Abstract
Background and Purpose
Recently, a new radiotherapy delivery technique has become clinically available – Volumetric Modulated Arc Therapy (VMAT). VMAT is the delivery of IMRT while the gantry is in motion using dynamic leaf motion. The perceived benefit of VMAT over IMRT is a reduction in delivery time. In this study, VMAT was compared directly with IMRT for a series of prostate cases.
Materials and Methods
For ten patients, a biologically optimised seven field IMRT plan was compared with a biologically optimised VMAT plan using the same planning objectives. The Pinnacle RTPS was used. The resultant target and organ at risk dose volume histograms (DVHs) were compared. The normal tissue complication probability (NTCP) for the IMRT and VMAT plans was calculated for three model parameter sets. The delivery efficiency and time for the IMRT and VMAT plans was compared.
Results
The VMAT plans resulted in a statistically significant reduction in the rectal V25Gy parameter of 8.2% on average over the IMRT plans. For one of the NTCP parameter sets the VMAT plans had a statistically significant lower rectal NTCP. These reductions in rectal dose were achieved using 18.6% fewer monitor units and a delivery time reduction of up to 69%.
Conclusion
VMAT plans resulted in reductions in rectal doses for all ten patients in the study. This was achieved with significant reductions in delivery time and monitor units. Given the target coverage was equivalent, the VMAT plans were superior.
Keywords: VMAT, IMRT, prostate, biological optimisation
1. Introduction
Intensity Modulated Radiation Therapy (IMRT) has been shown to be the preferred delivery method for prostate radiotherapy [1–7]. A new method of delivery has recently become available, Volumetric Modulated Arc Therapy (VMAT) [8–10]. VMAT is the delivery of IMRT while the linac is in rotation. This is essentially an open aperture IMRT arc technique. Parameters that can be varied are dose rate, gantry speed and number of arcs. One perceived benefit of VMAT is the increase in delivery efficiency.
Recent planning studies have compared VMAT with conventional delivery techniques such as IMRT, 3D conformal (3DCRT) and tomotherapy for prostate radiotherapy [11–13]. Wolff et al. [14] compared VMAT with static gantry IMRT, 3DCRT and serial tomotherapy and found the serial tomotherapy plans resulted in the highest plan quality and VMAT was the most efficient in terms of MU and treatment time. Zhang et. al. [15] compared VMAT with five field static gantry IMRT and found VMAT resulted in slightly better rectal sparing and used on average 55% fewer monitor units for delivery. Palma et al. [11] compared constant and variable dose rate VMAT with IMRT and 3DCRT plans for ten prostate patients. Improved rectal, bladder and femoral head sparing was observed with the VMAT plans over the IMRT and 3DCRT plans. Fewer monitor units were required with the VMAT plans than for the IMRT plans, but not for the 3DCRT plans. Kjaer-Kristoffersen et al. [16] achieved equal or better normal tissue sparing for prostate treatments with VMAT over conventional IMRT, however decreased target dose homogeneity was observed. A recent technical note by Bortfeld and Webb, and subsequent correspondence discussed any theoretical or technical advantages and disadvantages of VMAT[17–21].
The aims of recent studies in the literature have been steered towards the delivery efficiency of VMAT and whether equivalent target and organ-at-risk doses can be delivered while maintaining delivery efficiency. Previous literature has focused on Varian’s RapidArc technique and various in-house developed VMAT algorithms. A new VMAT algorithm, SmartArc™, was released with Version 9 of the Pinnacle RTPS. This study aims to evaluate SmartArc™ by comparing SmartArc™ VMAT plans with IMRT plans using a combination of dosimetric and biological endpoints, as well as delivery efficiency.
2. Methods and Materials
Treatment Planning
Ten sequential prostate radiotherapy patients were selected for analysis from those undergoing prostate radiotherapy at the Illawarra Cancer Care Centre. The CTV was the prostate, not including the seminal vesicles once separated from the prostate. A uniform 7mm CTV-PTV margin was used. The prescription dose was 78Gy in 39 fractions at the isocentre [22, 23]. The rectal volume was defined using full volume and the length from anal canal up to the anterior curve of rectum into sigmoid colon with a maximum distance of 11cm.
The Pinnacle RTPS (Philips Healtcare, Fitchburg, WI) with the biological optimisation toolkit was used [24]. All plans were created for a Varian 21EX (Varian Medical Systems, Palo Alto, USA) with 120 leaf multi-leaf collimator. For each patient two treatment plans were created. The first was a seven field IMRT plan from beam angles 120°, 80°, 40°, 0°, 320°, 280° and 240° (IEC convention [25]). The second plan was created with an alpha version of the Pinnacle SmartArc™ planning tool; This algorithm has been described and evaluated in a recent publication [8]. A dosimetric evaluation of this algorithm has been performed by Feygelman et. al. [26].
