google Scholar]
- 3.Li X, An HS, Ellman M, Phillips F, Thonar EJ, Park DK, et al. Action of fibroblast growth factor-2 on the intervertebral disc. Arthritis Res Ther. 2008;10:R48. doi: 10.1186/ar2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An H, Boden SD, Kang J, Sandhu HS, Abdu W, Weinstein J. Summary statement: emerging techniques for treatment of degenerative lumbar disc disease. Spine. 2003;28:S24–S25. doi: 10.1097/01.BRS.0000076894.33269.19. [DOI] [PubMed] [Google Scholar]
- 5.An HS, Thonar EJ, Masuda K. Biological repair of intervertebral disc. Spine. 2003;28:S86–S92. doi: 10.1097/01.BRS.0000076904.99434.40. [DOI] [PubMed] [Google Scholar]
- 6.Iannone F, Lapadula G. The pathophysiology of osteoarthritis. Aging Clin Exp Res. 2003;15:364–372. doi: 10.1007/BF03327357. [DOI] [PubMed] [Google Scholar]
- 7.Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther. 2007;9:R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Maitre CL, Pockert A, Buttle DJ, Freemont AJ, Hoyland JA. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soc Trans. 2007;35:652–655. doi: 10.1042/BST0350652. [DOI] [PubMed] [Google Scholar]
- 9.An HS, Masuda K, Inoue N. Intervertebral disc degeneration: biological and biomechanical factors. J Orthop Sci. 2006;11:541–552. doi: 10.1007/s00776-006-1055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osada R, Ohshima H, Ishihara H, Yudoh K, Sakai K, Matsui H, et al. Autocrine/paracrine mechanism of insulin-like growth factor-1 secretion, and the effect of insulin-like growth factor-1 on proteoglycan synthesis in bovine intervertebral discs. J Orthop Res. 1996;14:690–699. doi: 10.1002/jor.1100140503. [DOI] [PubMed] [Google Scholar]
- 11.Masuda K, Oegema TR, Jr, An HS. Growth factors and treatment of intervertebral disc degeneration. Spine. 2004;29:2757–2769. doi: 10.1097/01.brs.0000146048.14946.af. [DOI] [PubMed] [Google Scholar]
- 12.Im HJ, Pacione C, Chubinskaya S, Van Wijnen AJ, Sun Y, Loeser RF. Inhibitory effects of insulin-like growth factor-1 and osteogenic protein-1 on fibronectin fragment- and interleukin-1beta-stimulated matrix metalloproteinase-13 expression in human chondrocytes. J Biol Chem. 2003;278:25386–25394. doi: 10.1074/jbc.M302048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flechtenmacher J, Huch K, Thonar EJ, Mollenhauer JA, Davies SR, Schmid TM, et al. Recombinant human osteogenic protein 1 is a potent stimulator of the synthesis of cartilage proteoglycans and collagens by human articular chondrocytes. Arthritis Rheum. 1996;39:1896–1904. doi: 10.1002/art.1780391117. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, An HS, Song S, Toofanfard M, Masuda K, Andersson GB, et al. Growth factor osteogenic protein-1: differing effects on cells from three distinct zones in the bovine intervertebral disc. Am J Phys Med Rehabil. 2004;83:515–521. doi: 10.1097/01.phm.0000130031.64343.59. [DOI] [PubMed] [Google Scholar]
- 15.Takegami K, An HS, Kumano F, Chiba K, Thonar EJ, Singh K, et al. Osteogenic protein-1 is most effective in stimulating nucleus pulposus and annulus fibrosus cells to repair their matrix after chondroitinase ABC-induced in vitro chemonucleolysis. Spine J. 2005;5:231–238. doi: 10.1016/j.spinee.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Imai Y, Okuma M, An HS, Nakagawa K, Yamada M, Muehleman C, et al. Restoration of disc height loss by recombinant human osteogenic protein-1 injection into intervertebral discs undergoing degeneration induced by an intradiscal injection of chondroitinase ABC. Spine. 2007;32:1197–1205. doi: 10.1097/BRS.0b013e3180574d26. [DOI] [PubMed] [Google Scholar]
