Abstract
Objective
To examine the associations of cigarette smoking with rheumatoid arthritis (RA) in African Americans and to determine to whether this association is impacted by HLA-DRB1 shared epitope (SE).
Methods
Smoking status, cumulative smoking exposure, and SE status were measured in African American patients with RA and in healthy controls. Associations of smoking with RA were examined using age- and gender-adjusted logistic regression. Additive and multiplicative SE-smoking interactions were examined.
Results
After adjusting for age and gender, ever (OR = 1.45; 95% CI 1.07 to 1.97) and current smoking (OR = 1.56; 95% CI 1.07 to 2.26) were more common in African American RA cases (n = 605) than in controls (n = 255). The association of smoking with RA was limited to those with a cumulative exposure exceeding 10 pack-years, associations that were evident in both autoantibody positive and negative disease. There was evidence of a significant additive interaction between SE status and heavy smoking (≥ 10 pack-years) in RA risk (attributable proportion due to interaction [AP] of 0.58, p = 0.007) with an AP of 0.47 (p = 0.006) between SE status and ever smoking. There was no evidence of multiplicative interactions.
Conclusion
Among African Americans, cigarette smoking is associated not only with the risk of autoantibody positive RA but also with the risk of autoantibody negative disease. RA risk attributable to smoking is limited to African Americans with more than 10 pack-years of exposure and is more pronounced among individuals positive for HLA-DRB1 SE.
Keywords: rheumatoid arthritis, African Americans, cigarette smoking, rheumatoid factor, anti-CCP antibody, HLA-DRB1 shared epitope
Since initial reports published more than twenty years ago (1), cigarette smoking has repeatedly been shown to be associated with rheumatoid arthritis (RA) susceptibility (2–10), a risk most pronounced among heavy smokers (2, 11). Studies in populations of European ancestry have shown that the relationship of smoking with RA risk appears to be impacted by the presence of HLA-DRB1 shared epitope (SE) containing alleles (7, 12, 13), but the mechanisms underpinning this interaction have yet to be fully defined. The associations of cigarette smoking with disease risk in populations of European ancestry also appear to be limited to those developing seropositive RA, which is characterized by the presence of either rheumatoid factor (RF) or anti-cyclic citrullinated peptide (CCP) antibody in the serum (2, 6).
Prior reports examining the association of cigarette smoking with RA risk have almost exclusively involved populations of European ancestry. The lack of such studies among African Americans represents an important gap in our knowledge. Although smoking is less frequent in African Americans than in persons of European ancestry (14), smoking incidence appears to be increasing in this population (15) and concomitant rates of smoking cessation in African Americans are consistently lower compared to Caucasians (16). It is unknown whether smoking contributes to RA risk in African Americans and whether this risk is impacted by the presence of HLA-DRB1 SE, a genetic risk factor that is less prevalent in African Americans with RA than in individuals of European ancestry with RA (17). To address these knowledge gaps, we conducted a case-control study to examine the association of cigarette smoking with RA among African Americans, to assess the impact of cumulative exposure, and to define the extent to which this association is affected by HLA-DRB1 SE positivity.
Patients and Methods
Study population
RA cases and healthy controls were participants in the Consortium for the Longitudinal Evaluation of African-Americans with Early Rheumatoid Arthritis (CLEAR) (18–20). All cases satisfied the American College of Rheumatology (ACR) RA classification criteria (21) and all study participants self-reported African American race. Additional information regarding African American heritage (race/ethnicity of parents, grandparents) was not collected. This study included cases and controls from CLEAR-I (RA cases had ≤ 2 years disease duration from time of symptom onset) and CLEAR-II (cases with any disease duration).
African American controls were enrolled based on age, gender, and geographic residence and were recruited predominantly based on lists of telephone numbers from individuals residing in the same mailing zip codes as those of RA cases. These lists were obtained from Genesys / Marketing Systems Group (http://www.m-s-g.com/default.htm). Telephone numbers were selected from census tracts with high percentages of African Americans near the sites enrolling cases. Controls were selected within an age range of ± 10 years based on the mean age of RA cases at each site at a female to male ratio of 3:1 based on the anticipated gender distribution in cases. Potential controls were called by interviewers to determine eligibility and interest and lists of suitable control subjects were then distributed to the sites to arrange study visits.
RA cases and controls were enrolled through one of five sites: the University of Alabama at Birmingham (Birmingham, AL), Emory University (Atlanta, GA), Medical University of South Carolina (Charleston, SC), the University of North Carolina (Chapel Hill, NC), and Washington University (St. Louis, MO). The study was approved by the Institutional Review Board (IRB) at each participating center and all study subjects provided informed written consent prior to participation. Subjects missing data for either smoking status or cumulative smoking exposure were excluded from the analysis (11 RA cases and 7 controls excluded), leaving 605 RA cases and 255 healthy controls evaluable for this analysis.
