Abstract
During spinal neurogenesis, the p2 progenitor domain generates at least two subclasses of interneurons named V2a and V2b which are components of the locomotor central pattern generator. The winged-helix/forkhead transcription factor Foxn4 is expressed in a subset of p2 progenitors and required for specifying V2b interneurons. Here we report the generation of a Foxn4-Cre BAC transgenic mouse line that drives Cre recombinase expression mimicking endogenous Foxn4 expression pattern in the developing spinal cord. We used this transgenic line to map neuronal lineages derived from Foxn4-expressing progenitors and found that they gave rise to all neurons of the V2a, V2b as well as the newly identified V2c lineages. These data suggest that Foxn4 may be transiently expressed by all p2 progenitors and that the Foxn4-Cre line may serve as a useful genetic tool not only for lineage analysis but also for functional studies of genes and neurons involved in locomotion.
Keywords: Foxn4, transcription factor, V2 interneuron, CPG
INTRODUCTION
Spinal cord development begins with the formation of the neural tube, followed by the generation of distinct neuronal and glial cell types in the tube, and concludes with the establishment of functional sensory and motor circuits in the mature cord. The neuronal fates along the dorsoventral axis of the neural tube are controlled in part by signaling molecules secreted from the dorsal and ventral midlines in a concentration-dependent manner (Tanabe and Jessell, 1996; Ericson et al., 1997a; Ericson et al., 1997b; Briscoe et al., 1999; Briscoe et al., 2000; Jessell, 2000). For instance, the opposing activity gradients of Shh and BMPs/Wnts are shown to activate the expression of transcription factors (Liem et al., 1997; Lee et al., 1998; Lee et al., 2000; Liem et al., 2000; Mekki-Dauriac et al., 2002; Patten and Placzek, 2002), which in turn define different progenitor domains from which distinct neuronal cell types arise (Lee and Jessell, 1999; Briscoe et al., 2000; Jessell, 2000; Shirasaki and Pfaff, 2002; Caspary and Anderson, 2003).
In the ventral neural tube, the ventricular zone is organized into one motor neuron progenitor domain (pMN) and four interneuron progenitor domains (p0-p3) that give rise to motor neurons (MN) and several classes of interneurons (V0-V3), respectively (Burrill et al., 1997; Ericson et al., 1997a; Matise and Joyner, 1997; Pierani et al., 1999; Briscoe et al., 2000). Each of the progenitor domains is defined by the combinatorial activities of a set of transcription factors, in particular, the homeoproteins and bHLH factors (Pierani et al., 1999; Briscoe et al., 2000; Jessell, 2000; Zhou et al., 2000; Mizuguchi et al., 2001; Novitch et al., 2001; Zhou et al., 2001; Shirasaki and Pfaff, 2002; Muroyama et al., 2005). The p2 domain generates at least two molecularly distinct interneuron subclasses named V2a and V2b (Briscoe et al., 2000; Karunaratne et al., 2002; Li et al., 2005; Muroyama et al., 2005). V2a cells are mostly glutamatergic excitatory neurons defined by their expression of Chx10; whereas V2b cells are mostly inhibitory GABAergic/glycinergic neurons characterized by their expression of Gata2 and Gata3 (Briscoe et al., 2000; Karunaratne et al., 2002; Al-Mosawie et al., 2007; Lundfald et al., 2007). The binary V2a and V2b fates are selected by Dll-Notch signaling and consolidated by the activity of V2a- and V2b-specific LIM protein complexes involving Lhx3, LMO4, NLI, Scl, and Gata2 (Del Barrio et al., 2007; Peng et al., 2007; Joshi et al., 2009). Although the V2b function is currently unknown, V2a neurons have been shown recently to control speed-dependent left-right locomotor coordination (Crone et al., 2008; Crone et al., 2009).