- 17.Luyten FP, Hascall VC, Nissley SP, Morales TI, Reddi AH. Insulin-like growth factors maintain steady-state metabolism of proteoglycans in bovine articular cartilage explants. Arch Biochem Biophys. 1988;267:416–425. doi: 10.1016/0003-9861(88)90047-1. [DOI] [PubMed] [Google Scholar]
- 18.Gruber HE, Norton HJ, Hanley EN., Jr Anti-apoptotic effects of IGF-1 and PDGF on human intervertebral disc cells in vitro. Spine. 2000;25:2153–2157. doi: 10.1097/00007632-200009010-00002. [DOI] [PubMed] [Google Scholar]
- 19.Loeser RF, Pacione CA, Chubinskaya S. The combination of insulin-like growth factor 1 and osteogenic protein 1 promotes increased survival of and matrix synthesis by normal and osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003;48:2188–2196. doi: 10.1002/art.11209. [DOI] [PubMed] [Google Scholar]
- 20.Hauselmann HJ, Aydelotte MB, Schumacher BL, Kuettner KE, Gitelis SH, Thonar EJ. Synthesis and turnover of proteoglycans by human and bovine adult articular chondrocytes cultured in alginate beads. Matrix. 1992;12:116–129. doi: 10.1016/s0934-8832(11)80053-3. [DOI] [PubMed] [Google Scholar]
- 21.Gruber HE, Hoelscher GL, Leslie K, Ingram JA, Hanley EN., Jr Three-dimensional culture of human disc cells within agarose or a collagen sponge: assessment of proteoglycan production. Biomaterials. 2006;27:371–376. doi: 10.1016/j.biomaterials.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 22.Muddasani P, Norman JC, Ellman M, van Wijnen AJ, Im HJ. Basic fibroblast growth factor activates the MAPK and NFkappaB pathways that converge on Elk-1 to control production of matrix metalloproteinase-13 by human adult articular chondrocytes. J Biol Chem. 2007;282:31409–31421. doi: 10.1074/jbc.M706508200. [DOI] [PubMed] [Google Scholar]
- 23.Im HJ, Muddasani P, Natarajan V, Schmid TM, Block JA, Davis F, et al. Basic fibroblast growth factor stimulates matrix metalloproteinase-13 via the molecular cross-talk between the mitogen-activated protein kinases and protein kinase Cdelta pathways in human adult articular chondrocytes. J Biol Chem. 2007;282:11110–11121. doi: 10.1074/jbc.M609040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urban JP, McMullin JF. Swelling pressure of the lumbar intervertebral discs: influence of age, spinal level, composition, and degeneration. Spine. 1988;13:179–187. doi: 10.1097/00007632-198802000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Loeser RF, Shanker G, Carlson CS, Gardin JF, Shelton BJ, Sonntag WE. Reduction in the chondrocyte response to insulin-like growth factor 1 in aging and osteoarthritis: studies in a non-human primate model of naturally occurring disease. Arthritis Rheum. 2000;43:2110–2120. doi: 10.1002/1529-0131(200009)43:9<2110::AID-ANR23>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 26.Knudson CB. Hyaluronan receptor-directed assembly of chondrocyte pericellular matrix. J Cell Biol. 1993;120:825–834. doi: 10.1083/jcb.120.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmierer B, Hill CS. Kinetic analysis of Smad nucleocytoplasmic shuttling reveals a mechanism for transforming growth factor beta-dependent nuclear accumulation of Smads. Mol Cell Biol. 2005;25:9845–9858. doi: 10.1128/MCB.25.22.9845-9858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97:33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 29.Starkman BG, Cravero JD, Delcarlo M, Loeser RF. IGF-I stimulation of proteoglycan synthesis by chondrocytes requires activation of the PI 3-kinase pathway but not ERK MAPK. Biochem J. 2005;389:723–729. doi: 10.1042/BJ20041636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 31.Nishihara A, Fujii M, Sampath TK, Miyazono K, Reddi AH. Bone morphogenetic protein signaling in articular chondrocyte differentiation. Biochem Biophys Res Commun. 2003;301:617–622. doi: 10.1016/s0006-291x(02)03068-1. [DOI] [PubMed] [Google Scholar]