Smoking status
Information regarding smoking status (current, former, never) was collected at the time of enrollment, and among ever smokers, pack-years of smoking served as the measure of cumulative exposure. Never smoking was defined as having smoked fewer than 100 cigarettes in the subject’s lifetime. Former smokers included individuals smoking ≥ 100 cigarettes over the subject’s lifetime but who quit smoking any time prior to study enrollment. Based on recent reports examining the association of heavy smoking with RA risk among women of European ancestry (11), ever smokers were further categorized based on the magnitude of cumulative exposure (< 10 pack-years and ≥ 10 pack-years). Information specific to ‘second-hand’ or other environmental smoking exposures were not collected as part of this study.
Autoantibody measurement
Autoantibody measurements including anti-CCP antibody and RF, were performed as previously reported using commercially available ELISA kits (18). Anti-CCP antibody (IgG, Diastat, Axis-Shield Diagnostics Ltd., Dundee, Scotland, UK) was measured in arbitrary units (U) per ml and was considered to be positive at a cut-off value ≥ 5 U/ml (18). RF (IgM, INOVA Diagnostics Inc., San Diego, CA, USA) was measured in international units (IU) per ml and was considered positive at concentrations ≥ 9.5 IU/ml (18).
HLA-DRB1 genotyping
High resolution HLA-DRB1 genotyping was performed as previously described, with a previous report showing a higher frequency of SE-containing alleles in African American cases compared to controls(22). HLA-DRB1 SE status was not available for 13 cases (2% of all cases) and 5 controls (2%); these subjects were excluded from analyses that included SE status.
Ancestral Informative Markers (AIMs)
To examine potential ancestral differences in cases and controls, DNA samples from a subset of CLEAR RA cases (n = 561) and controls (n = 231) were genotyped using a custom Illumina chip with 3,317 AIM markers in the laboratory of Dr. Peter Gregersen as part of the International MHC and Autoimmunity Genetics Network (IMAGEN) (23). The percent European ancestry for each participant was calculated based on the AIM genotypes using the software package Structure Version 2.3.1 (24). Simulations were run assuming two founding populations, 10,000 burnins, and 1,000 subsequent replicates to generate the estimates.
Statistical analysis
Subject characteristics (RA cases vs. controls) including the level of European ancestry were compared using descriptive statistics, the Chi-square test for dichotomous variables, and the Student’s t-test for continuous variables. Associations of smoking (current and former vs. never) with case status were examined using unconditional logistic regression, adjusting for age and gender given the differences in these characteristics between cases and controls. To account for effects of cumulative exposure, we examined the associations of heavy smoking (≥ 10 pack-years) with RA risk referent to individuals reporting never smoking combined with individuals reporting < 10 pack-years of smoking. In additional analyses, we examined the aforementioned smoking variables with the risk of autoantibody positive and negative disease, examining associations with anti-CCP antibody and RF positive disease in separate models. Given the small proportion of healthy controls positive for anti-CCP antibody or RF, all controls (n = 255) were included in analyses examining associations of smoking with autoantibody positive and negative RA.
To explore the potential interactions between smoking and HLA-DRB1 SE, additional models stratified by SE status (0 vs. 1 or 2 alleles) were examined. A potential ‘dose effect’ of SE was not examined given the low proportion of African American RA cases homozygous for HLA-DRB1 SE. Analyses stratified by SE status and examining the risk of autoantibody positive and negative RA were considered exploratory. For stratified analyses with small ‘cell sizes’, we also examined the associations of smoking with disease risk using Firth’s penalized likelihood approach, which is an alternative method of addressing issues of small sample sizes and resulting bias in parameter estimates (25, 26).
SE-smoking interactions were examined in two ways. First, we evaluated evidence of departure from additivity using methods previously described by Rothman and colleagues (27). This method has been used in other major epidemiological studies in RA examining additive interactions of HLA-DRB1 SE and smoking (2, 6, 11, 28). Using the methods detailed by Andersson et al (29), we calculated the attributable proportion (AP) due to interaction as the primary measure of additive interaction (AP = 0 corresponds to no interaction and an AP = 1.0 corresponds to ‘complete’ additive interaction). Secondary measures of additive interaction included the relative excess risk due to interaction (RERI), and the synergy index (SI) (29). Confidence intervals (95% CIs) were calculated for the AP, RERI, and SI using the method of Hosmer and Lemeshow (30). A p-value < 0.05 for AP was considered to represent statistically significant additive interaction. Multiplicative interaction was then assessed by modeling the SE-smoking product term in age- and gender-adjusted logistic regression models. To optimize study power, interactions were limited to dichotomous variables (SE-positive vs. SE-negative, ever vs. never smoking, and ≥ 10 pack-years vs. never / < 10 pack-years) and to two-way interactions.
We calculated minimal detectable odds ratios (ORs) for the main effects of ever smoking and heavy smoking with 80% power using a statistical threshold of α = 0.05 (one-sided), assuming that 10% of the variability in multivariate analyses would be explained by covariates. Based on the number of cases and controls available and the smoking exposures observed, the study was powered to detect minimal detectable OR of 1.45 for ever smoking with similar power for the associations of heavy smoking. Based on the assumption that the presence of one risk factor in isolation (SE or smoking) would have an OR = 2.0, we had 78% power to detect a SI of 2.55 for the interaction between SE and ever smoking and 60% power to detect a SI of 2.88 for the interaction between SE and heavy smoking. All analyses were conducted using SAS v9.2 (SAS Institute Inc., Cary, NC).