Foxn4 is a winged-helix/forkhead transcription factor transiently expressed in a subset of progenitors during spinal neurogenesis and retinogenesis. It has been shown by gene targeting and gain-of-function analyses to play a crucial role in the generation of amacrine and horizontal cells during retinal development (Li et al., 2004). In the spinal cord, Foxn4 is expressed by a subset of cells in the p2 progenitor domain (Li et al., 2005). Foxn4 inactivation causes a complete loss of V2b neurons with a concomitant fate-switch to V2a neurons whereas its misexpression promotes the V2b fate (Li et al., 2005; Del Barrio et al., 2007), thus demonstrating both the necessity and sufficiency for Foxn4 to promote V2b neuron differentiation. Foxn4 acts genetically upstream of the bHLH factors Mash1 and Scl, both of which are required for specifying the V2b fate (Li et al., 2005; Muroyama et al., 2005), as well as controls the expression of Dll4, a ligand for Notch signaling (Del Barrio et al., 2007).
Delineation of neuronal lineages is important for providing a framework to understand the molecular basis of neurogenesis. Our previous immunostaining and knockin analyses have localized Foxn4 to a subset of p2 progenitors and identified a subset of V2a and V2b cells as the progeny of Foxn4-expressing progenitors (Li et al., 2005; Del Barrio et al., 2007), implicating the expression of Foxn4 only by a subset of p2 progenitors. However, due to the transient nature of Foxn4 expression, a possibility exists that Foxn4 may be transiently expressed by all p2 progenitors which give rise to all V2a and V2b cells. Thus, the neural lineages of Foxn4-expressing progenitors have yet to be clearly described during spinal cord development. In this work, we generated a Foxn4-Cre BAC transgenic mouse line and employed the Cre-loxP fate-mapping strategy to determine the cell lineages derived from Foxn4-expressing progenitors in the ventral spinal cord. Our results indicate that the Foxn4-Cre transgene can drive Cre recombinase expression mimicking that of the endogenous Foxn4 in the developing spinal cord and therefore should be suitable for conditional deletion/ablation of genes and neurons in the V2 domain.
RESULTS AND DISCUSSION
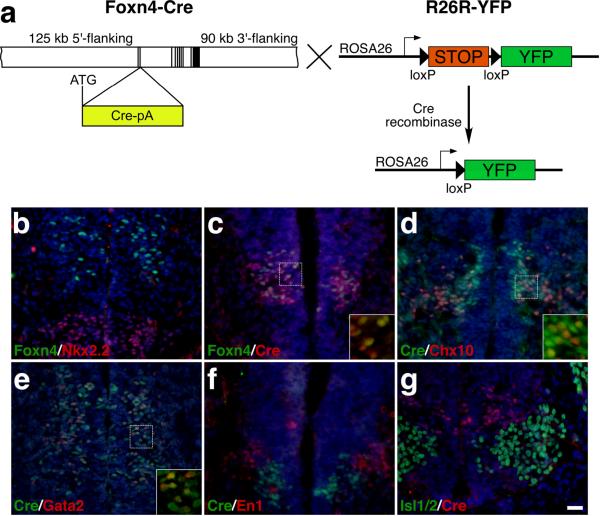
To generate the Foxn4-Cre mice, a BAC containing the Foxn4 locus was modified by recombineering in E. coli to insert the Cre coding region at the Foxn4 translation initiation site (Fig. 1a) (Copeland et al., 2001; Gong et al., 2002; Warming et al., 2005; Yang et al., 2006). This BAC transgenic construct contains ~125 kb 5′ flanking sequence, all exons and introns, and ~90 kb 3′ flanking sequence (Fig. 1a), thereby increasing the likelihood of sufficient regulatory information to recapitulate the endogenous Foxn4 expression pattern as demonstrated for many other genes (Yang et al., 1997; Heintz, 2001; Battiste et al., 2007). We obtained three founder lines for this transgenic construct and all of them displayed a similar Cre expression pattern in the developing ventral spinal cord (Fig. 1c-g).
