Abstract
Gait and mobility problems are prominent features of Parkinson’s Disease (PD), and are difficult to observe clinically in early stages of PD. We previously reported that gait changes were measurable in early to mid-stage PD subjects, when we used inertial sensors during an instrumented Timed Up and Go test (iTUG). With the advent of wearable inertial sensors, home assessment of mobility has become possible. We tested six people with early PD and eight control subjects using the iTUG in the home and laboratory. Our objectives were to 1) investigate the feasibility of testing subjects at home, and 2) compare performance at home versus laboratory. We found that home iTUG testing is feasible and the patients with PD were more affected than the healthy control subjects when tested at home.
Keywords: Parkinson’s Disease, home, instrumented, Timed Up and Go
1. INTRODUCTION
Gait and mobility problems are prominent features of Parkinson’s Disease (PD), and a major cause of disability [1]. Such deficits start early in PD, but are not clinically observable until patients reach moderate to advanced disease stages. Only motion analysis techniques have been able to detect early deficits [2]. However, motion analysis usually has to be performed in sophisticated laboratories, and is cumbersome, expensive and time consuming. With the advent of small, wearable inertial sensors the assessment of mobility at home has become possible [3, 4]. Assessing mobility at home may be advantageous over the laboratory because it is more convenient, practical and ecological. However, no studies have reported whether quantitative mobility assessment in the home is feasible in PD and whether results are the same as those obtained in the laboratory.
The Timed Up and Go (TUG) test has been extensively used to assess balance and mobility in moderate-to-severe stage PD. Previous work by our research group, though, has shown that the sole stopwatch measure of the TUG duration was not sensitive to detect abnormalities in early-to-mid stage PD. In order to make the TUG test more sensitive and reliable, we used inertial sensors to instrument the TUG (iTUG) test [2]. Several metrics from the iTUG were shown to be sensitive and reliable to detect early gait and mobility changes in PD in a laboratory setting [5].
There are potentially a wide range of activities that could be recorded in the home using inertial sensors to measure mobility. In this pilot study, we used the iTUG to directly compare the same test collected in the laboratory and in the home to see if test location makes a difference. We also investigated the feasibility of inertial sensor testing in the home.
2. METHODS
2.1. Subjects
Six subjects with PD (57.3 ± 8.6 years old; 3M/3F) and 8 (63.7 ± 5.9; 2M/6F) healthy controls participated in this study. The groups were similar in height and weight (p>0.05). Patients were in early-to-mid-stages of PD, with a Unified Parkinson’s Disease Rating Scale Motor of 28.6 ± 15 and a Hoehn and Yahr of 1.9 ± 0.7. Three patients had been taking anti-parkinsonism medication, two for 1 month and the other for 8 months. All participants provided informed consent approved by the Institutional Review Board.
2.2. Apparatus and Protocol
Subjects wore a portable data-logger on a waist belt (Physilog®) [3] with five inertial sensors attached to their body [3-5]. Two uni-axial gyroscopes (range 600°/s) were attached to the anterior shank, 4 cm above the ankle joint. Two 2-dimensional (2-D) gyroscopes (range ± 1200 °/s), that measured respectively roll (axial rotation) and pitch (flexion extension) angular velocity, were attached to the dorsum of each wrist. One sensor, which contained a 2-D gyroscope (range ± 400 °/s, pitch and roll axis) and a 3-D accelerometer (range ± 2g), was attached to the chest on the sternum; 2 cm below the sternal notch. Data were recorded at a sampling rate of 200 Hz, with 16 bits/sample and stored in a flash memory card.
Subjects performed the iTUG test at home within 24 hours before or after laboratory testing. For consistency of data collection, same researcher administered all tests in the home and laboratory to ensure appropriate placement of the sensors and minimize bias. Thus, all measurement procedures were the same and only the data collection location was different. The iTUG test consists of standing up from a chair, walking 7 meters, turning, walking back to the chair and sitting down [2, 5]. Three trials were collected at each test location.
Our laboratory has both natural and fluorescent lighting on an open space of 9 meters by 4 meters over a linoleum floor. The distance walked in the laboratory was 7 meters. The distance walked at home varied between 5-7 meters because most homes could not accommodate 7 meters of straight walking. When the distance was shorter than 7 meters, subjects performed an extra trial so that total number of gait cycles remained comparable with laboratory trials. Criteria for selecting home settings were: 1) sufficient walking space to allow at least a 5-meter walk and turn, 2) even floor surface, and 3) quiet environment.