Results
There were 605 RA cases and 255 healthy controls included in the analysis. Characteristics of cases and controls are shown in Table 1. There were more women among RA cases than among controls (84% vs. 76%, p = 0.004). RA cases were also slightly older than controls (mean 54 vs. 52 years, p = 0.048) and were much more likely to have at least one HLA-DRB1 SE containing allele (40% vs. 23%, p = 0.0001). Only 5% of African American RA cases were homozygous for HLA-DRB1 SE. Among RA cases, the mean (± SD) disease duration was 6.3 ± 8.7 years (1.0 ± 0.6 years in CLEAR-I and 11.2 ± 9.9 years in CLEAR-II) and most were positive for anti-CCP antibody (67%) or RF (76%). Mean (± SD) levels of European admixture based on AIM genotyping did not differ between RA cases (0.14 ± 0.13) and controls (0.13 ± 0.11) (p = 0.59).
Table 1.
Characteristics of African American rheumatoid arthritis (RA) cases and healthy controls; means (± SD) or %
| RA cases (n = 605) |
Controls (n = 255) |
P-value | |
|---|---|---|---|
| Female sex | 84 | 76 | 0.004 |
| Age, years | 54 (13) | 52 (13) | 0.048 |
| Disease duration at baseline visit, years † | 6.3 (9) | ---- | ---- |
| Shared epitope positive (1 or 2 copies) | 40 | 23 | 0.0001 |
| Anti-CCP antibody positive* | 67 | 4 | < 0.0001 |
| RF positive* | 76 | 16 | < 0.0001 |
| Smoking status | |||
| Never | 48 | 56 | |
| Former | 25 | 22 | |
| Current | 27 | 23 | 0.055 |
| Cumulative smoking exposure | |||
| Never or < 10 pk-yrs | 72 | 85 | |
| Ever, ≥ 10 pk-yrs | 28 | 15 | < 0.0001 |
CCP = cyclic citrullinated peptide; RF = rheumatoid factor; HLA-DRB1 shared epitope (SE) data were not available for 13 (2%) of RA cases and 5 (2%) healthy controls; p-values calculated using Chi-square test or Student’s t-test.
Bimodal disease duration: for CLEAR-I mean (± SD) = 1.0 (0.6) years; for CLEAR-II mean 11.2 (9.9)
Smoking status and the frequency of heavy smoking (defined as ≥ 10 pack-years) among RA cases and controls are shown in Table 1. Compared to healthy controls, RA cases were slightly more likely to report former or current smoking and less likely to be never smokers (p = 0.055). Among those reporting a history of ever smoking, heavy smoking was much more common in RA cases (54% of ever smokers) than in controls (35% of ever smokers).
After adjusting for age and sex, RA cases were much more likely than controls to report current smoking vs. never smoking (OR = 1.56; 95% CI 1.07 to 2.26) with a non-significant trend towards higher rates of former smoking in cases (Table 2). As anticipated, the association of ever smoking (OR = 1.45; 95% CI 1.07 to 1.97) with overall RA, referent to never smoking, was intermediate to associations of current and former smoking. Associations of smoking status with RF/anti-CCP antibody positive and negative RA are shown in Table 2. The association of smoking with RA was greatest among heavy smokers (vs. never smokers, OR = 2.37; 95% CI 1.56 to 3.60), an association that was significant for both autoantibody positive and negative disease (Table 2), whether based on anti-CCP antibody or RF status. In contrast, there were no associations of lower cumulative smoking exposure (< 10 pack-years) with RA (Table 2).
Table 2.
Age- and gender-adjusted associations of cigarette smoking with rheumatoid arthritis in African Americans.