FIG. 1.
Generation of Foxn4-Cre transgenic mice that express Cre recombinase in the p2/V2 domain of the spinal cord. (a) The Cre-loxP system for conditional activation of reporter YFP expression using Foxn4-Cre and R26R-YFP mice. To generate the Foxn4-Cre BAC, a BAC containing the Foxn4 locus was modified by recombineering to insert the Cre coding region (Cre-pA) at the Foxn4 translation initiation site (ATG) to generate the Foxn4-Cre BAC. The exons are indicated by vertical black bars and the estimated lengths of 5′ and 3′ flanking sequences are also indicated. The transgenic line was crossed with R26R-YFP mice to activate YFP expression in the spinal cord. (b-g) Spinal cord sections from E10.5 wild-type (b) and Foxn4-Cre (c-g) embryos were labeled by double-immunofluorescence using the indicated antibodies and weakly counterstained with nuclear DAPI. There is colocalization between Cre and Foxn4, Chx10 and Gata2 in cells of the p2/V2 domain but no colocalization between Cre and En1 or Isl1/2. Insets in (c-e) show corresponding outlined regions at a higher magnification. Scale bar equals 25 μm (b-g).
To ask whether Foxn4-Cre can mimic the spatial expression pattern of the endogenous Foxn4 gene (Fig. 1b), we carried out double-immunolabeling between Cre and markers for p2/V2 and adjacent domains. In the ventral spinal cord of E10.5 Foxn4-Cre transgenic embryos, Cre was seen in clusters of cells at the p2/V2 position, many of which co-expressed Foxn4, Chx10 or Gata2 (Fig. 1c-e). The presence of Cre in Chx10+ and Gata2+ cells is likely due to the perdurability of Cre since Foxn4 is normally expressed only in progenitors but not in V2a and V2b interneurons (Li et al., 2005). This perdurability may also explain why Cre is present in more cells than Foxn4 (Fig. 1c). There was no expression of Cre in En1+ V1 cells or in Isl1/2+ motor neurons (Fig. 1f,g). Thus, the Foxn4-Cre transgene appears to be able to largely recapitulate the expression pattern of the endogenous Foxn4 gene in the developing spinal cord. However, given the presence of Cre in more numerous cells than Foxn4, there is still a possibility that the BAC used may not contain all the regulatory elements necessary to completely mimic endogenous Foxn4 expression.
To determine the neuronal offspring of Foxn4-expressing progenitors, we crossed the Foxn4-Cre line with the conditional reporter R26R-YFP mice, in which all cells would constitutively express YFP following a Cre-mediated recombination event to remove a transcription stop cassette (Fig. 1a) (Srinivas et al., 2001). When spinal cord sections from E14.5 Foxn4-Cre;R26R-YFP embryos were double-labeled with an anti-GFP antibody and the nuclear marker DAPI, we found that Foxn4-expressing progenitors gave rise to many YFP+ cells scattered within the ventral gray matter with their axons projected into the ventrolateral funiculus (Fig. 2a,e). The ventral commissure appeared to be largely devoid of YFP+ processes (Fig. 2a,e), consistent with the finding that V2 interneurons are mostly non-commissural neurons that project ipsilaterally (Al-Mosawie et al., 2007; Lundfald et al., 2007).
FIG. 2.
Foxn4-expressing cells give rise to all interneurons of the three known V2 lineages. (a-p) Spinal cord sections from E14.5 (a-h) and E12.5 (i-p) Foxn4-Cre;R26R-YFP embryos were stained by double-immunofluorescence using the indicated antibodies. Sections in (a,e,i,m) were also weakly counterstained with DAPI. Note the colocalization between YFP and Chx10 in V2a neurons (a-d), between YFP and Gata2 in V2b neurons (e-h), and between YFP and Sox1 in V2c neurons (indicated by arrows) (i-l). YFP and Isl1/2 are not colocalized in motor neurons (m-p). The dashed ovals in (i) outline the regions where Sox1-immunoreactive V2c cells are located. CC, central canal; VLF, ventrolateral funiculus; VZ, ventricular zone. Scale bar equals 50 μm (a,e,i,m), 12.5 μm (b-d,f-h,j-l), and 12 μm (n-p).