The SmartArc™ planning tool optimisation process is as follows. The initial arc parameters (such as arc length, delivery time, number of arcs) are set by the user. The arc length is split into a finite number of fields, spaced equally around the arc with a separation of 24°. Intensity modulation is performed on each field, resulting in intensity maps spaced every 24°. Intensity maps are converted into 2–4 leaf and jaw segments per map. The segments satisfy static machine constraints. The segments are distributed around the arc length. This is done by taking the two segments with the highest number of leaf pairs and repositioning them one third of the initial angle spacing (8°) to the left and right of the initial angle. Segments are then created and inserted at angles evenly between the existing segments to match the user-selected final angle spacing. These segments are created by linearly interpolating between the existing segments. This results in the desired gantry angle spacing, with a segment (or control point at each angle). For example, for an arc length of 360° and a final gantry angle spacing of 4°, 91 control points are created and placed every 4° around the arc. There are 91 control points as the arc is not the full 360°, rather 359.9°. Therefore an extra control point is required to describe the control point for the gantry angle 359.9°. The machine parameters for the MLC and jaw segments are then optimised using a gradient based algorithm. The optimisation takes into account gantry speed, dose rate, total arc delivery time and maximum leaf travel speed. The jaw positions are set. For machines with static jaws, the jaw positions are set to the maximum segment size. For machines with dynamic jaws, the jaw positions are set for each segment.
During the optimisation, the algorithm employs a modified pencil beam dose calculation method - the Singular Value Decomposition method [27]. This decreases the dose calculation time during the iterations. Full collapsed cone convolution calculations are performed during the optimisation (if selected by the user) and at the end of the optimisation iterations [28]. Segment weight optimisation is also performed on the final segments.
A single arc utilising the full 360° was employed. Dose rate modulation was used and a maximum delivery time of 120s was set. The same number of iterations (50) was run for the IMRT and VMAT plans.
For both the IMRT and VMAT plans the optimisation objectives given in Table 1 were used. Biological optimisation with maximum generalized EUD objective was used on the rectum in all plans. Maximum generalized EUD objective with a=3 works to reduce the volumes receiving mid-low doses. The maximum generalized EUD objective was coupled with a maximum dose objective to ensure the high dose region was minimised. A maximum DVH objective was used for the bladder, with the volume receiving 50Gy set to as low as possible (ALAP) without compromising PTV coverage. A maximum dose objective of 50Gy was set for the femoral heads.
Table 1.
Optimisation objectives for all IMRT and VMAT plans
| ROI | Type | Dose (Gy) | Volume | Weight | a |
|---|---|---|---|---|---|
| PTV | Min Dose | 78 | - | 100 | - |
| Max Dose | 81 | - | 100 | - | |
| Rectum | Max gEUD | 30–40 | - | 30 | 3 |
| Max Dose | 76 | - | 30 | - | |
| Bladder | Max DVH | 50 | ALAP | 3 | - |
| Femoral Heads | Max Dose | 50 | - | 2.5 | - |
Plan Analysis
All plans were imported into the Computational Environment for Radiotherapy Research (CERR) (University of Washington in St. Louis, USA) [29]. The coverage of the PTV was compared using the D95% (dose to 95% of the PTV), V95% (volume of PTV with dose > 95% of the prescription (74.1Gy)), mean dose and standard deviation.
Cumulative dose volume histograms (DVHs) were compared for the PTV, rectum, bladder and femoral heads.
Comparison between the plans was also done using a biological end point parameter, Normal Tissue Complication Probability (NTCP), calculated for the rectum. The Lyman-Kutcher-Burman (LKB) NTCP model was chosen [30, 31]. To minimize the any impact of LKB model parameters, three sets of LKB model parameters were chosen from the literature, all representing ≥ Grade 2 rectal toxicity. The NTCP parameters are given in Table 2. Although the LKB model parameters by Tucker et al [32] have recently been updated [33], the NTCP1 values in Table 1 were used so as to represent a range of model parameters. The NTCPs were calculated using the CERR toolkit.
Table 2.
NTCP calculation parameters
Delivery efficiency was assessed by comparing the number of monitor units (MUs) and delivery time. The average number of MUs for each IMRT and VMAT plan was compared. In our clinic, 1MU is equal to 1cGy at Dmax (1.5cm) depth in water for a 10×10cm2 beam with 6MV photons. The delivery time for each plan was measured on a Varian 21EX as the time from the start of the first beam to the end of the last beam. This was compared in the context of total ‘in-room’ time for prostate radiotherapy patients using the average in-room time for all of 2009 from Illawarra Cancer Care Centre. The average in-room time was taken for patients that were registered with and without fiducial markers. The Wilcoxon matched-pair signed-rank test was used to compare the DVHs, NTCP, MU and delivery time results between IMRT and VMAT plans with a statistical significance threshold of p ≤ 0.05.