2.3. Outcomes and Data Analysis
We used a Matlab program to automatically detect, separate and analyze different components of gait and postural transition measures (sit-to-stand and turning) during the iTUG. More details on the algorithms used to measure the gait parameters can be found in our previous work [4,5]. We previously found stride length, stride velocity, cadence, peak arm swing velocity on the more affected side (MAS), and turning velocity to be the most sensitive variables in early PD [2], so we used these parameters for comparisons here. Our previous work has shown that these parameters have good to excellent test-retest reliability, with stride velocity ρ=0.78, stride length ρ=0.67, cadence ρ=0.94, and peak arm swing velocity ρ=0.90 and turning velocity ρ=0.86 [5]. Below is a definition of each parameter:
Stride Length: distance between two consecutive strikes of the same foot, presented as a percentage of the subject’s height (%height).
Stride Velocity: stride length in centimeters divided by stride time in seconds, presented as a percentage of the subjects’ height (%height/sec).
Cadence: number of steps per minute.
Peak arm swing velocity on the more affected side (MAS): The maximum angular velocity achieved during the swing phase (deg/sec). The 2 axes of the forearm gyroscopes were combined.
Turning velocity: The maximum achieved angular velocity of trunk rotation in the yaw axis during 180-degree turns (deg/sec).
The peak arm swing velocity on MAS was determined based on a sum of bradykinesia sub-scores of the Motor UPDRS (items 23 – finger tapping, 24 – hand open and close, 25 – hand pronation/supination, and 26 – leg agility). The MAS corresponded to the side with higher sum of bradykinesia sub-scores. For control subjects, the average of both sides was used for comparison. This classification has been used in previous work [2].
2.4. Statistical Analysis
A Repeated Measures ANOVA was run with group (PD vs Control) as a between-group factor, and location (Laboratory vs Home) as a within-group factor for each parameter (α = 0.05). Tukey-Kramer tests were run as post-hoc comparisons.
3. RESULTS
The distances walked at home were shorter than the laboratory, and similar between groups: PD = 5.9 ± 0.5 meters, Control = 5.9 ± 0.6 meters. The characteristics of the houses were: 3 Control and 3 PD group houses had hardwood or laminate floors, while the remaining houses had carpeted floors; all had both natural and artificial lighting; all had an armless firm chair for performing the iTUG; and all but one house had walls or furniture within 2 feet of the turning position. These findings show that home testing was feasible because the homes provided enough space and met the criteria to accommodate testing.
Results comparing home versus laboratory are summarized in Figure 1 and Table 1. There was a significant group effect for stride velocity (p=0.03, Fig.1A, Table 1), cadence (p=0.001, Fig.1C, Table 1), peak arm swing velocity MAS (p=0.002, Fig.1D, Table 1) and turning velocity (p=0.003, Fig.1E, Table 1). There was a significant interaction effect for stride velocity (p=0.02, Fig.1A, Table 1) and stride length (p=0.002 Fig.1B, Table 1). The interaction effect indicates the difference between groups depends on location; with the Control group performing similarly in both settings and PD group walking significantly slower at home. There was a significant location effect for turning velocity (p=0.002 Fig.1E, Table 1). A post-hoc analysis showed that the location effect was significant for the Control group only.
Figure 1. Comparison of gait measurements between groups and locations on the iTUG test.
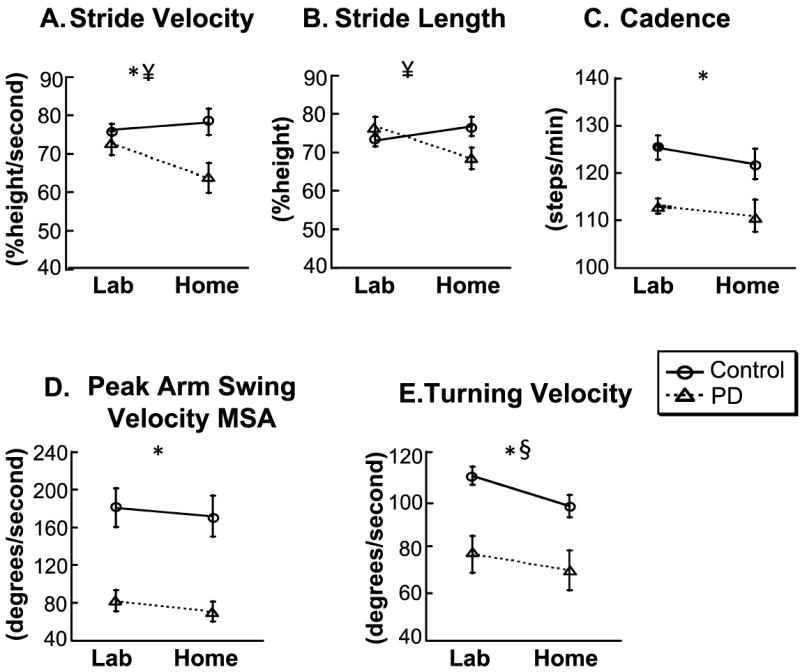
Symbols: *group effect, ¥ interaction effect, § location effect. Fig 1A and B shows that patients walk slower and take shorter steps than controls at home, whereas in the laboratory there is no difference between the groups. Fig. 1 C and D shows that patients walk with slower cadence and swing their most affected arm slower than controls independent of the location. Fig. 1E shows that patients turn slower than controls in the lab and at home. A post-hoc analysis revealed that the location effect is only for controls: controls turn slower at home when compared to the laboratory, whereas there is no difference on location for the PD group.
