Abstract
Objective
To examine the association between alcohol consumption and markers of inflammation in pre-clinical rheumatoid arthritis (RA) patients.
Methods
We studied 174 incident RA cases with stored blood collected 1–16 years prior to RA symptoms (“pre-clinical RA”) from the Nurses’ Health Study. Alcohol intake was measured using a detailed food frequency questionnaire every two years prior to blood collection. Plasma was tested for biomarkers of inflammation: high-sensitivity C-reactive protein (hsCRP), anti–cyclic citrullinated peptide (anti-CCP) antibodies, interleukin-6 (IL-6) and soluble tumor necrosis factor receptor II (sTNFRII). Generalized additive models were used to uncover structure in the relationship between each biomarker and cumulative average alcohol intake. Then general linear models were used for multivariable adjusted analyses with appropriate polynomial terms of alcohol consumption.
Results
After controlling for age at blood collection, smoking, parity and duration of breastfeeding, menopausal status, oral contraceptive use, BMI and the time between blood collection and RA onset, we found that the daily alcohol consumption showed a U-shaped association with IL-6 levels in RA patents prior to symptoms. We also found an inverse relationship between alcohol intake and sTNFRII levels but no associations with hsCRP and anti-CCP levels.
Conclusion
Alcohol consumption was associated with markers of inflammation including IL-6 and sTNFRII in RA patients prior to symptoms.
INTRODUCTION
Rheumatoid arthritis (RA) is an autoimmune disease, characterized by chronic, destructive, debilitating arthritis, that affects approximately 1% of the adult population (1, 2). The cause of RA is unknown, but it is considered to be a multifactorial disease, resulting from the interaction of both genetic and environmental factors. (1). Epidemiological studies have suggested that moderate alcohol consumption may decrease the risk for RA and RA progression (3–5). One possible mechanism for this inverse association is that moderate alcohol consumption may be associated with reduced levels of inflammatory biomarkers (6). Alcohol has been shown to diminish the response to antigens in animals as well as in humans,(7, 8) and to suppress the synthesis of proinflammatory cytokines and chemokines, such as TNFα , IL-6 and IL-8 both in vivo and in vitro in alveolar macrophages and human blood monocytes (9, 10). Studies have shown elevated autoantibodies and markers of inflammation in RA cases where blood was collected prior to RA symptoms (eg. “pre-clinical RA”) compared with matched controls (11–14). However, little is known about the possible anti-inflammatory effect of alcohol consumption in pre-clinical RA. Our objective was to investigate the influence of alcohol consumption on autoantibodies and markers of inflammation including anti–cyclic citrullinated peptide (anti-CCP) antibodies, interleukin-6 (IL-6), soluble tumor necrosis factor receptor II (sTNFRII) and high-sensitivity C-reactive protein (hsCRP) in women with preclinical RA enrolled in two Nurses’ Health Study cohorts (NHS and NHS II).
METHODS
Study design and participants
The Nurses’ Health Study (NHS) was established in 1976 and enrolled 121,700 US female registered nurses, ages 30–55 years. A second NHS cohort, the NHS II, was established in 1989 and enrolled 116,609 female registered nurses, ages 25–42 years. All women completed an initial questionnaire and have been followed biennially in the combined NHS cohorts by questionnaire to update exposures and disease diagnoses. From 1989 through 1990, 32,826 participants ages 43–70 years in the NHS provided plasma samples in heparinized tubes. From 1996 through 1999, 29,611 participants ages 32–51 years in NHS II provided blood samples. Collection and storage procedures for NHS II were similar to those described above for the NHS. All aspects of this study were approved by the Partners HealthCare Institutional Review Board.
RA case identification in NHS and NHS II is a 2-step process. Two board-certified rheumatologists trained in chart abstraction conducted independent medical record reviews based on the American College of Rheumatology (ACR) classification criteria for RA. Detailed procedures were reported in previous research (12, 15). After excluding all prevalent RA cases at the time of blood collection, we included 174 incident RA cases in the study (126 in NHS, 48 in NHS II) with a stored blood sample collected at least 3 months prior to the date of the first RA symptom documented in the medical record.