3. Results
PTV Coverage
The PTV coverage data is presented in Table 3. The PTV coverage for the IMRT and VMAT plans is approximately equal for all parameters, with the exception of the D95% parameter. The VMAT plans resulted in a statistically significantly lower dose to 95% of the PTV volume, although the difference is very small in absolute terms.
Table 3.
PTV coverage for the IMRT and VMAT plans. D95% = dose to 95% of PTV volume; V95% = volume receiving 95% of prescription dose (74.1Gy); mean = mean PTV dose; St. Dev = Standard deviation of the PTV dose
| Plan | D95% | V95% | Mean | St. Dev | ||||
|---|---|---|---|---|---|---|---|---|
| Value | p | Value | p | Value | p | Value | p | |
| IMRT | 76.98 | 0.005 < p < 0.01 | 0.99 | > 0.2 | 79.60 | > 0.2 | 1.58 | > 0.2 |
| VMAT | 76.63 | 0.99 | 79.60 | 1.68 | ||||
Dose-Volume Histograms
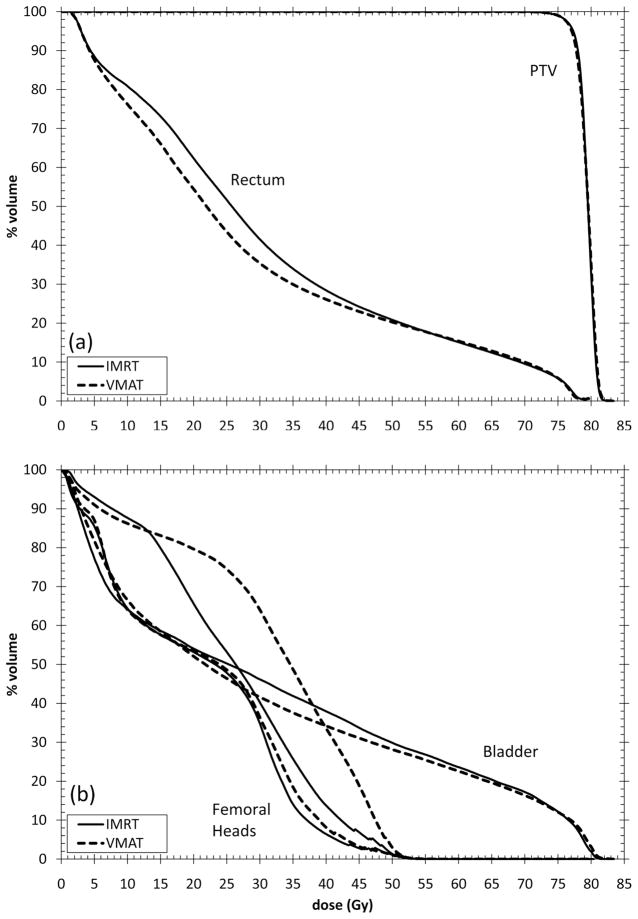
Example IMRT and VMAT dose distributions from one patient are given in Figure 1. The average cumulative DVHs for the PTV and rectum are given in Figure 2a (individual patient DVHs are found in supplementary materials). For equivalent PTV coverage, VMAT plans on average result in lower rectal volumes for doses <50Gy. VMAT and IMRT plans have similar volumes receiving doses >50Gy. The average cumulative DVHs for the femoral heads and bladder are given in Figure 2b. VMAT and IMRT resulted in similar average DVHs. Both VMAT and IMRT plans resulted in similar maximum femoral head dose however VMAT plans resulted in higher volumes receiving doses over the whole range of 0– 50Gy. Table 4 shows average DVH parameters for the IMRT and VMAT plans for the rectum. A statistically significant reduction in the rectal V25Gy parameter was observed with VMAT. This reduction was small (8.21%); its clinical relevance could be debated. The VMAT plans also resulted in a statistically significant increase in the rectal V70Gy parameter of 0.45%. There was no difference in the rectal V50Gy, V60Gy or V75Gy parameters.
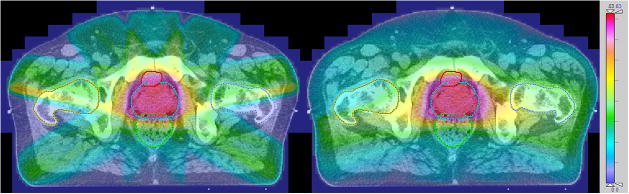
Figure 1.
Example dose distributions for IMRT (left) and VMAT. Dose scale on the right is in Gy.
Figure 2.
Average cumulative DVHs of a) PTV and rectum and b) bladder and femoral heads for IMRT and VMAT plans. Individual patient DVHs can be found in supplementary materials.
Table 4.