Double-immunostaining of E14.5 spinal cord sections with antibodies against V2 markers revealed the expression of YFP in all Chx10+ and Gata2+ cells (Figs. 2a-h, 3a), indicating that all V2a and V2b interneurons arise from Foxn4-expressing progenitors. However, only about 37.2% and 25.5% of YFP+ cells co-expressed Chx10 and Gata2, respectively (Figs. 2a-h, 3b), suggesting that many V2 interneurons may completely downregulate the expression of V2 markers by E14.5 and/or Foxn4 may be expressed in additional unidentified V2 interneurons or glial cells. In spinal cord sections of E12.5 Foxn4-Cre; R26R-YFP embryos, we investigated whether YFP+ cells co-express Sox1, which is found in a small subset of interneurons that are derived from the p2 domain and designated as V2c (Panayi et al., 2010). As shown in Fig. 2I-L and by quantification (Fig. 3a), all ventrolaterally located Sox1+ cells were immunoreactive for GFP, demonstrating that Foxn4-expressing progenitors also give rise to all Sox1+ V2c interneurons. Approximately 4% of all YFP+ cells co-expressed Sox1 in the ventrolateral area (Fig. 3b), consistent with V2c cells forming a very small subclass of V2 interneurons (Panayi et al., 2010). By contrast, motor neurons are not generated from the p2 progenitor domain and correspondingly we did not observe YFP+ cells that co-expressed the motor neuron marker Isl1/2 (Fig. 2m-p).
FIG. 3.

Percentages of V2 marker-positive cells that are immunoreactive for GFP (a), and GFP-positive cells that are immunoreactive for V2 cell markers (b) in Foxn4-Cre;R26R-YFP spinal cords. Each histogram in (b) represents the mean±SD for 3 different spinal cords. Note that Chx10+ and Gata2+ cells were counted at E14.5 but counts of Sox1+ cells were obtained at E12.5 when these cells could be optimally labeled.
Foxn4 is seen only in a limited number of p2 progenitors at a given stage during spinal cord development (Fig. 1b,c) (Li et al., 2005). Moreover, the progeny marked by β-gal expressed from the knock-in lacZ reporter in Foxn4lacZ/+ spinal cords encompasses only a proportion of V2a and V2b interneurons (Li et al., 2005; Del Barrio et al., 2007). These observations raise questions about whether all p2 progenitors transiently express Foxn4 and whether Foxn4-expressing progenitors give rise to all V2 interneurons or only a subset. Our lineage analyses suggest that all V2a, V2b and V2c interneurons arise from p2 progenitors that express Foxn4 at some point in life. Consistent with this, there is a complete absence of V2b and V2c neurons in the spinal cord of Foxn4 null mutant mice (Li et al., 2005; Panayi et al., 2010). Thus, despite the apparent expression of Foxn4 only in a subset of p2 progenitors (Li et al., 2005), a broader than anticipated complement of V2 interneurons arise from Foxn4-expressing progenitors. Similarly, a recent fate-mapping study has revealed that Ascl1-expressing progenitors in the spinal cord generate oligodendrocytes more extensively than previously appreciated (Battiste et al., 2007).