4. DISCUSSION
The objectives of this pilot study were to investigate the feasibility of administering the iTUG in the home and to compare performance in the home versus the laboratory. Our results show that home testing is feasible, and that the home environment affected subjects’ performance on the iTUG, more so for the PD group than the Control group. Our main findings shows that when tested in the laboratory, subjects with PD and control subjects walk with similar step length and velocity, but when tested at home, PD group walked significantly slower with shorter steps than the control group (Fig. 1A,B, Table 1). These preliminary results indicate not only that the iTUG can identify differences between PD and healthy subjects, but also that differences are more apparent at home than in the laboratory; i.e. patients with PD performed worse at home. Because home characteristics were similar between groups, we are confident that the differences found between groups cannot be attributed to home differences, but to the effects of the disease. Our findings also suggest that the home environment makes mobility testing more sensitive than the laboratory environment. Several factors may contribute to our findings:
1) Home environments are more cluttered and constrained than gait laboratories
The houses we visited varied in characteristics (e.g. flooring, lighting, open spaces, furniture arrangement), whereas our gait laboratory was a “standard” area with ample open space, flat linoleum floor and good lighting. Constraints in the environment are known to affect gait in PD. For example, freezing of gait usually is triggered by narrow spaces such as doorways or spaces between furniture [6]. Studies investigating the circumstances of falls in PD have shown that 80% of all fall events happen at home, and fallers are ambulant in 45% of falls [7].
2) People may feel more comfortable and relaxed at home
The laboratory is a formal and non-familiar environment, which may increase alertness and stress. Whereas at home patients may be more relaxed and thus exhibit their typical posture and gait. In a laboratory environment, increased anxiety and attention, as well as increased effort to impress the examiner, may increase dopamine levels in patients with PD [8].
3) Patients may be more distracted at home than in the laboratory
Performance of a cognitive task while walking can interfere with gait in PD subjects even more so than in healthy subjects [9]. The home environment may naturally engender shared attentional resources between walking and a mental task. For example, while walking subjects could have been thinking of a “to do” list, concerns about the home conditions, or the presence of a visitor in their home. In contrast, in the laboratory, they may have automatically directed their attention to the task so that they were more focused on physical performance with less extraneous mental activity.
This pilot study is the first to investigate the use of portable inertial sensors for mobility testing at home and to compare it to the laboratory. One limitation of this study is the small sample size. However, this was meant to be a preliminary study, involving only a few subjects. More studies should follow to confirm our findings in larger groups. In addition, the inherent day-to-day variability in motor performance in PD may raise the question whether the differences we found are due to environmental constrains or the natural variability in PD. However, it is not likely that the variability is the cause of our results because it is more predominant in moderate-to-late stages of the disease, rather than early stages [2], which is the focus of our sample. In addition, our data showed similar changes across PD subjects, whereas day-to-day fluctuations would likely cause some PD subjects to be better and others to be worse when tested in the home.
Inertial systems are a promising technology that has great advantages over other technologies used for mobility assessment at home. Other studies have used accelerometry [10], infrared beams [11], and activity monitors [12], however, these systems provide limited quantitative information on gait and mobility. Inertial sensors are becoming smaller, lighter, more comfortable to wear and practical to operate. However, it remains to be demonstrated whether patients could administer the iTUG by themselves given appropriate instructions and software. In the future, continuous monitoring of gait, turning and arm swing while subjects go about their normal daily activities may be most advantageous because of the large amounts of data that can be gathered throughout the day, rather than performing a scripted task like the TUG.