Summary of average DVH parameters over the ten patients
| ROI | Parameter | IMRT | VMAT | p-value |
|---|---|---|---|---|
| Rectum | V25Gy | 51.67 | 43.46 | < 0.01 |
| V50Gy | 20.84 | 20.27 | not significant | |
| V60Gy | 15.12 | 15.40 | not significant | |
| V70Gy | 9.56 | 10.01 | < 0.01 | |
| V75Gy | 5.85 | 5.89 | not significant |
The dose to the femoral heads is greater with the VMAT plans. In addition, an asymmetric dose distribution was observed with the VMAT plans in which the left side of the patient received a greater dose than the right side, leading to an increase in dose to the left femoral head (Figure 2b). The femoral head dose still satisfied the dose-volume optimisation objectives for both the IMRT and VMAT plans.
NTCP Comparisons
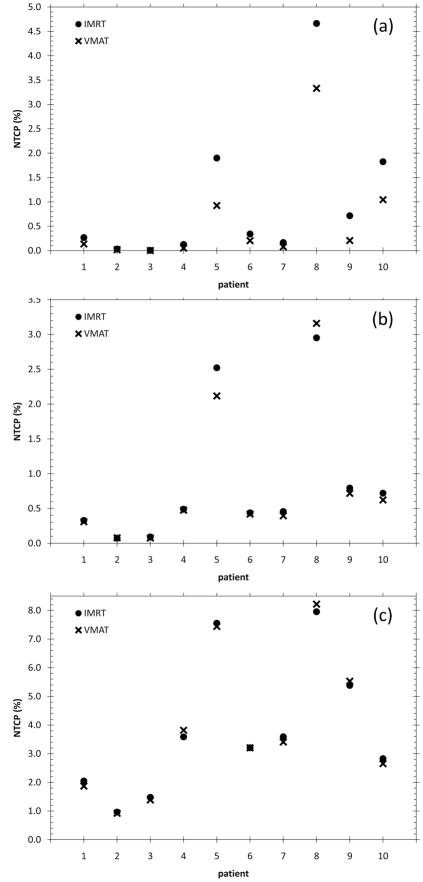
Figure 3 shows the calculated NTCP for all ten patients in the study. Figure 3a, Figure 3b and Figure 3c show the NTCPs for parameter set NTCP1, NTCP2 and NTCP3 respectively. For parameter set NTCP1, VMAT results in a lower NTCP for all ten patients. Parameter set NTCP1 has an n value that corresponds to more parallel tissue architecture. As a result, the gains in rectal DVH with VMAT at the low-mid dose ranges are reflected in NTCP reductions. For six out of ten patients however, the NTCP reductions are extremely small. For Parameter set NTCP2, VMAT has a higher rectal NTCP for one patient. There is minimal gain, if at all, for nine out of the ten patients. Parameter set NTCP2 has an n value reflecting more serial organ architecture. As VMAT and IMRT rectal DVHs are very similar at high range doses then no gain in NTCP is made with VMAT or IMRT. This is further accentuated with parameter set NTCP3, which has an even lower n value. The average NTCPs for all ten patients are given Table 5.
Figure 3.
NTCPs for IMRT and VMAT plans for all 10 patients (a) NTCP1 (b) NTCP2 and (c) NTCP3
Table 5.
Summary of average NTCPs for IMRT and VMAT plans
| Parameter | IMRT | VMAT | p-value |
|---|---|---|---|
| NTCP1 (%) | 0.91 | 0.55 | < 0.01 |
| NTCP2 (%) | 0.81 | 0.77 | not significant |
| NTCP3 (%) | 3.57 | 3.58 | not significant |
Delivery Efficiency
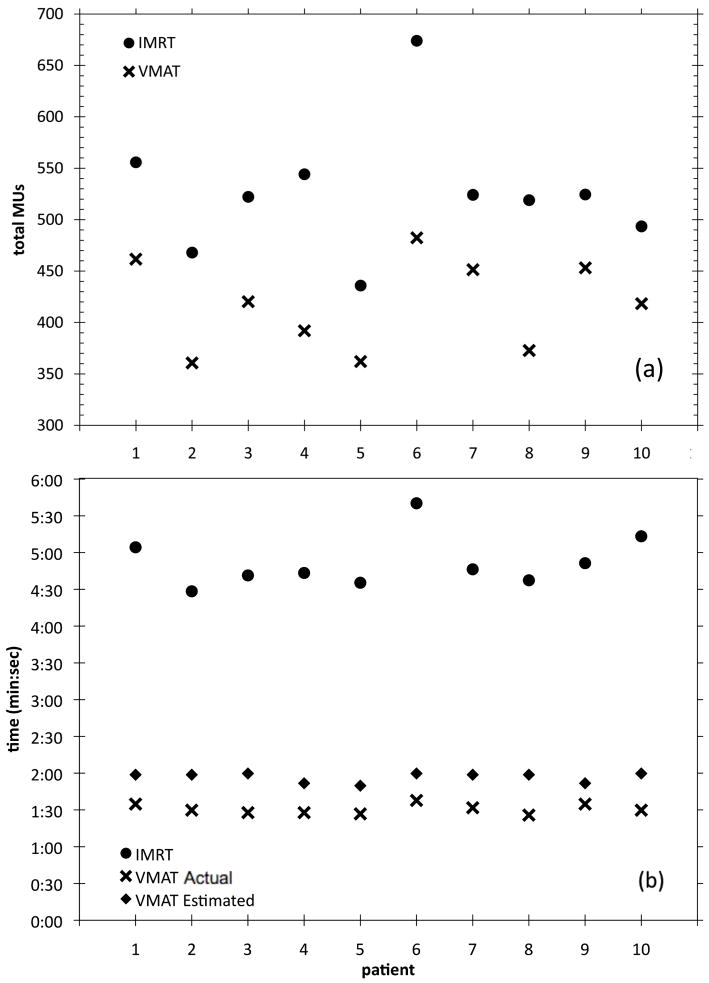
Figure 4 shows the required monitor units for delivery of each plan. The average MU and delivery time data is given in Table 6. Averaged over all ten patients, VMAT required 18.6% fewer monitor units than IMRT (521MU vs 424MU respectively, p < 0.01) for delivery of a 2Gy fraction. The total ‘beam-on’ time for all ten patients is shown in Figure 4b. The actual VMAT delivery time was less than the RTPS-estimated delivery time. This is similar to results presented by Feygelman et. al. [26]. The average delivery time for the IMRT plans (min:sec) was 4:52 ± 0:21 and the average delivery time for the VMAT plans was 1:30 ± 0:03 (p < 0.01). At our institution, the average in-room time for prostate treatment, not including beam delivery time, is 6:42. This means that when the beam delivery time is included, the total in-room time for prostate irradiation would be 11:34 for IMRT treatments and 8:12 for VMAT treatments. This represents a reduction in in-room time of 29.1% when using VMAT. For patients whose setup includes registration using implanted fiducial markers in the prostate, the total in-room treatment time would be 13:51 and 10:29 for IMRT and VMAT respectively, representing a reduction in in-room time of 24.3% with VMAT.