V2 neurons constitute part of the locomotor central pattern generator (CPG) network in the spinal cord, which includes also V0, V1 and V3 interneurons and motor neurons (Goulding, 2009). The CPG is an intrinsic neural network capable of generating rhythmic patterns of motor activity without sensory inputs. At present, the developmental, cellular and physiological events leading to locomotor rhythm still remain poorly defined. Gene targeting experiments have shown that V0 neurons are essential for proper coordination of left-right locomotor activity (Lanuza et al., 2004). Genetic ablation of V1 cells demonstrates a key role for these interneurons in the generation of fast locomotor outputs (Gosgnach et al., 2006). V2a cells are mostly glutamatergic excitatory interneurons (Al-Mosawie et al., 2007; Lundfald et al., 2007). Selective ablation of V2a interneurons by diphtheria toxin results in disrupted left-right coordination at high speeds and increased variability in frequency and amplitude of locomotor rhythm (Crone et al., 2008; Crone et al., 2009). This subclass of interneurons is therefore required for maintaining left-right alternation at high speeds of locomotion as well as for stabilizing the locomotor rhythm. V2b cells on the other hand are mostly GABAergic/glycinergic inhibitory interneurons (Al-Mosawie et al., 2007; Lundfald et al., 2007). It is currently unknown how the V2b and V2c subclasses of V2 interneurons participate in the CPG network. In this regard, our Foxn4-Cre transgenic line should provide a useful genetic tool not only for lineage analysis but also for domain- and neuron-specific gene manipulation to study functions of genes and neurons in the V2 domain.
MATERIALS AND METHODS
Generation of Transgenic Mice by BAC Recombineering
A Foxn4 BAC (Clone-ID: RP23-154G3) was purchased from Invitrogen. The Foxn4-Cre BAC transgenic construct was prepared primarily following the online BAC recombineering protocols (http://web.ncifcrf.gov/research/brb/recombineeringInformation.aspx) (Copeland et al., 2001). Two adapters located in the exon with the translation start site were used for homologous recombination, which resulted in the immediate control of Cre gene under the endogenous Foxn4 promoter without any extra sequence. The two adapters are from two pairs of oligos: 5′end: 5′ CCGCTCGAGATTGTCCTGCAATTGTGACCCTTTTGG 3′ and 5′ CGCAAGCTTATTTCCTTAGGGATGAAAAAAAAAAG 3′ and 3′ end: 5′ CGGACTAGTATAGAAAGTGGCATTTGGTCCAGAATG 3′and 5′ TCCCCGCGGGTATTCCTGTGGAGAGCAGTGGTGG 3′. Three Foxn4-Cre transgenic founder lines were produced at the transgenic/knockout core facility of the Cancer Institute of New Jersey using F1 (C57BL/6×CBA) with the finished construct. All three lines exhibited a similar reporter expression pattern in the spinal cord and the final analysis was mainly based on one line. The conditional reporter R26R-YFP mice were purchased from the Jackson Laboratory (Srinivas et al., 2001).
Immunofluorescence
Staged mouse embryos were collected and fixed with 4% PFA/PBS and processed for cryosections. Immunofluorescent staining of cryosections was then carried out as previously described (Li et al., 2004). The antibodies used in the immunostaining analysis are: anti-GFP (for YFP) (goat, 1:1000, Abcam), anti-GFP (for YFP) (rabbit, 1:400, MBL International), anti-Foxn4 (rabbit, 1:50) (Li et al., 2004), anti-Cre (mouse, 1:1000, Covance), anti-Cre (rabbit, 1:10000, EMD Chemicals), anti-Chx10 (sheep, 1:1600, Exalpha Biologicals), anti-Gata2 (rabbit, 1:200, Santa Cruz Biotechnology), anti-Gata2 (guinea pig, 1:2000) (Peng et al., 2007), anti-En1 [mouse, 1:25, Developmental Studies Hybridoma Bank (DSHB)], anti-Nkx2.2 (mouse, 1:50, DSHB), anti-Isl1 (mouse, 1:50, DSHB), and anti-Sox1 (guinea pig, 1:500) (Panayi et al., 2010).
Quantification
To quantify YFP+ cells and YFP+ cells colocalized with cell type-specific markers in the spinal cord, three comparable samples of each type were counted for positively labeled cells on serial semi-sections located at the thoracic region. The results were analyzed statistically.