Assessment of alcohol consumption
Alcohol consumption was assessed every two years with a semi-quantitative food frequency questionnaire including separate items for beer, white wine, red wine, and liquor started from 1980 in NHS and 1989 in NHS II. We specified standard portions as a glass, bottle, or can of beer; a 4-oz glass of wine; and a shot of liquor. For each beverage, participants were asked to estimate their average consumption over the past year. The estimated alcohol content of each beverage was 13.2 g per bottle or can of beer, 10.8 g per glass of wine, and 15.1 g per standard drink of liquor. Total alcohol intake was recorded as the sum of these 3 beverages. The food frequency questionnaires were updated across different survey years. The reproducibility and validity of the assessment of alcohol intake were evaluated among 173 Boston-area participants who completed written one-week dietary records every three months for a year during which time they weighed and measured all their food and drinks. The correlation of alcohol intake on the questionnaire with alcohol intake on the dietary records was 0.9 (16). We used the repeated alcohol measurements prior to blood draw. Daily alcohol intake was estimated based on the cumulative average method widely used for diet intake in NHS (17). More weight was given to the most recent alcohol intake in calculation of the cumulative average. For example, during 1980–1984 time period, the cumulative average alcohol intake will be calculated as the following: [1984 alcohol + (1980 alcohol + 1982 alcohol)/2]/2. Detailed information of cumulative average method was documented elsewhere (17). The use of cumulative average method instead of a single measurement will reduce within-subject variation and best represent overall long-term alcohol consumption.
Information on potential confounding variables
All exposure information was self-reported on the mailed questionnaires administered every 2 years since 1976 in the NHS and since 1989 in the NHS II. Cigarette smoking is a strong environmental risk factor for RA (18–20) and is associated with biomarker levels (21–23). We adjusted for smoking status evaluated by pack-years of smoking (product of years of smoking and packs of cigarettes per day). Reproductive covariates were chosen based on the past findings of associations between reproductive factors and the risk of RA in the NHS including parity and duration of breastfeeding (nulliparous, parous and no breastfeeding, parous and 1–12 months breastfeeding, parous and >12 months breastfeeding), menopausal status and oral contraceptive use (15). Other potential confounders we adjusted for included age at blood draw, body mass index (BMI) and the time between blood collection and RA onset. All the covariates were measured in the questionnaire prior to the blood draw.
Laboratory assays
The laboratory selected for NHS cohorts has high assay precision and runs internal positive and negative quality control samples daily. The laboratory has undergone rigorous blinded pilot testing with aliquots from NHS quality control specimens in which aliquots were divided into 2 masked samples (12). Anti–CCP was tested using the second generation Diastat ELISA (Axis-Shield Diagnostics, Dundee, UK), with positive anti-CCP defined as >5 units/ml. High-sensitivity C-reactive protein in mg/dl was measured by high-sensitivity latex-enhanced immunonephelometric assay on a BN II analyzer (Dade-Behring, Newark, DE). IL-6 (pg/ml) was measured with an ultrasensitive quantitative sandwich enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN). In this study, we measured sTNFRII as a proxy for TNFα because TNFα cannot be reliably measured in stored plasma as it degrades rapidly. Coefficients of variation were 0.07–17.7% for IL-6, 4.0–13.6% for sTNFRII, and 1.6–5.2% for CRP. The detailed laboratory procedures and quality controls have been reported previously (12),
Statistical analysis
Descriptive statistics such as minimum, maximum, range, median, mean and standard deviation for each biomarker (IL-6, sTNFRII, hsCRP, and anti-CCP antibodies), frequencies and percentages for each categorical variable were used to summarize the data as well as detect outliers and missing values. The normality of the distribution of primary outcomes of interest was examined with a normal probability plot. Natural log transformed IL-6 and hsCRP were used in the analysis based on their distributions. Chi-square tests, t-tests or non-parametric tests were used for unadjusted analysis as well as evaluation of potential confounders when applicable.
We used semi-parametric generalized additive models to uncover structure in the relationship between each inflammatory marker and alcohol intake based on the method of cubic smoothing splines. Then general linear models were used with an appropriate polynomial term for alcohol intake. We computed the β coefficients over 1 gram/day change of alcohol intake to evaluate the independent association between alcohol consumption and the four inflammatory biomarkers. Adjusted variables for potential confounding included age, cigarette smoking, reproductive covariates, BMI, the time between blood collection and RA onset. The validity of model assumptions was evaluated using analysis of residuals. All p values were calculated with two-sided significance level of 0.05. Data analyses were performed using SAS 9.1.2 (SAS Institute, Inc, Cary, North Carolina).