- 32.Matsaba T, Ramoshebi LN, Crooks J, Ripamonti U. Transforming growth factor-beta1 supports the rapid morphogenesis of heterotopic endochondral bone initiated by human osteogenic protein-1 via the synergistic upregulation of molecular markers. Growth Factors. 2001;19:73–86. doi: 10.3109/08977190109001077. [DOI] [PubMed] [Google Scholar]
- 33.Motazed R, Colville-Nash P, Kwan JT, Dockrell ME. BMP-7 and proximal tubule epithelial cells: activation of multiple signaling pathways reveals a novel anti-fibrotic mechanism. Pharm Res. 2008;25:2440–2446. doi: 10.1007/s11095-008-9551-1. [DOI] [PubMed] [Google Scholar]
- 34.An HS, Takegami K, Kamada H, Nguyen CM, Thonar EJ, Singh K, et al. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine. 2005;30:25–31. doi: 10.1097/01.brs.0000148002.68656.4d. [DOI] [PubMed] [Google Scholar]
- 35.Masuda K, Imai Y, Okuma M, Muehleman C, Nakagawa K, Akeda K, et al. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine. 2006;31:742–754. doi: 10.1097/01.brs.0000206358.66412.7b. [DOI] [PubMed] [Google Scholar]
- 36.Takegami K, Thonar EJ, An HS, Kamada H, Masuda K. Osteogenic protein-1 enhances matrix replenishment by intervertebral disc cells previously exposed to interleukin-1. Spine. 2002;27:1318–1325. doi: 10.1097/00007632-200206150-00014. [DOI] [PubMed] [Google Scholar]
- 37.Gruber HE, Hoelscher GL, Ingram JA, Bethea S, Hanley EN. IGF-1 rescues human intervertebral annulus cells from in vitro stress-induced premature senescence. Growth Factors. 2008;26:220–225. doi: 10.1080/08977190802273814. [DOI] [PubMed] [Google Scholar]
- 38.Pattison ST, Melrose J, Ghosh P, Taylor TK. Regulation of gelatinase-A (MMP-2) production by ovine intervertebral disc nucleus pulposus cells grown in alginate bead culture by Transforming Growth Factor-beta(1)and insulin like growth factor-I. Cell Biol Int. 2001;25:679–689. doi: 10.1006/cbir.2000.0718. [DOI] [PubMed] [Google Scholar]
- 39.Chubinskaya S, Hakimiyan A, Pacione C, Yanke A, Rappoport L, Aigner T, et al. Synergistic effect of IGF-1 and OP-1 on matrix formation by normal and OA chondrocytes cultured in alginate beads. Osteoarthritis Cartilage. 2007;15:421–430. doi: 10.1016/j.joca.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baserga R, Hongo A, Rubini M, Prisco M, Valentinis B. The IGF-I receptor in cell growth, transformation and apoptosis. Biochim Biophys Acta. 1997;1332:F105–F126. doi: 10.1016/s0304-419x(97)00007-3. [DOI] [PubMed] [Google Scholar]
- 41.Pratsinis H, Kletsas D. PDGF, bFGF and IGF-I stimulate the proliferation of intervertebral disc cells in vitro via the activation of the ERK and Akt signaling pathways. Eur Spine J. 2007;16:1858–1866. doi: 10.1007/s00586-007-0408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gajewska M, Motyl T. IGF-binding proteins mediate TGF-beta 1-induced apoptosis in bovine mammary epithelial BME-UV1 cells. Comp Biochem Physiol C Toxicol Pharmacol. 2004;139:65–75. doi: 10.1016/j.cca.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Qureshi HY, Ricci G, Zafarullah M. Smad signaling pathway is a pivotal component of tissue inhibitor of metalloproteinases-3 regulation by transforming growth factor beta in human chondrocytes. Biochim Biophys Acta. 2008;1783:1605–1612. doi: 10.1016/j.bbamcr.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Chen WH, Lo WC, Lee JJ, Su CH, Lin CT, Liu HY, et al. Tissue-engineered intervertebral disc and chondrogenesis using human nucleus pulposus regulated through TGF-beta1 in platelet-rich plasma. J Cell Physiol. 2006;209:744–754. doi: 10.1002/jcp.20765. [DOI] [PubMed] [Google Scholar]