ACKNOWLEDGEMENTS
We thank Drs. Stavros Malas (Cyprus Institute of Neurology and Genetics) and Kamal Sharma (University of Chicago) for the anti-Sox1 and anti-Gata2 antibodies, respectively, and Drs. Kangxin Jin and Huijun Luo for thoughtful comments on the manuscript. This work was supported by the National Institutes of Health (EY015777 and EY012020 to M.X.) and the New Jersey Commission on Spinal Cord Research (09-3087-SCR-E-0 to M.X.).
Contract grant sponsor: NIH; Contract grant number: EY015777 and EY012020; Contract grant sponsor: New Jersey Commission on Spinal Cord Research; Contract grant number: 09-3087-SCR-E-0.
LITERATURE CITED
- Al-Mosawie A, Wilson JM, Brownstone RM. Heterogeneity of V2-derived interneurons in the adult mouse spinal cord. Eur J Neurosci. 2007;26:3003–3015. doi: 10.1111/j.1460-9568.2007.05907.x. [DOI] [PubMed] [Google Scholar]
- Battiste J, Helms AW, Kim EJ, Savage TK, Lagace DC, Mandyam CD, Eisch AJ, Miyoshi G, Johnson JE. Ascl1 defines sequentially generated lineage-restricted neuronal and oligodendrocyte precursor cells in the spinal cord. Development. 2007;134:285–293. doi: 10.1242/dev.02727. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Pierani A, Jessell TM, Ericson J. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–445. doi: 10.1016/s0092-8674(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Sussel L, Serup P, Hartigan-O’Connor D, Jessell TM, Rubenstein JL, Ericson J. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999;398:622–627. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]
- Burrill JD, Moran L, Goulding MD, Saueressig H. PAX2 is expressed in multiple spinal cord interneurons, including a population of EN1+ interneurons that require PAX6 for their development. Development. 1997;124:4493–4503. doi: 10.1242/dev.124.22.4493. [DOI] [PubMed] [Google Scholar]
- Caspary T, Anderson KV. Patterning cell types in the dorsal spinal cord: what the mouse mutants say. Nat Rev Neurosci. 2003;4:289–297. doi: 10.1038/nrn1073. [DOI] [PubMed] [Google Scholar]
- Copeland NG, Jenkins NA, Court DL. Recombineering: a powerful new tool for mouse functional genomics. Nat Rev Genet. 2001;2:769–779. doi: 10.1038/35093556. [DOI] [PubMed] [Google Scholar]
- Crone SA, Quinlan KA, Zagoraiou L, Droho S, Restrepo CE, Lundfald L, Endo T, Setlak J, Jessell TM, Kiehn O, Sharma K. Genetic ablation of V2a ipsilateral interneurons disrupts left-right locomotor coordination in mammalian spinal cord. Neuron. 2008;60:70–83. doi: 10.1016/j.neuron.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Crone SA, Zhong G, Harris-Warrick R, Sharma K. In mice lacking V2a interneurons, gait depends on speed of locomotion. J Neurosci. 2009;29:7098–7109. doi: 10.1523/JNEUROSCI.1206-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Barrio MG, Taveira-Marques R, Muroyama Y, Yuk DI, Li S, Wines-Samuelson M, Shen J, Smith HK, Xiang M, Rowitch D, Richardson WD. A regulatory network involving Foxn4, Mash1 and delta-like 4/Notch1 generates V2a and V2b spinal interneurons from a common progenitor pool. Development. 2007;134:3427–3436. doi: 10.1242/dev.005868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson J, Briscoe J, Rashbass P, van Heyningen V, Jessell TM. Graded Sonic Hedgehog signaling and the specification of cell fate in the ventral neural tube. Cold Spring Harb Symp Quant Biol. 1997a;62:451–466. [PubMed] [Google Scholar]
- Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, van Heyningen V, Jessell TM, Briscoe J. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell. 1997b;90:169–180. doi: 10.1016/s0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- Gong S, Yang XW, Li C, Heintz N. Highly efficient modification of bacterial artificial chromosomes (BACs) using novel shuttle vectors containing the R6Kgamma origin of replication. Genome Res. 2002;12:1992–1998. doi: 10.1101/gr.476202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosgnach S, Lanuza GM, Butt SJ, Saueressig H, Zhang Y, Velasquez T, Riethmacher D, Callaway EM, Kiehn O, Goulding M. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature. 2006;440:215–219. doi: 10.1038/nature04545. [DOI] [PubMed] [Google Scholar]
- Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci. 2009;10:507–518. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N. BAC to the future: the use of BAC transgenic mice for neuroscience research. Nat Rev Neurosci. 2001;2:861–870. doi: 10.1038/35104049. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Joshi K, Lee S, Lee B, Lee JW, Lee SK. LMO4 controls the balance between excitatory and inhibitory spinal V2 interneurons. Neuron. 2009;61:839–851. doi: 10.1016/j.neuron.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunaratne A, Hargrave M, Poh A, Yamada T. GATA proteins identify a novel ventral interneuron subclass in the developing chick spinal cord. Dev Biol. 2002;249:30–43. doi: 10.1006/dbio.2002.0754. [DOI] [PubMed] [Google Scholar]
- Lanuza GM, Gosgnach S, Pierani A, Jessell TM, Goulding M. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron. 2004;42:375–386. doi: 10.1016/s0896-6273(04)00249-1. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Dietrich P, Jessell TM. Genetic ablation reveals that the roof plate is essential for dorsal interneuron specification. Nature. 2000;403:734–740. doi: 10.1038/35001507. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Jessell TM. The specification of dorsal cell fates in the vertebrate central nervous system. Annu Rev Neurosci. 1999;22:261–294. doi: 10.1146/annurev.neuro.22.1.261. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Mendelsohn M, Jessell TM. Neuronal patterning by BMPs: a requirement for GDF7 in the generation of a discrete class of commissural interneurons in the mouse spinal cord. Genes Dev. 1998;12:3394–3407. doi: 10.1101/gad.12.21.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Misra K, Matise MP, Xiang M. Foxn4 acts synergistically with Mash1 to specify subtype identity of V2 interneurons in the spinal cord. Proc Natl Acad Sci U S A. 2005;102:10688–10693. doi: 10.1073/pnas.0504799102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Mo Z, Yang X, Price SM, Shen MM, Xiang M. Foxn4 controls the genesis of amacrine and horizontal cells by retinal progenitors. Neuron. 2004;43:795–807. doi: 10.1016/j.neuron.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Liem KF, Jr., Jessell TM, Briscoe J. Regulation of the neural patterning activity of sonic hedgehog by secreted BMP inhibitors expressed by notochord and somites. Development. 2000;127:4855–4866. doi: 10.1242/dev.127.22.4855. [DOI] [PubMed] [Google Scholar]
- Liem KF, Jr., Tremml G, Jessell TM. A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell. 1997;91:127–138. doi: 10.1016/s0092-8674(01)80015-5. [DOI] [PubMed] [Google Scholar]
- Lundfald L, Restrepo CE, Butt SJ, Peng CY, Droho S, Endo T, Zeilhofer HU, Sharma K, Kiehn O. Phenotype of V2-derived interneurons and their relationship to the axon guidance molecule EphA4 in the developing mouse spinal cord. Eur J Neurosci. 2007;26:2989–3002. doi: 10.1111/j.1460-9568.2007.05906.x. [DOI] [PubMed] [Google Scholar]