RESULTS
In the NHS and NHS II, we confirmed 174 incident RA cases with stored blood collected prior to onset of the first RA symptoms, hereafter referred to as preclinical RA. The average age in years at blood draw in NHS was 56.1 and 44.3 in NHS II. The average time between blood collection and the onset of RA symptoms was 6.5 years (range 0.3–15.7 years). Overall 26.2% of women in NHS and 43.8% in NHS II did not consume any alcohol before blood collection. Table 1 presents the general characteristics of the preclinical RA cases at time of blood collection according to alcohol consumption level in NHS and NHS II.
Table 1.
Characteristics of the rheumatoid arthritis cases at time of blood collection in NHS
| Covariates | Alcohol Consumption (gram/day) |
|||
|---|---|---|---|---|
| 0 (n=54) | 0.1–5.0 (n=74) | 5.1–10.0 (n=23) | >10.0 (n=23) | |
| Age at blood collection, mean (SD) years | 51.0(8.2) | 53.7(8.6) | 53.6(8.7) | 53.4(7.1) |
| Age at RA diagnosis, mean (SD) years | 57.4(9.9) | 59.7(10.4) | 60.4(11.0) | 61.9(7.5) |
| Body mass index, mean (SD) | 28.0(5.7) | 27.0(5.4) | 27.4(6.1) | 26.1(4.7) |
| Ever smoke, % | 37.04 | 52.70 | 69.57 | 78.26 |
| Contraceptive use, % | 72.22 | 63.51 | 73.91 | 73.91 |
| Menopausal, % | 38.89 | 62.16 | 69.57 | 56.52 |
| Parity and breastfeeding, % | ||||
| Nulliparous | 9.26 | 8.11 | 17.39 | 4.35 |
| Parous/0–3 months | 27.78 | 33.78 | 26.09 | 17.39 |
| Parous/4–11 months | 42.59 | 50.00 | 36.13 | 56.52 |
| Parous/12- months | 20.37 | 8.11 | 17.39 | 21.74 |
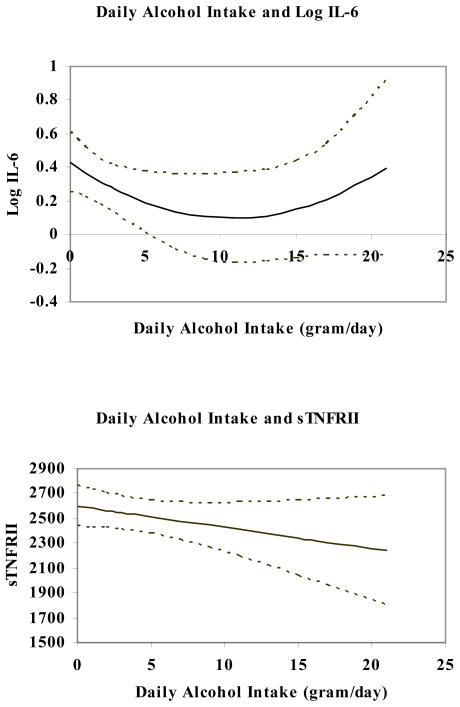
Figure 1 shows the unadjusted associations between daily alcohol intake and levels of log transformed IL-6 (log IL-6) and sTNFRII. Daily alcohol intake followed a U-shaped association with log IL-6, with minimum values at an alcohol intake of 10–12 grams daily (~1 drink/day). sTNFRII levels were lower with increased daily alcohol intake (p<0.01). The confident limits represent the variability of the relationships. Table 2 give adjusted means of each inflammation marker according to daily alcohol consumption. The parametric polynomial regression model confirmed the pattern of the quadratic relationship with IL-6 after adjustment for the potential confounders including age at blood draw, cigarette smoking, reproductive covariates, BMI and the time between blood collection and RA onset. Our adjusted analyses also confirmed that daily alcohol intake was associated with decreased level of sTNFRII (p=0.03). However, no clear statistical associations were found between alcohol consumption and hsCRP or anti-CCP levels.
Figure 1.
The unadjusted relationship between IL-6 / sTNFRII and daily alcohol intake (with 95% confidence limits) in preclinical rheumatoid arthritis patients
Table 2.
Adjusted means (SD) for biomarkers of inflammation according to daily alcohol intake *
| Inflammatory Markers | Daily Alcohol Intake, means (SE) in grams |
P for linear term | P for quadratic term | |||
|---|---|---|---|---|---|---|
| 0 | 0.1–5.0 | 5.1–10.0 | >10.0 | |||
| Hs-CRP, mg/dl† | 0.48(0.20) | 0.49(0.14) | 0.62(0.26) | 0.65(0.26) | 0.687 | - |
| IL-6, pg/ml† | 0.61(0.12) | 0.35(0.11) | 0.16(0.17) | 0.41(0.18) | 0.005 | 0.006 |
| sTNFRII, pg/ml | 2690(124.5) | 2443(111.6) | 2471(184.1) | 2167(192.7) | 0.030 | - |
| Anti-CCP, units/ml | 9.17(3.16) | 11.62(2.76) | 12.82(4.86) | 12.04(4.94) | 0.507 | - |
Adjusted for age at blood collection, smoking, time between blood collection and RA onset, parity and duration of breastfeeding, menopausal status, oral contraceptive use and BMI.