Figure 4.
(a) Total MU for all ten patients and (b) total ‘beam-on’ time for all ten patients including the Pinnacle-estimated VMAT delivery time
Table 6.
Delivery efficiency: Average required MUs and delivery time for IMRT and VMAT plans
| Plan | Average MU ± SD | p-value | Average delivery time ± SD (min:sec) |
|---|---|---|---|
| IMRT | 526 ± 63 | < 0.01 | 4:52 ± 0:21 |
| VMAT | 417 ± 44 | 1:31 ± 0:03 |
4. Discussion
An average reduction in the rectal V25Gy values over the ten patients of 8.21% was observed. This reduction in V25Gy came at the expense of a minor increase in V70Gy of 0.45%, averaged over the ten patients. It can be argued that the gains made with the V25Gy parameter are greater than the increase in rectal toxicity probability with the higher V70Gy. This is seen in the NTCP values for parameter sets NTCP2 and NTCP3, where no statistically significant increases in rectal NTCP were observed.
The observed increase in femoral head dose is expected due to the VMAT utilising delivery angles that result in delivery of target dose through the femoral heads; the gantry angles used in the IMRT plans limit delivery through the whole femoral head. Nevertheless, the VMAT plans still met the dose volume objectives set in optimisation. The reason for the asymmetric dose distribution was not clear. Investigation for a subset of patients into gantry rotation direction did not affect the asymmetry of the dose distribution. However, further investigation of placing an extra dose-volume objective on the ‘hot’ femoral head was found to reduce any asymmetry whilst conserving the gains with VMAT in the low dose region of the rectum.
The three parameter sets used allow some estimate of the range of NTCP values that might be experienced for grade 2 rectal toxicities, given the variability in the published model parameters. The value of n is 1.03, 0.24 and 0.084 for NTCP1, NTCP2 and NTCP3 respectively. Consequently, each parameter set penalises the rectal DVHs according to the rectum being more parallel (high n) or serial (low n). It should also be acknowledged that clinically, there may be various toxicity end points to be considered. Each of these end points will be characterised by its own set of NTCP model parameters. As a result, the clinical selection of a plan based on NTCP could involve the assessment of a range of NTCP values related to various toxicity end points, as suggested by Rancati et al. [34]. These NTCP values may span a similar range to the uncertainty in the NTCP for any single endpoint such as the range of grade 2 rectal toxicities which we have calculated here.
The high dose region of the rectum is included in the PTV, so the only reduction in NTCP due to this dose can be made by reducing the CTV-PTV margin. It is very hard to achieve reduction of the dose to this region with technique changes. Therefore, as the NTCP parameter set becomes more weighted towards the small volume of the rectum receiving high doses, no change in NTCP is seen when technique is changed.