More research is warranted to determine the specific environmental factors that influence gait and dynamic posture in both healthy people and patients with PD. Future research is also necessary to 1) develop and test a self-administered iTUG, 2) evaluate the reliability and sensitivity of the home-based iTUG, and 3) determine the validity of the iTUG to monitor the progression of mobility deficits.
In conclusion, this study demonstrated that the use of wearable inertial sensors to quantify the TUG test at home is both feasible and sensitive to detect mobility deficits in patients with early PD.
Acknowledgments
We thank our research subjects, Triana Nagel for assisting with subject scheduling and Prof. K. Aminian for providing the Physilog system. This research was supported by the Kinetics Foundation, the National Institutes on Aging (006457) and the Oregon Center for Aging and Technology (AG024978). This research was also supported in part by the Intramural Research Program of the NIH, Clinical Center, Rehabilitation Medicine Department. Dr. Horak was a consultant for the Kinetics Foundation.
Footnotes
This potential conflict of interest has been reviewed and managed by OHSU.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marras C, Rochon P, Lang AE. Predicting motor decline and disability in Parkinson disease: a systematic review. Arch Neurol. 2002;59(11):1724–8. doi: 10.1001/archneur.59.11.1724. [DOI] [PubMed] [Google Scholar]
- 2.Zampieri C, Salarian A, Carlson-Kuhta P, Aminian K, Nutt JG, Horak FB. The instrumented Timed Up and Go test: Potential outcome measure for disease modifying therapies in Parkinson’s Disease. JNNP. 2010;81:171–6. doi: 10.1136/jnnp.2009.173740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aminian K, Najafi B, Bula C, Leyvraz PF, Robert P. Spatio-temporal parameters of gait measured by an ambulatory system using miniature gyroscopes. J Biomech. 2002;35:689–99. doi: 10.1016/s0021-9290(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 4.Salarian A, Russmann H, Vingerhoets FJ, Burkhard PR, Aminian K. Ambulatory monitoring of physical activities in patients with Parkinson’s disease. IEEE Trans Biom Eng. 2007;54(12):2296–9. doi: 10.1109/tbme.2007.896591. [DOI] [PubMed] [Google Scholar]
- 5.Salarian A, Horak FB, Zampieri C, Carlson-Kuhta P, Nutt JG, Kamiar A. iTUG, a Sensitive and Reliable Measure of Mobility. IEEE Trans Neural Syst Rehabil Eng. 2010;18(3):303–10. doi: 10.1109/TNSRE.2010.2047606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okuma Y, Yanagisawa N. The clinical spectrum of freezing of gait in Parkinson’s disease. Mov Disord. 2008;23(Suppl 2):S426–30. doi: 10.1002/mds.21934. [DOI] [PubMed] [Google Scholar]
- 7.Ashburn A, Stack E, Ballinger C, Fazakarley L, Fitton C. The circumstances of falls among people with Parkinson’s disease and the use of Falls Diaries to facilitate reporting. Disabil Rehabil. 2008;30(16):1205–12. doi: 10.1080/09638280701828930. [DOI] [PubMed] [Google Scholar]
- 8.De La Fuente-Fernandez R, Stoessl AJ. The placebo effect in Parkinson’s disease. Trends in Neurosciences. 2002;25(6):302–6. doi: 10.1016/s0166-2236(02)02181-1. [DOI] [PubMed] [Google Scholar]
- 9.Hausdorff JM, Balash J, Giladi N. Effects of cognitive challenge on gait variability in patients with Parkinson’s disease. J Geriatr Psychiatry Neurol. 2003;16(1):53–8. doi: 10.1177/0891988702250580. [DOI] [PubMed] [Google Scholar]
- 10.Keijsers NL, Horstink MW, Gielen SC. Ambulatory motor assessment in Parkinson’s disease. Mov Disord. 2006;21(1):34–44. doi: 10.1002/mds.20633. [DOI] [PubMed] [Google Scholar]
- 11.Pavel M, Hayes T, Tsay I, Erdogmus D, Paul A, Larimer N, et al. Continuous assessment of gait velocity in Parkinson’s disease from unobtrusive measurements. Conf Proc IEEE Eng Med Biol Soc. 2007:700–3. [Google Scholar]
- 12.White DK, Wagenaar RC, Del Olmo ME, Ellis TD. Test-retest reliability of 24 hours of activity monitoring in individuals with Parkinson’s disease in home and community. Neurorehabil Neural Repair. 2007;21(4):327–40. doi: 10.1177/1545968306297867. [DOI] [PubMed] [Google Scholar]