| All RA | Anti-CCP positive | Anti-CCP negative | RF positive | RF negative | ||
|---|---|---|---|---|---|---|
| Odds Ratio (95% Confidence Interval) | ||||||
| Smoking status | No. Cases / Controls |
|||||
| Never | 289 / 142 | Ref. | Ref. | Ref. | Ref. | Ref. |
| Former | 153 / 55 | 1.34 (0.91 to 1.97) | 1.42 (0.94 to 2.14) | 1.19 (0.73 to 1.94) | 1.44 (0.96 to 2.16) | 1.07 (0.63 to 1.82) |
| Current | 163 / 58 | 1.56 (1.07 to 2.26) | 1.57 (1.05 to 2.34) | 1.47 (0.92 to 2.35) | 1.66 (1.12 to 2.44) | 1.31 (0.77 to 2.21) |
| Never | 289 / 142 | Ref. | Ref. | Ref. | Ref. | Ref. |
| Ever | 316 / 113 | 1.45 (1.07 to 1.97) | 1.49 (1.07 to 2.08) | 1.32 (0.90 to 1.96) | 1.55 (1.12 to 2.14) | 1.18 (0.77 to 1.81) |
| Cumulative exposure | ||||||
| Never | 289 / 142 | Ref. | Ref. | Ref. | Ref. | Ref. |
| Ever, < 10 pk-yrs | 145 / 74 | 1.00 (0.71 to 1.43) | 1.05 (0.72 to 1.55) | 0.94 (0.59 to 1.49) | 1.08 (0.75 to 1.57) | 0.81 (0.49 to 1.36) |
| Ever, ≥ 10 pk-yrs | 171 / 39 | 2.37 (1.56 to 3.60) | 2.40 (1.54 to 3.74) | 2.11 (1.27 to 3.51) | 2.51 (1.63 to 3.87) | 1.93 (1.11 to 3.35) |
| Never / < 10 pk-yrs | 434 / 216 | Ref. | Ref. | Ref. | Ref. | Ref. |
| Ever, ≥ 10 pk-yrs | 171 / 39 | 2.37 (1.60 to 3.52) | 2.35 (1.55 to 3.58) | 2.16 (1.33 to 3.50) | 2.43 (1.62 to 3.66) | 2.07 (1.23 to 3.50) |
CCP = cyclic citrullinated peptide; RF = rheumatoid factor
Because analyses revealed that lower cumulative smoking exposures (< 10 pack-years) were not associated with an increased risk of RA relative to never smoking, never smokers and ever smokers with < 10 pack-years were combined in the subsequent analyses accounting for cumulative smoking exposure. Compared to never smokers and those smoking < 10 pack-years combined, heavy smoking was significantly associated with the development of both anti-CCP antibody positive (OR = 2.35; 95% CI 1.55 to 3.58) and anti-CCP antibody negative RA (OR = 2.16; 95% CI 1.33 to 3.50), results that were similar to those corresponding to RF positive and RF negative RA (Table 2). Age- and sex-adjusted associations of heavy smoking with overall RA were examined separately and found to be significant in both CLEAR-I (OR = 2.15; 95% CI 1.23 to 3.75) and CLEAR-II (OR = 2.48; 95% CI 1.39 to 4.42) (data not shown).
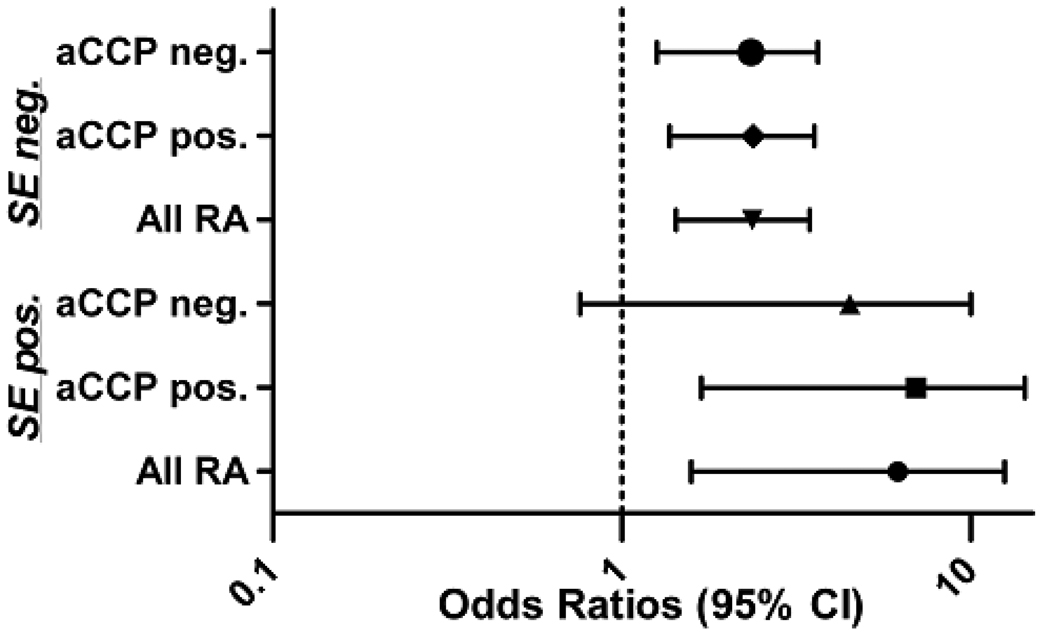
Age- and sex-adjusted associations of heavy smoking with RA, stratified by HLA-DRB1 SE status, are shown in Figure 1. Based on the lower frequency of SE positivity among cases and controls combined (35% of total participants SE positive), coupled with the small number of controls with heavy smoking exposure (n = 39), confidence intervals were universally wider for analyses limited to SE positive individuals compared to analyses in SE negative individuals. Among African Americans with 1 or 2 SE alleles, heavy smoking was associated with a more than 4-fold increased risk of RA (OR = 4.44; 95% CI 1.58 to 12.51). Among SE negative African Americans, the association of heavy smoking was less striking although it remained statistically significant (OR = 2.22; 95% CI 1.43 to 3.45). Associations of heavy smoking with anti-CCP antibody positive and negative RA stratified by SE status were similar to estimates observed for overall disease risk (Figure 1) as were results based on RF positive and negative disease (data not shown). Results of these stratified analyses were not changed after application of Firth’s penalized likelihood approach (25, 26) to account for small cell sizes (data not shown).
Figure 1.