The MU reduction observed in this study is consistent with reported results for prostate irradiation [11, 15]. The results in this study however show a smaller reduction in required monitor units; any number of factors can lead variations in delivery efficiency, as discussed by Ost et al. [35] and Palma et al. [36]. A number of possibilities may have led to a lower reduction in required MU compared with other studies. This study compares VMAT plans with step and shoot static gantry angle IMRT, which is an efficient method of delivering IMRT. Previous studies [11] have used sliding window techniques which require significantly more MU for delivery.
The reduction in the required monitor units was supported by a 69% reduction in the ‘beam-on’ time required with VMAT. When added to the time taken for patient setup, it is estimated that the total in-room time would be reduced on average by up to 29% per patient. This is a significant gain in delivery efficiency, which would increase patient throughput significantly. Additionally, institutions not yet employing IMRT for localised prostate radiotherapy due to increases in delivery time over 3DCRT, may view VMAT as an attractive modality to improve plan quality whilst maintaining delivery efficiency.
5. Conclusion
This study has compared IMRT and VMAT plans for ten prostate patients. We have shown that for equivalent target coverage, a small reduction in rectal volumes receiving mid-low doses, specifically the V25Gy parameter, can be achieved using a single-arc VMAT for prostate radiotherapy. A small increase in the V70Gy parameter was observed with VMAT, with no changes to the V50Gy, V60Gy and V75Gy parameters. The VMAT plans required on average 18.6% fewer monitor units per fraction. A statistically significant average reduction in rectal NTCP was achieved for one rectal NTCP parameter set that penalises volumes receiving low-mid doses, although the gains were extremely small in six out of the ten patients. In general, provided the isodose maps and DVHs show similar target coverage, the rectal DVH reduction and delivery efficiency gains observed with VMAT plans result in a superior plan.
Supplementary Material
Figure 5: PTV and rectal DVHs for all 10 patients
Acknowledgments
The authors would like to acknowledge Philips Radiation Oncology Systems for a research license of the Pinnacle RTPS with the SmartArc and Biological Optimisation toolboxes and Insight Oceania for hardware support. The authors would also like to thank Australian Rotary Health, the NSW Cancer Institute Clinical Leaders Program and United States National Institute of Health R01-CA106835 for funding for this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kupelian PA, Reddy CA, Carlson TP, Altsman KA, Willoughby TR. Preliminary observations on biochemical relapse-free survival rates after short-course intensity-modulated radiotherapy (70 Gy at 2.5 Gy/fraction) for localized prostate cancer. International Journal of Radiation Oncology Biology Physics. 2002;53(4):904–912. doi: 10.1016/s0360-3016(02)02836-5. [DOI] [PubMed] [Google Scholar]
- 2.Kupelian PA, Reddy CA, Carlson TP, Willoughby TR. Dose/volume relationship of late rectal bleeding after external beam radiotherapy for localized prostate cancer: Absolute or relative rectal volume? Cancer Journal. 2002;8(1):62–66. doi: 10.1097/00130404-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Namiki S, Ishidoya S, Tochigi T, Kawamura S, Kuwahara M, Terai A, Yoshimura K, Numata I, Satoh M, Saito S, Takai Y, Yamada S, Arai Y. Health-related quality of life after intensity modulated radiation therapy for localized prostate cancer: Comparison with conventional and conformal radiotherapy. Japanese Journal of Clinical Oncology. 2006;36(4):224–230. doi: 10.1093/jjco/hyl002. [DOI] [PubMed] [Google Scholar]
- 4.Sanguineti G, Cavey ML, Endres EJ, Franzone P, Barra S, Parker BC, Marcenaro M, Colman M, Agostinelli S, Foppian F, Vitale V. Does treatment of the pelvic nodes with IMRT increase late rectal toxicity over conformal prostate only radiotherapy to 76 Gy? Strahlentherapie Und Onkologie. 2006;182(9):543–549. doi: 10.1007/s00066-006-1586-9. [DOI] [PubMed] [Google Scholar]
- 5.Veldeman L, Madani I, Hulstaert F, De Meerleer G, Mareel M, De Neve W. Evidence behind use of intensity-modulated radiotherapy: a systematic review of comparative clinical studies. The Lancet Oncology. 2008;9(4):367–375. doi: 10.1016/S1470-2045(08)70098-6. [DOI] [PubMed] [Google Scholar]