- Matise MP, Joyner AL. Expression patterns of developmental control genes in normal and Engrailed-1 mutant mouse spinal cord reveal early diversity in developing interneurons. J Neurosci. 1997;17:7805–7816. doi: 10.1523/JNEUROSCI.17-20-07805.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekki-Dauriac S, Agius E, Kan P, Cochard P. Bone morphogenetic proteins negatively control oligodendrocyte precursor specification in the chick spinal cord. Development. 2002;129:5117–5130. doi: 10.1242/dev.129.22.5117. [DOI] [PubMed] [Google Scholar]
- Mizuguchi R, Sugimori M, Takebayashi H, Kosako H, Nagao M, Yoshida S, Nabeshima Y, Shimamura K, Nakafuku M. Combinatorial roles of olig2 and neurogenin2 in the coordinated induction of pan-neuronal and subtype-specific properties of motoneurons. Neuron. 2001;31:757–771. doi: 10.1016/s0896-6273(01)00413-5. [DOI] [PubMed] [Google Scholar]
- Muroyama Y, Fujiwara Y, Orkin SH, Rowitch DH. Specification of astrocytes by bHLH protein SCL in a restricted region of the neural tube. Nature. 2005;438:360–363. doi: 10.1038/nature04139. [DOI] [PubMed] [Google Scholar]
- Novitch BG, Chen AI, Jessell TM. Coordinate regulation of motor neuron subtype identity and pan-neuronal properties by the bHLH repressor Olig2. Neuron. 2001;31:773–789. doi: 10.1016/s0896-6273(01)00407-x. [DOI] [PubMed] [Google Scholar]
- Panayi H, Orford M, Panayiotou E, Genethliou N, Mean R, Lapathitis G, Li S, Xiang M, Kessaris N, Richardson WD, Malas S. SOX1 is required for the specification of a novel p2-derived interneuron subtype in the mouse ventral spinal cord. J Neurosci. 2010 doi: 10.1523/JNEUROSCI.2402-10.2010. (in revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten I, Placzek M. Opponent activities of Shh and BMP signaling during floor plate induction in vivo. Curr Biol. 2002;12:47–52. doi: 10.1016/s0960-9822(01)00631-5. [DOI] [PubMed] [Google Scholar]
- Peng CY, Yajima H, Burns CE, Zon LI, Sisodia SS, Pfaff SL, Sharma K. Notch and MAML signaling drives Scl-dependent interneuron diversity in the spinal cord. Neuron. 2007;53:813–827. doi: 10.1016/j.neuron.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierani A, Brenner-Morton S, Chiang C, Jessell TM. A sonic hedgehog-independent, retinoid-activated pathway of neurogenesis in the ventral spinal cord. Cell. 1999;97:903–915. doi: 10.1016/s0092-8674(00)80802-8. [DOI] [PubMed] [Google Scholar]
- Shirasaki R, Pfaff SL. Transcriptional codes and the control of neuronal identity. Annu Rev Neurosci. 2002;25:251–281. doi: 10.1146/annurev.neuro.25.112701.142916. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe Y, Jessell TM. Diversity and pattern in the developing spinal cord. Science. 1996;274:1115–1123. doi: 10.1126/science.274.5290.1115. [DOI] [PubMed] [Google Scholar]
- Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 2005;33:e36. doi: 10.1093/nar/gni035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XW, Model P, Heintz N. Homologous recombination based modification in Escherichia coli and germline transmission in transgenic mice of a bacterial artificial chromosome. Nat Biotechnol. 1997;15:859–865. doi: 10.1038/nbt0997-859. [DOI] [PubMed] [Google Scholar]
- Yang Z, Jiang H, Chachainasakul T, Gong S, Yang XW, Heintz N, Lin S. Modified bacterial artificial chromosomes for zebrafish transgenesis. Methods. 2006;39:183–188. doi: 10.1016/j.ymeth.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Choi G, Anderson DJ. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron. 2001;31:791–807. doi: 10.1016/s0896-6273(01)00414-7. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wang S, Anderson DJ. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 2000;25:331–343. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]