Age- and gender-adjusted associations of heavy smoking (≥ 10 pack-years vs. never or < 10 pack-years) with the risk of overall rheumatoid arthritis (RA), anti-CCP antibody positive RA, and anti-CCP antibody negative RA in African Americans; analyses stratified by HLA-DRB1 shared epitope (SE) status; Odds ratios (ORs) and 95% confidence intervals (CIs) are shown; SE status missing for 13 (2%) of RA cases and 5 (2%) healthy controls.
There was evidence of significant additive interaction of HLA-DRB1 SE status with heavy smoking in overall RA disease (AP = 0.58; 95% CI 0.16 to 0.99, p = 0.007), an interaction that was less striking but still significant with ever smoking (AP = 0.47; 95% CI 0.14 to 0.80, p = 0.006) (Table 3). Corresponding measures of SI and RERI are shown in Table 3. There was no evidence of multiplicative interaction between HLA-DRB1 SE and heavy smoking (p = 0.38) or ever smoking (p = 0.17) in overall disease risk. APs corresponding to HLA-DRB1-smoking interactions for anti-CCP antibody positive and anti-CCP antibody negative RA are shown in Table 3, with similar values for measures of interaction corresponding to RF positive and RF negative RA (data not shown). Additive interactions between HLA-DRB1 SE and smoking were stronger for seropositive RA when compared to seronegative RA. Results of these analyses were not changed when the amount of European admixture based on AIM genotyping was included as a covariate in the models (data not shown).
Table 3.
Measures interaction between HLA-DRB1 shared epitope (SE) containing alleles (1 or 2 copies vs. none) and smoking in rheumatoid arthritis risk in African Americans*
| All RA | ||
|---|---|---|
| Measures of Additive Interaction | Ever smoking /SE | ≥ 10 pack-years /SE |
| Attributable proportion† (AP) (95% CI) | 0.47 (0.14 to 0.80) | 0.58 (0.16 to 0.99) |
| P-value for interaction | Padd = 0.006 | Padd = 0.007 |
| Relative excess risk due to interaction (95% CI) | 2.04 (−0.36 to 4.43) | 4.86 (−3.08 to 12.80) |
| Synergy Index (95% CI) | 2.55 (0.99 to 6.60) | 2.88 (0.92 to 9.04) |
| Measures of Multiplicative Interaction | ||
| P-value of product term | Pmult = 0.17 | Pmult = 0.38 |
| Anti-CCP Antibody Positive RA | ||
| Measures of Additive Interaction | ||
| Attributable proportion† (AP) (95% CI) | 0.53 (0.22 to 0.83) | 0.63 (0.25 to 1.00) |
| P-value for interaction | Padd = 0.001 | Padd = 0.001 |
| Relative excess risk due to interaction (95% CI) | 3.16 (−0.22 to 6.54) | 7.16 (−3.76 to 18.08) |
| Synergy Index (95% CI) | 2.70 (1.13 to 6.45) | 3.17 (1.04 to 9.62) |
| Measures of Multiplicative Interaction | ||
| P-value of product term | Pmult = 0.13 | Pmult = 0.36 |
| Anti-CCP Antibody Negative RA | ||
| Measures of Additive Interaction | ||
| Attributable proportion (AP)† (95% CI) | 0.04 (−0.77 to 0.86) | 0.24 (−0.69 to 1.17) |
| P-value for interaction | Padd = 0.92 | Padd = 0.61 |
| Relative excess risk due to interaction (95% CI) | 0.08 (−1.39 to 1.54) | 0.81 (−3.17 to 4.80) |
| Synergy Index (95% CI) | 1.11 (0.15 to 8.38) | 1.52 (0.26 to 9.04) |
| Measures of Multiplicative Interaction | ||
| P-value of product term | Pmult = 0.96 | Pmult = 0.87 |
Additive interaction examined by assessing for evidence of departure from additivity in age- and sex-adjusted models; Multiplicative interaction examined by modeling SE-smoking product term. Smoking examined as ever vs. never smoking and heavy smoking (≥ 10 pack-years) vs. other (never or < 10 pack-years).
AP = 0 corresponds to no interaction and an AP = 1.0 corresponds to ‘complete’ additive interaction
Discussion
To our knowledge, this is the first study to show an association of cigarette smoking with RA in African Americans. We found that this association is most striking in heavy smokers and those with HLA-DRB1 SE containing alleles. Among African Americans with a cumulative smoking history exceeding 10 pack-years, the risk of RA is increased by more than two-fold, and this risk is increased to more than four-fold in the presence of SE alleles. In contrast, the risk of RA among ever smokers with a cumulative exposure of less than 10 pack-years appears to be negligible. Assuming that the smoking behaviors reported by controls in this study (where 15% reported a smoking history of more than 10 pack-years) reflect those of African Americans nationally, the attributable risk of RA due to heavy smoking exposure in this population may be as high as 16%. Said in another way, our results suggest that approximately 1 in 6 new cases of RA occurring in African Americans could be prevented through smoking cessation or by limiting cumulative smoking exposure in this population to less than 10 pack-years. In light of reports suggesting that smoking is on the rise among African Americans (15), our results suggest that RA incidence and disease burden may increase in this population over the next decades.