- 6.Zelefsky MJ, Fuks Z, Happersett L, Lee HJ, Ling CC, Burman CM, Hunt M, Wolfe T, Venkatraman E, Jackson A, Skwarchuk M, Leibel SA. Clinical experience with intensity modulated radiation therapy (IMRT) in prostate cancer. Radiotherapy and Oncology. 2000;55(3):241–249. doi: 10.1016/s0167-8140(99)00100-0. [DOI] [PubMed] [Google Scholar]
- 7.Zelefsky MJ, Fuks Z, Hunt M, Lee HJ, Lombardi D, Ling CC, Reuter VE, Venkatraman ES, Leibel SA. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. Journal of Urology. 2001;166(3):876–881. [PubMed] [Google Scholar]
- 8.Bzdusek K, Friberger H, Eriksson K, Hardemark B, Robinson D, Kaus M. Development and Evaluation of an Efficient Approach to Volumetric Arc Therapy Planning. Medical Physics. 2009;36(6):2328–2339. doi: 10.1118/1.3132234. [DOI] [PubMed] [Google Scholar]
- 9.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Medical Physics. 2008;35(1):310–317. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 10.Yu CX. Intensity-modulated arc therapy with dynamic multileaf collimation - An alternative to tomotherapy. Physics in Medicine and Biology. 1995;40(9):1435–1449. doi: 10.1088/0031-9155/40/9/004. [DOI] [PubMed] [Google Scholar]
- 11.Palma D, Vollans E, James K, Nakano S, Moiseenko V, Shaffer R, McKenzie M, Morris J, Otto K. Volumetric modulated arc therapy for delivery of prostate radiotherapy: Comparison with intensity-modulated radiotherapy and three-dimensional conformal radiotherapy. International Journal of Radiation Oncology Biology Physics. 2008;72(4):996–1001. doi: 10.1016/j.ijrobp.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 12.Palma D, Vollans E, James K, Nakano S, Moiseenko V, Shaffer R, McKenzie M, Morris J, Otto K. Volumetric Modulated Arc Therapy (VMAT) for delivery of prostate radiotherapy: Reduction in treatment time and monitor unit requirements compared to intensity modulated radiotherapy. 50th Annual Meeting of the American-Society-for-Therapeutic-Radiology-and Oncology; 2008; Boston, MA. [Google Scholar]
- 13.Wolff D, Stieler F, Abo-Madyan Y, Polednik M, Steil V, Mai S, Wenz F, Lohr F. Volumetric intensity modulated arc therapy (VMAT) vs. serial tomotherapy and segmental (step and shoot) IMRT for treatment of prostate cancer. 50th Annual Meeting of the American-Society-for-Therapeutic-Radiology-and Oncology; 2008; Boston, MA. [DOI] [PubMed] [Google Scholar]
- 14.Wolff D, Stieler F, Welzel G, Lorenz F, Abo-Madyan Y, Mai S, Herskind C, Polednik M, Steil V, Wenz F, Lohr F. Volumetric modulated arc therapy (VMAT) vs. serial tomotherapy, step-and-shoot IMRT and 3D-conformal RT for treatment of prostate cancer. Radiother Oncol. 2009;93(2):226–33. doi: 10.1016/j.radonc.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Zhang P, Happersett L, Hunt M, Jackson A, Zelefsky M, Mageras G. Volumetric modulated arc therapy: planning and evaluation for prostate cancer cases. Int J Radiat Oncol Biol Phys. 2010;76(5):1456–62. doi: 10.1016/j.ijrobp.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 16.Kjaer-Kristoffersen F, Ohlhues L, Medin J, Korreman S. RapidArc volumetric modulated therapy planning for prostate cancer patients. Symposium of the Nordic-Association-of-Clinical-Physics; 2008; Aarhus, DENMARK. [DOI] [PubMed] [Google Scholar]
- 17.Bortfeld T, Webb S. Single-Arc IMRT? Physics in Medicine and Biology. 2009;54(1):N9–N20. doi: 10.1088/0031-9155/54/1/N02. [DOI] [PubMed] [Google Scholar]
- 18.Bortfeld T, Webb S. Reply to ‘Comments on ‘Single-Arc IMRT? Physics in Medicine and Biology. 2009;54(8):L35–L36. doi: 10.1088/0031-9155/54/8/L01. [DOI] [PubMed] [Google Scholar]
- 19.Bortfeld T, Webb S. Reply to ‘Letter to the Editor on’ Single-Arc IMRT? Physics in Medicine and Biology. 2009;54(8):L43–L44. doi: 10.1088/0031-9155/54/8/L03. [DOI] [PubMed] [Google Scholar]
- 20.Otto K. Letter to the Editor on ‘Single-Arc IMRT?’. Physics in Medicine and Biology. 2009;54(8):L37–L41. doi: 10.1088/0031-9155/54/8/L03. [DOI] [PubMed] [Google Scholar]
- 21.Verbakel W, Senan S, Lagerwaard FJ, Cuijpers JP, Slotman BJ. Comments on ‘Single-Arc IMRT?’. Physics in Medicine and Biology. 2009;54(8):L31–L34. doi: 10.1088/0031-9155/54/8/L01. [DOI] [PubMed] [Google Scholar]
