Abstract
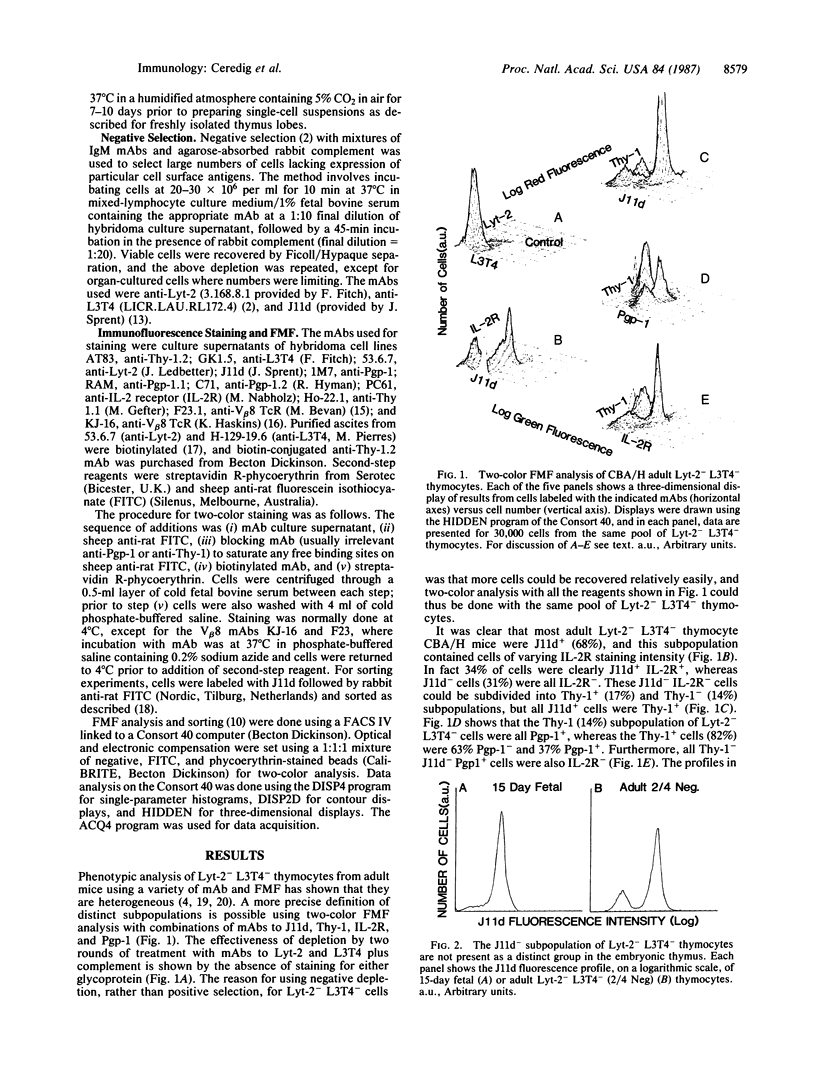
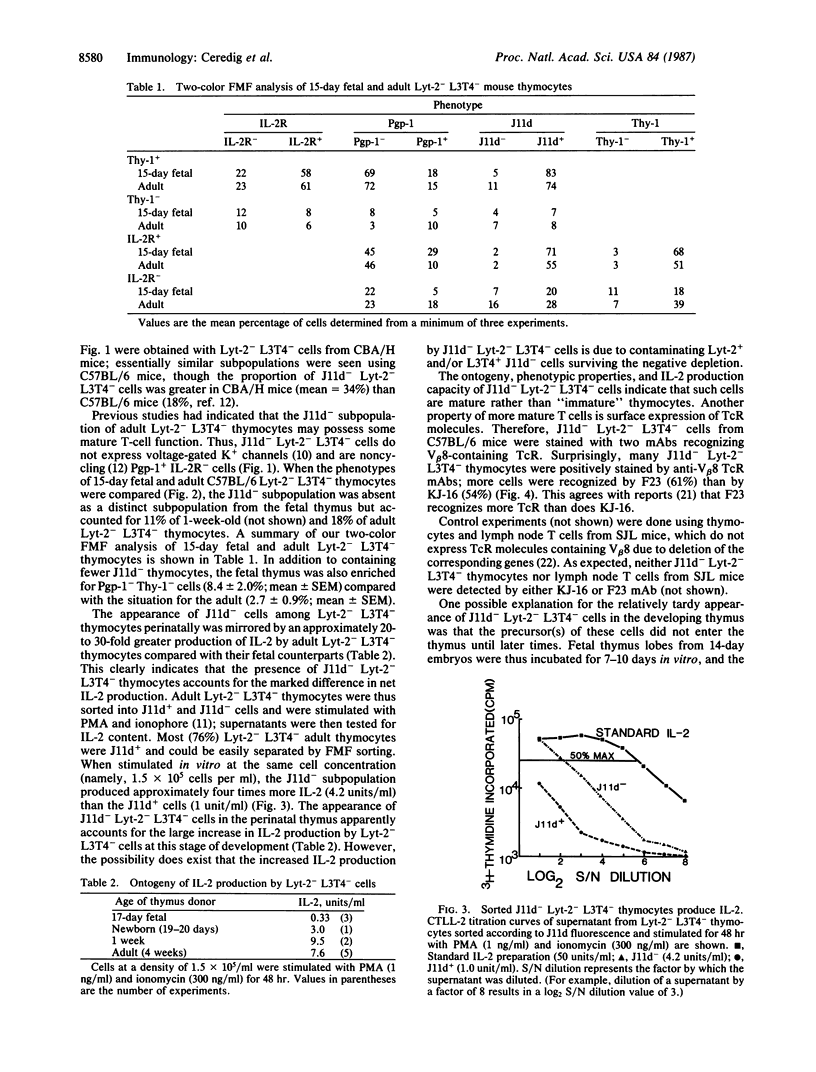
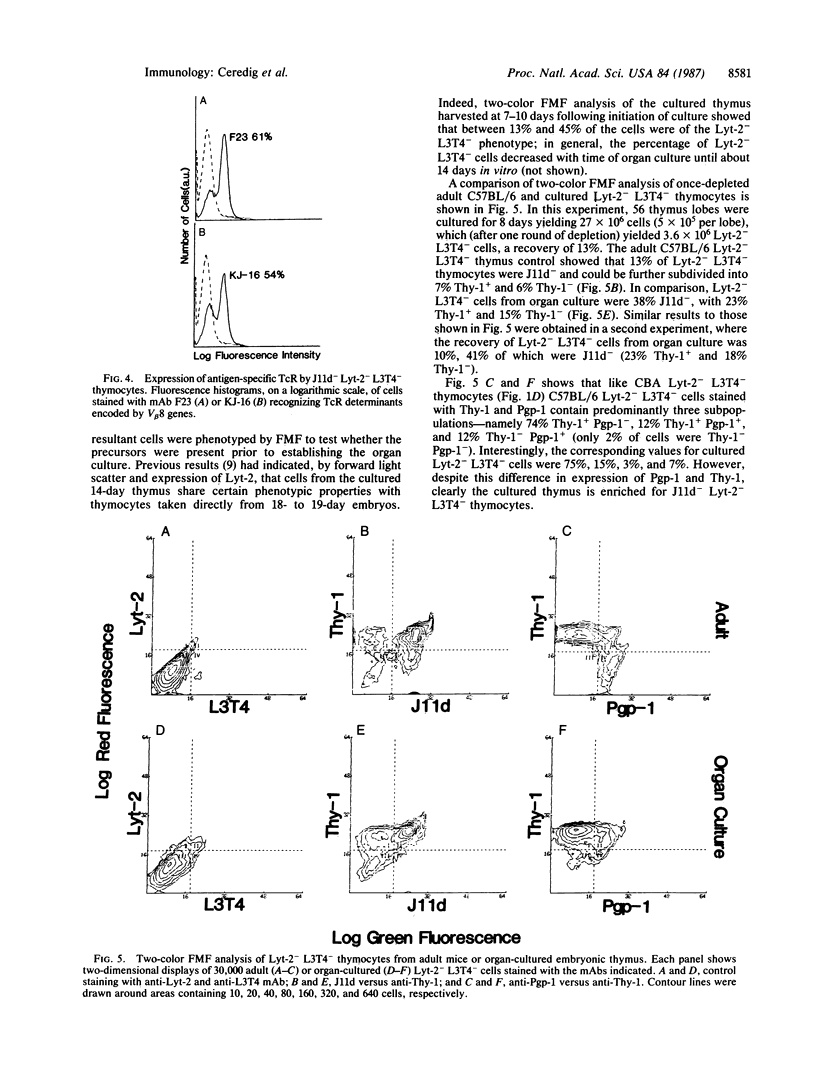
Two-color flow microfluorometry using monoclonal antibodies to cell surface determinants has shown that a subpopulation of mouse Lyt-2- L3T4- thymocytes, comprising 18% of Lyt-2- L3T4- cells in adult C57BL/6 mice, appears in the thymus late during fetal development. These Lyt-2- L3T4- cells are characterized by lack of expression of determinants recognized by monoclonal antibody J11d, a phenotype characteristic of more "mature" functional T cells. This J11d- subpopulation of Lyt-2- L3T4- thymocytes has now been shown to produce significant quantities of interleukin 2 following mitogen stimulation and to express T-cell receptor molecules recognized by monoclonal antibodies KJ-16 and F23.1. Furthermore, culturing of fetal thymus lobes has shown that precursors of this subpopulation of Lyt-2- L3T4- thymocytes are already present in thymus at 14 days of embryonic development and are thus derived from an intrathymic precursor cell. So, within the mouse thymus, phenotypic changes and acquisition of mature T-cell characteristics occur within a subpopulation of cells originally thought of as exclusively "immature."