It is worth noting that some uncertainty remains regarding the optimal method to model gene-environment interactions (31). In contrast to prior studies that have examined smoking-SE interactions in RA risk using only additive interaction (2, 6), we have examined measures of both additive and multiplicative interaction. Multiplicative interaction refers to the inclusion of a product term in regression analyses to generate an ‘optimal fit’ of the data in the statistical model. It is important to note that the absence of multiplicative interaction does not exclude the existence of important biologic or additive interactions, which in the case of this study show that at least one ‘pathway’ to RA development in African Americans requires the presence of two risk factors (i.e. heavy smoking and HLA-DRB1 SE).
The results presented here are similar to a recent report from the Nurses’ Health Study (NHS), a nested case-control study of more than 100,000 women of European ancestry in the U.S. including 680 women with incident RA (11). In the NHS study (11), investigators found evidence of significant additive interaction between HLA-DRB1 and heavy smoking (> 10 pack-years) in overall RA risk with an AP of 0.39 (95% CI 0.08 to 0.69, p = 0.01). Although the NHS study did not yield evidence of multiplicative interaction in overall disease risk (p = 0.14), there was evidence of a significant multiplicative interaction between HLA-DRB1 SE and heavy smoking in the development of seropositive RA (a phenotype based on RF status in some patients and anti-CCP antibody status in others) (11). The absence of multiplicative interaction in our study of African Americans may relate to the smaller number of study participants and limited power relative to the larger NHS study. NHS investigators also found no evidence of additive interaction between HLA-DRB1 SE and ever smoking status in disease risk (AP = 0.23; 95% CI −0.14 to 0.61, p = 0.23). Evidence of additive interaction in CLEAR between SE and ever smoking was similarly attenuated compared to interaction between SE and heavy smoking, although still statistically significant in CLEAR. Results from the NHS suggest that accounting for cumulative exposure is essential in assessing the role of cigarette smoking and gene-smoking interactions in RA. In light of our results, these conclusions can now be extended to African Americans. Failure to account for smoking “dose” could explain the lack of substantial SE-smoking interaction found in other North American cohorts (28).
Biologic interactions between HLA-DRB1 SE alleles and smoking in RA risk have been shown in several epidemiological investigations, although ours is the first study to exclusively involve African Americans. Taken together, these studies suggest that smoking may trigger initial inflammatory events in RA that are HLA-DRB1 dependent. Previous studies, which involved populations of European ancestry, have shown that SE-smoking interactions are most evident in the development of anti-CCP antibody positive disease. These results have been interpreted to mean that smoking either upregulates citrullination or enhances the immunogenicity of citrullinated peptides in the context of select HLA alleles. However, our study of African Americans showed that the associations of heavy smoking with RA are similar for autoantibody positive and negative disease, although risk estimates were consistently higher for seropositive RA. This finding is in direct contrast to results from reports involving populations of European ancestry (2, 11) and one that will require replication in separate study populations. These results are consistent with a prior case-only analysis done in a subset of CLEAR-I patients showing no association of smoking with anti-CCP antibody positivity (32). In the present study of African Americans, heavy smoking was associated with a significant and more than two-fold increased risk for both anti-CCP antibody negative RA and RF negative RA. By contrast, in a large national case-control study from Sweden, Klareskog and colleagues found no associations of smoking with the risk of anti-CCP antibody negative RA, regardless of HLA DRB1 SE status (2). Reasons for this apparent discrepancy are unknown, but it is possible that there are other genetic and/or environmental factors that could mediate the effect of smoking in autoantibody negative RA and the prevalence of these as of yet undefined factors could vary markedly in prevalence based on race/ethnicity. In addition to differences in the study populations and accounting for cumulative exposures, variations in study design, ascertainment of smoking status, and different methods of analysis could also serve to explain differences across published reports.
Limitations to this study are those inherent to its case-control design. These include possible recall bias and the possibility of a “healthy responder” bias among controls. This latter concern is mitigated by the recruitment and enrollment of healthy controls residing in the same census tracts as RA cases, individuals similar to cases in regards to sociodemographic characteristics and other “unmeasured confounders”. Similarities between cases and controls were further borne out at the genetic level, with examinations of AIMS showing very similar levels of European admixture in both groups.
The case-control design also prohibits conclusions regarding the ‘direction’ of the associations examined, although for all RA cases included in this study initial smoking exposure preceded disease onset, in most cases by many years. We also found no major differences in risk estimates corresponding to heavy smoking in analyses limited to CLEAR-I (RA cases with disease duration < 2 years) and analyses limited to CLEAR-II (RA cases with any disease duration), suggesting that recall bias and protopathic bias (disease onset leading to exposure) are less likely issues. If a protopathic bias had been operative in these findings, we would have expected to have observed much stronger associations of heavy smoking with RA in CLEAR-II, a cohort that included RA patients with established disease and a much longer time interval between disease onset and study enrollment. Despite these potential limitations, this effort represents the largest study to date examining the impact of smoking and gene-smoking interactions in RA in a well characterized African American population, a group that has been vastly understudied in RA epidemiology.
In summary, cigarette smoking is significantly associated with RA in African Americans, an association that is most pronounced with a cumulative smoking history exceeding 10 pack-years. Similar to reports involving populations of European ancestry, the risk attributed to smoking is highest in African Americans positive for HLA-DRB1 SE alleles with evidence of a significant biologic interaction between SE and heavy smoking in RA risk.