- 22.Kuban DA, Tucker SL, Dong L, Starkschall G, Huang EE, Cheung MR, Lee AK, Pollack A. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. International Journal of Radiation Oncology Biology Physics. 2008;70(1):67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 23.Pollack A, Zagars GK, Starkschall G, Antolak JA, Lee JJ, Huang E, von Eschenbach AC, Kuban DA, Rosen I. Prostate cancer radiation dose response: Results of the M. D. Anderson phase III randomized trial. International Journal of Radiation Oncology Biology Physics. 2002;53(5):1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 24.Biological Optimization using the Equivalent Uniform Dose (EUD) in Pinnacle3. RaySearch Laboratories AB; Stockholm, Sweden: 2003. [Google Scholar]
- 25.I.E. Commission. IEC1217. International Electrotechnical Commission; 1996. Radiotherapy equipment - Coordinates, movements and scales. [Google Scholar]
- 26.Feygelman V, Zhang G, Stevens C. Initial dosimetric evaluation of SmartArc - a novel VMAT treatment planning module implemented in a multi-vendor delivery chain. J Appl Clin Med Phys. 2010;11(1):99–116. doi: 10.1120/jacmp.v11i1.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bortfeld T, Schlegel W, Rhein B. Decomposition of pencil beam kernels for fast dose calculations in 3-dimensional treatment planning. Medical Physics. 1993;20(2):311–318. doi: 10.1118/1.597070. [DOI] [PubMed] [Google Scholar]
- 28.McNutt T. Dose calculations: collapsed cone convolution superposition and delta pexel beam. Philips; 2002. [Google Scholar]
- 29.Deasy JO, Blanco AI, Clark VH. CERR: A computational environment for radiotherapy research. Medical Physics. 2003;30(5):979–985. doi: 10.1118/1.1568978. [DOI] [PubMed] [Google Scholar]
- 30.Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation - the effective volume method. International Journal of Radiation Oncology Biology Physics. 1989;16(6):1623–1630. doi: 10.1016/0360-3016(89)90972-3. [DOI] [PubMed] [Google Scholar]
- 31.Lyman JT. Complication probability as assessed from dose volume histograms. Radiation Research. 1985;104(2):S13–S19. [PubMed] [Google Scholar]
- 32.Tucker SL, Dong L, Cheung R, Johnson J, Mohan R, Huang EH, Liu HH, Thames HD, Kuban D. Comparison of rectal dose-wall histogram versus dose-volume histogram for modeling the incidence of late rectal bleeding after radiotherapy. Int J Radiat Oncol Biol Phys. 2004;60(5):1589–601. doi: 10.1016/j.ijrobp.2004.07.712. [DOI] [PubMed] [Google Scholar]
- 33.Tucker SL, Dong L, Bosch WR, Michalski J, Winter K, Lee AK, Cheung MR, Kuban DA, Cox JD, Mohan R. Fit of a generalized Lyman Normal-Tissue Complication Probability (NTCP) model to grade >= 2 late rectal toxicity data from patients treated on protocol RTOG 94-06. 49th Annual Meeting of the American-Society-for-Therapeutic-Radiology-and-Oncology; 2007; Los Angeles, CA. [Google Scholar]
- 34.Rancati T, Fiorino C, Gagliardi G, Cattaneo GM, Sanguineti G, Borca VC, Cozzarini C, Fellin G, Foppiano F, Girelli G, Menegotti L, Piazzolla A, Vavassori V, Valdagni R. Fitting late rectal bleeding data using different NTCP models: results from an Italian multi-centric study (AIROPROS0101) Radiother Oncol. 2004;73(1):21–32. doi: 10.1016/j.radonc.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Ost P, Fonteyne V, De Neve W, De Gersem W, De Wagter C, Vandecasteele K, Duprez F, De Meerleer G. Volumetric modulated arc therapy for delivery of prostate radiotherapy: In regard to Palma et al. (Int J Radiat Oncol Biol Phys 2008;70:996-1001) International Journal of Radiation Oncology Biology Physics. 2009;73(4):1286–1286. doi: 10.1016/j.ijrobp.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Palma D, Moiseenko V, Vollans E, McKenzie M, Morris J, Otto K. Volumetric modulated arc therapy for delivery of prostate radiotherapy: In regard to Palma et al. (Int J Radiat Oncol Biol Phys 2008;70:996-1001) Reply. International Journal of Radiation Oncology Biology Physics. 2009;73(4):1287–1287. doi: 10.1016/j.ijrobp.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 37.Sohn M, Yan D, Liang J, Meldolesi E, Vargas C, Alber M. Incidence of late rectal bleeding in high-dose conformal radiotherapy of prostate cancer using equivalent uniform dose-based and dose-volume-based normal tissue complication probability models. Int J Radiat Oncol Biol Phys. 2007;67(4):1066–73. doi: 10.1016/j.ijrobp.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 5: PTV and rectal DVHs for all 10 patients