Natural log transformed scale
DISCUSSION
Excessive alcohol consumption depresses the immune system and increases the propensity to severe bacterial infections (8, 24). However, it has been documented that light to moderate alcohol consumption has protective effects against several diseases including chronic heart diseases and ischemic stroke (25). An indication that alcohol consumption may also influence the risk of human RA has come from several epidemiological studies (3, 4, 26). No clear-cut mechanism has been identified to explain this relationship. One hypothesis is that light to moderate alcohol consumption may exert anti-inflammatory effects (6). However, no studies have assessed the effects of alcohol consumption on inflammatory markers in pre-clinical RA patients. Using blood samples from the prospective Nurses’ Health Study, we found alcohol intake showed an apparent U-shaped association with IL-6 in preclinical RA patients. And sTNFRII was negatively associated with daily alcohol consumption. We failed to find alcohol intake had significant effects on hsCRP and anti-CCP levels.
Effects of alcohol consumption on the immune function are well known and depend in part on the amount and duration of alcohol consumption. Chronic alcohol consumption modulates various components of the immune system. Associations between moderate alcohol intake and lower levels of inflammation markers have been found in the general population (27). Several studies have demonstrated that moderate alcohol consumption was associated with lower levels of CRP in a U-shaped or J-shaped manner, but with a weaker association among women than men(6, 28). Alcohol has been shown to suppress the synthesis of pro-inflammatory cytokines and chemokines, such as TNF-α and IL-6 both in vivo and/or in vitro in alveolar macrophages and human blood monocytes (9, 27). Notably, addition of alcohol to the drinking water of mice was recently shown to reduce clinical signs of arthritis as well as joint destruction (29). The beneficial effects may be mediated by up-regulation of testosterone production, which in turn inhibits NFkB activation, leading to decreased cytokine/chemokine production and decreased chemotactic activity of leukocytes (29). A recently published study indicates a trend toward reduced radiographic progression in alcohol drinkers compared with nondrinkers, specifically in occasional and daily alcohol consumers (5). In this analysis, we confirmed that light to moderate alcohol consumption (<12 grams daily) was associated with lower levels of IL-6 and sTNFRII in pre-clinical incident RA patients, similar to findings in general population. We did not find significant association between alcohol consumption and hsCRP or anti-CCP. The possible reason may be that nurses in this study consume less alcohol beverages than other populations reported. Thus we could not assess the effect of high levels of alcohol intake, since there were few heavy drinkers in our sample.
One advantage of the present study is that it was population-based and included only incidence cases with definite RA as determined by two board-certified rheumatologists based on ACR classification criteria for RA. In contrast to other studies, we collected information on alcohol use and measured inflammatory markers before the occurrence of RA to reduce recall bias that is inherent in most retrospective designs. This study had some limitations. First, information on alcohol consumption was self-reported, perhaps leading to some misclassification bias. However, the cumulative average estimate of consumption may be more representative for regular alcohol exposure than a one-time measure. Second, the relatively small number of incident RA cases limits the power to correct for multiple tests and detect modest effects of alcohol consumption on markers of inflammation. Therefore, our findings should be considered as exploratory results. Another limitation is that we were unable to adjust for confounding factors due to infections and other inflammatory conditions, although we did exclude subjects with self-reported SLE or other CTDs prior to blood sample collection.
In conclusion, alcohol intake is associated with IL-6 in a U-shaped manner before RA onset, and sTNFRII was negatively associated with daily alcohol consumption. These associations suggest that the alcohol anti-inflammatory effect might be the link between moderate alcohol consumption and possible decreased risk of RA. Alcohol intake alone, however, may not be powerful enough to influence risk of RA. While long-term randomized controlled trials of alcohol are the ideal setting for studying this association, they are unlikely to take place for ethical reasons. Thus, there is clearly a need for further observational cohort studies, as well as for animal and experimental studies on the cellular and molecular level, to understand these relationships. Although alcohol in moderation may be protective against inflammatory diseases, such as cardiovascular disease and possibly RA, alcohol abuse is a major public health and social problem with far-reaching consequences.