Acknowledgements
The CLEAR Registry is an NIH-sponsored resource, with clinical data, DNA, and other biological samples available to approved users. Details on obtaining data or biological samples are available at the following website: http://www.dom.uab.edu/rheum/CLEAR%20home.htm.
The CLEAR investigators are: University of Alabama at Birmingham: S. Louis Bridges, Jr., MD, PhD, Director; George Howard, DrPH, Co-Director; Graciela S. Alarcón, MD, MPH. Emory University: Doyt L. Conn, MD. University of North Carolina: Beth L. Jonas, MD; Leigh F. Callahan, PhD. Medical University of South Carolina: Edwin A. Smith, MD. Washington University: Richard D. Brasington, Jr., MD. University of Nebraska: Ted R. Mikuls, MD, MSPH. University of Pittsburgh: Larry W. Moreland, MD, Co-Director.
We gratefully acknowledge CLEAR Registry staff and coordinators at the following sites: University of Alabama at Birmingham: Stephanie Ledbetter, MS; Zenoria Causey, MS; Selena Luckett, RN, CRNC; Laticia Woodruff, RN, MSN; Candice Miller; Emory University: Joyce Carlone, RN, RNP; Karla Caylor, BSN, RN; Sharon Henderson, RN; University of North Carolina: Diane Bresch, RN; Medical University of South Carolina: Trisha Sturgill; Washington University: Teresa Arb.
We also gratefully acknowledge the following physicians who enrolled patients into the CLEAR Registry: Jacob Aelion, MD, Jackson, TN; Charles Bell, Birmingham, AL; Sohrab Fallahi, MD, Montgomery, AL; Richard Jones, PhD, MD, Tuscaloosa, AL; Maura Kennedy, MD, Birmingham, AL; Adahli Estrada Massey, MD, Auburn, AL; John Morgan, MD, Birmingham, AL; Donna Paul, MD, Montgomery, AL; Runas Powers, MD, Alexander City, AL; William Shergy, MD, Huntsville, AL; Cornelius Thomas, MD, Birmingham, AL; Ben Wang, MD, Memphis, TN.
Grant support: The CLEAR Registry is supported by NIH grant N01-AR-02247. Dr. Mikuls’ work was supported by the Nebraska Arthritis Outcomes Research Center and by grants from NIH/NIAMS (RO3-AR-054539 and K23-AR-050004), the Arthritis Foundation (National and Nebraska Chapters), and the Veterans Affairs Office of Research & Development (VA Merit). The CLEAR Registry is also supported by NIH N01-AR-6-2278 (SL Bridges, Jr., PI), by the University of Alabama at Birmingham General Clinical Research Center, and a grant from NIH/NCRC (M01-RR-00032).
References
- 1.Vessey MP, Villard-Mackintosh L, Yeates D. Oral contraceptives, cigarette smoking and other factors in relation to arthritis. Contraception. 1987;35:457–464. doi: 10.1016/0010-7824(87)90082-5. [DOI] [PubMed] [Google Scholar]
- 2.Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 3.Karlson E, Lee I, Cook N, Manson J, Buring J, Hennekens C. A retrospective cohort study of cigarette smoking and risk of rheumatoid arthritis in female health professionals. Arthritis Rheum. 1999;42:910–917. doi: 10.1002/1529-0131(199905)42:5<910::AID-ANR9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 4.Heliovaara M, Aho K, Aromaa A, Knekt P, Reunanen A. Smoking and risk of rheumatoid arthritis. J Rheumatol. 1993;20:1830–1835. [PubMed] [Google Scholar]
- 5.Symmons DP, Bankhead C, Harrison BJ, et al. Blood transfusion, smoking, and obesity as risk factors for the development of rheumatoid arthritis: results from a primary care-based incident case-control study in Norfolk, England. Arthritis Rheum. 1997;40:1955–1961. doi: 10.1002/art.1780401106. [DOI] [PubMed] [Google Scholar]
- 6.Padyukov L, Silva C, Stolt P, Alfredsson L, Klareskog L. A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum. 2004;50:3085–3092. doi: 10.1002/art.20553. [DOI] [PubMed] [Google Scholar]
- 7.Criswell LA, Merlino LA, Cerhan JR, et al. Cigarette smoking and the risk of rheumatoid arthritis among postmenopausal women: results from the Iowa Women's Health Study. Am J Med. 2002;15:465–471. doi: 10.1016/s0002-9343(02)01051-3. [DOI] [PubMed] [Google Scholar]
- 8.Silman A, Newman J, MacGregor A. Cigarette smoking increases the risk of rheumatoid arthrits. Arthritis Rheum. 1996;39(5):732–735. doi: 10.1002/art.1780390504. [DOI] [PubMed] [Google Scholar]
- 9.Voigt L, Koepsell T, Nelson JL, Dugowson C, Daling J. Smoking, obesity, alcohol consumption, and the risk of rheumatoid arthritis. Epidemiology. 1994;5:525–532. [PubMed] [Google Scholar]
- 10.Uhlig T, Hagen K, Kvien T. Current tobacco smoking, formal education, and the risk of rheumatoid arthritis. J Rheumatol. 1999;26:47–54. [PubMed] [Google Scholar]