Acknowledgments
We thank the participants in the NHS and NHS II cohorts for their dedication and continued participation in these longitudinal studies, as well as the NHS staffs in the Channing Laboratory, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School for their assistance with this project. We also thank Gideon Aweh for assistance with NHS data.
Supported by the NIH (grants P01 CA87969, CA49449, CA67262, K24 AR055989).
Footnotes
AUTHOR CONTRIBUTIONS:
Study design. Lu, Karlson, Solomon, Costenbader.
Acquisition of data. Lu, Karlson, Keenan, Chibnik.
Analysis and interpretation of data. Lu, Solomon, Karlson,
Manuscript preparation. Lu, Karlson, Solomon, Costenbader.
Statistical analysis. Lu, Keenan
References
- 1.Alamanos Epidemiology of adult rheumatoid arthritis. Autoimmun Rev. 2005;4(3):130–6. doi: 10.1016/j.autrev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Gabriel SE, Crowson CS, O’Fallon WM. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955–1985. Arthritis Rheum. 1999;42(3):415–420. doi: 10.1002/1529-0131(199904)42:3<415::AID-ANR4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 3.Kallberg H, Jacobsen S, Bengtsson C, Pedersen M, Padyukov L, Garred P, et al. Alcohol consumption is associated with decreased risk of rheumatoid arthritis: results from two Scandinavian case-control studies. Ann Rheum Dis. 2009;68(2):222–7. doi: 10.1136/ard.2007.086314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedersen M, Jacobsen S, Klarlund M, Pedersen BV, Wiik A, Wohlfahrt J, et al. Environmental risk factors differ between rheumatoid arthritis with and without auto-antibodies against cyclic citrullinated peptides. Arthritis Res Ther. 2006;8(4):R133. doi: 10.1186/ar2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nissen MJGC, Scherer A, Finckh A. The Effect of Alcohol on Radiographic Progression in Rheumatoid Arthritis. Arthritis Rheum. 2010;62(5):1256–1272. doi: 10.1002/art.27388. [DOI] [PubMed] [Google Scholar]
- 6.Imhof A, Froehlich M, Brenner H, Boeing H, Pepys MB, Koenig W. Effect of alcohol consumption on systemic markers of inflammation. Lancet. 2001;357(9258):763–7. doi: 10.1016/S0140-6736(00)04170-2. [DOI] [PubMed] [Google Scholar]
- 7.Cooper CL, Cameron DW. Effect of alcohol use and highly active antiretroviral therapy on plasma levels of hepatitis C virus (HCV) in patients coinfected with HIV and HCV. Clin Infect Dis. 2005;41 (Suppl 1):S105–9. doi: 10.1086/429506. [DOI] [PubMed] [Google Scholar]
- 8.Boe DM, Nelson S, Zhang P, Quinton L, Bagby GJ. Alcohol-induced suppression of lung chemokine production and the host defense response to Streptococcus pneumoniae. Alcohol Clin Exp Res. 2003;27(11):1838–45. doi: 10.1097/01.ALC.0000095634.82310.53. [DOI] [PubMed] [Google Scholar]
- 9.Nelson S, Bagby GJ, Bainton BG, Summer WR. The effects of acute and chronic alcoholism on tumor necrosis factor and the inflammatory response. J Infect Dis. 1989;160(3):422–9. doi: 10.1093/infdis/160.3.422. [DOI] [PubMed] [Google Scholar]
- 10.Szabo G, Mandrekar P, Catalano D. Inhibition of superantigen-induced T cell proliferation and monocyte IL–1 beta, TNF-alpha, and IL-6 production by acute ethanol treatment. J Leukoc Biol. 1995;58(3):342–50. doi: 10.1002/jlb.58.3.342. [DOI] [PubMed] [Google Scholar]
- 11.Chibnik LB, Mandl LA, Costenbader KH, Schur PH, Karlson EW. Comparison of threshold cutpoints and continuous measures of anti-cyclic citrullinated peptide antibodies in predicting future rheumatoid arthritis. J Rheumatol. 2009;36(4):706–11. doi: 10.3899/jrheum.080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlson EW, Chibnik LB, Tworoger SS, Lee IM, Buring JE, Shadick NA, et al. Biomarkers of inflammation and development of rheumatoid arthritis in women from two prospective cohort studies. Arthritis Rheum. 2009;60(3):641–52. doi: 10.1002/art.24350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielen MM, van Schaardenburg D, Reesink HW, Twisk JW, van de Stadt RJ, van der Horst-Bruinsma IE, et al. Increased levels of C-reactive protein in serum from blood donors before the onset of rheumatoid arthritis. Arthritis Rheum. 2004;50(8):2423–7. doi: 10.1002/art.20431. [DOI] [PubMed] [Google Scholar]