- 11.Karlson EW, Chang SC, Cui J, et al. Gene-environment interaction between HLA-DRB1 shared epitope and heavy cigarette smoking in predicting incident RA. Ann Rheum Dis. 2009 doi: 10.1136/ard.2008.102962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costenbader K, Feskanich D, Mandl L, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119:503. doi: 10.1016/j.amjmed.2005.09.053. e1–9. [DOI] [PubMed] [Google Scholar]
- 13.Stolt P, Bengtsson C, Nordmark B, et al. Quantification of the influence of cigarette smoking on rheumatoid arthritis: results from a population based case-control study, using incident cases. Ann Rheum Dis. 2003;62:835–841. doi: 10.1136/ard.62.9.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gazes PC, Lackland DT, Mountford WK, Gilbert GE, Harley RA. Comparison of cardiovascular risk factors for high brachial pulse pressure in blacks versus whites (Charleston Heart Study, Evans County Study, NHANES I and II Studies) Am J Cardiol. 2008;102(11):1514–1517. doi: 10.1016/j.amjcard.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Tobacco use among high school students -- United States, 1997. MMWR. 1998;46:433–440. [Google Scholar]
- 16.U.S. Department of Health and Human Services. U.S. Department of Health and Human Services and Center for Disease Control and Prevention) Tobacco Use Among U.S, Racial/Ethnic Groups -- African Americans, American Indian and Alaska Natives, Asian Americans and Pacific Islanders, and Hispanics. 1998 [Google Scholar]
- 17.Del Rincon I, Battafarano DF, Arroyo RA, Murphy FT, Fischbach M, Escalante A. Ethnic variation in the clinical manifestations of rheumatoid arthritis: role of HLA-DRB1 alleles. Arthritis Rheum. 2003;49(2):200–208. doi: 10.1002/art.11000. [DOI] [PubMed] [Google Scholar]
- 18.Mikuls T, Holers VM, Parrish LA, et al. Anti-cyclic citrullinated peptide antibody and rheumatoid factor isotypes in African Americans with early rheumatoid arthritis. Arthritis Rheum. 2006;54:3057–3059. doi: 10.1002/art.22200. [DOI] [PubMed] [Google Scholar]
- 19.Mikuls TR, Saag KG, Curtis J, et al. Prevalence of osteoporosis and osteopenia among African Americans with early rheumatoid arthritis: the impact of ethnic-specific normative data. J Natl Med Assoc. 2005;97:1155–1160. [PMC free article] [PubMed] [Google Scholar]
- 20.Bridges SL, Jr, Hughes LB, Mikuls TR, et al. Early rheumatoid arthritis in African-Americans: the CLEAR Registry. Clin Exp Rheumatol. 2003;21(5 Suppl 31):S138–S145. [PubMed] [Google Scholar]
- 21.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 22.Hughes LB, Morrison D, Kelley JM, et al. The HLA-DRB1 shared epitope is associated with susceptibility to rheumatoid arthritis in African Americans through European genetic admixture. Arthritis Rheum. 2008;58(2):349–358. doi: 10.1002/art.23166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rioux JD, Goyette P, Vyse TJ, et al. Mapping of multiple susceptibility variants within the MHC region for 7 immune-mediated diseases. Proc Natl Acad Sci U S A. 2009;106(44):18680–18685. doi: 10.1073/pnas.0909307106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinze G. A comparative investigation of methods for logistic regression with separated or nearly separated data. Stat Med. 2006;25:4216–4226. doi: 10.1002/sim.2687. [DOI] [PubMed] [Google Scholar]
- 26.Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med. 2002;21(16):2409–2419. doi: 10.1002/sim.1047. [DOI] [PubMed] [Google Scholar]
- 27.Rothman K, Greenland S, Walker A. Concepts of interaction. Am J Epidemiol. 1980;112:467–470. doi: 10.1093/oxfordjournals.aje.a113015. [DOI] [PubMed] [Google Scholar]
- 28.Lee HS, Irigoyen P, Kern M, et al. Interaction between smoking, the shared epitope, and anti-cyclic citrullinated peptide: a mixed picture in three large North American rheumatoid arthritis cohorts. Arthritis Rheum. 2007;56(6):1745–1753. doi: 10.1002/art.22703. [DOI] [PubMed] [Google Scholar]
- 29.Andersson T, Alfredsson L, Kallberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20(7):575–579. doi: 10.1007/s10654-005-7835-x. [DOI] [PubMed] [Google Scholar]
- 30.Hosmer D, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3:452–456. doi: 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Ahlbom A, Alfredsson L. Interaction: a word with two meanings creates confusion. Eur J Epidemiol. 2005;20:563–564. doi: 10.1007/s10654-005-4410-4. [DOI] [PubMed] [Google Scholar]
- 32.Mikuls T, Hughes L, Westfall AO, et al. Cigarette smoking, disease severity, and autoantibody expression in African Americans with recent-onset rheumatoid arthritis. Ann Rheum Dis. 2008;67:1529–1534. doi: 10.1136/ard.2007.082669. [DOI] [PMC free article] [PubMed] [Google Scholar]