- 14.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48(10):2741–9. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 15.Karlson EW, Mandl LA, Hankinson SE, Grodstein F. Do breast-feeding and other reproductive factors influence future risk of rheumatoid arthritis? Results from the Nurses’ Health Study. Arthritis Rheum. 2004;50(11):3458–67. doi: 10.1002/art.20621. [DOI] [PubMed] [Google Scholar]
- 16.Giovannucci E, Colditz G, Stampfer MJ, Rimm EB, Litin L, Sampson L, et al. The assessment of alcohol consumption by a simple self- administered questionnaire. Am J Epidemiol. 1991;133(8):810–817. doi: 10.1093/oxfordjournals.aje.a115960. [DOI] [PubMed] [Google Scholar]
- 17.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–40. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 18.Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119(6):503, e1–9. doi: 10.1016/j.amjmed.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 19.Criswell LA, Merlino LA, Cerhan JR, Mikuls TR, Mudano AS, Burma M, et al. Cigarette smoking and the risk of rheumatoid arthritis among postmenopausal women: results from the Iowa Women’s Health Study. Am J Med. 2002;112(6):465–471. doi: 10.1016/s0002-9343(02)01051-3. [DOI] [PubMed] [Google Scholar]
- 20.Karlson EW, Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH. A retrospective cohort study of cigarette smoking and risk of rheumatoid arthritis in female health professionals. Arthritis Rheum. 1999;42(5):910–917. doi: 10.1002/1529-0131(199905)42:5<910::AID-ANR9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 21.Beulens JW, Rimm EB, Hu FB, Hendriks HF, Mukamal KJ. Alcohol consumption, mediating biomarkers, and risk of type 2 diabetes among middle-aged women. Diabetes Care. 2008;31(10):2050–5. doi: 10.2337/dc08-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukamal KJ, Conigrave KM, Mittleman MA, Camargo CA, Jr, Stampfer MJ, Willett WC, et al. Roles of drinking pattern and type of alcohol consumed in coronary heart disease in men. N Engl J Med. 2003;348(2):109–18. doi: 10.1056/NEJMoa022095. [DOI] [PubMed] [Google Scholar]
- 23.Pai JK, Hankinson SE, Thadhani R, Rifai N, Pischon T, Rimm EB. Moderate alcohol consumption and lower levels of inflammatory markers in US men and women. Atherosclerosis. 2006;186(1):113–20. doi: 10.1016/j.atherosclerosis.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 24.Cooper C, MacPherson P, Angel JB. Liver toxicity resulting from syphilis and Jarish–Herxheimer reaction in cases of coinfection with HIV and hepatitis C virus. Clin Infect Dis. 2005;40(8):1211–2. doi: 10.1086/428848. [DOI] [PubMed] [Google Scholar]
- 25.Corrao G, Bagnardi V, Zambon A, La Vecchia C. A meta–analysis of alcohol consumption and the risk of 15 diseases. Prev Med. 2004;38(5):613–9. doi: 10.1016/j.ypmed.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 26.Hazes JM, Dijkmans BA, Vandenbroucke JP, de Vries RR, Cats A. Lifestyle and the risk of rheumatoid arthritis: cigarette smoking and alcohol consumption. Ann Rheum Dis. 1990;49(12):980–982. doi: 10.1136/ard.49.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imhof A, Koenig W. Alcohol inflammation and coronary heart disease. Addict Biol. 2003;8(3):271–7. doi: 10.1080/13556210310001602176. [DOI] [PubMed] [Google Scholar]
- 28.Albert MA, Glynn RJ, Ridker PM. Alcohol consumption and plasma concentration of C-reactive protein. Circulation. 2003;107(3):443–7. doi: 10.1161/01.cir.0000045669.16499.ec. [DOI] [PubMed] [Google Scholar]
- 29.Jonsson IM, Verdrengh M, Brisslert M, Lindblad S, Bokarewa M, Islander U, et al. Ethanol prevents development of destructive arthritis. Proc Natl Acad Sci U S A. 2007;104(1):258–63. doi: 10.1073/pnas.0608620104. [DOI] [PMC free article] [PubMed] [Google Scholar]