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behlke M. A., Henkel T. J., Anderson S. J., Lan N. C., Hood L., Braciale V. L., Braciale T. J., Loh D. Y. Expression of a murine polyclonal T cell receptor marker correlates with the use of specific members of the V beta 8 gene segment subfamily. J Exp Med. 1987 Jan 1;165(1):257–262. doi: 10.1084/jem.165.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J., Symington F. W., McKearn T. J., Sprent J. A monoclonal antibody discriminating between subsets of T and B cells. J Immunol. 1981 Dec;127(6):2496–2501. [PubMed] [Google Scholar]
- Budd R. C., Miescher G. C., Howe R. C., Lees R. K., Bron C., MacDonald H. R. Developmentally regulated expression of T cell receptor beta chain variable domains in immature thymocytes. J Exp Med. 1987 Aug 1;166(2):577–582. doi: 10.1084/jem.166.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceredig R., Glasebrook A. L., MacDonald H. R. Phenotypic and functional properties of murine thymocytes. I. Precursors of cytolytic T lymphocytes and interleukin 2-producing cells are all contained within a subpopulation of "mature" thymocytes as analyzed by monoclonal antibodies and flow microfluorometry. J Exp Med. 1982 Feb 1;155(2):358–379. doi: 10.1084/jem.155.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceredig R., MacDonald H. R. Intrathymic differentiation: some unanswered questions. Surv Immunol Res. 1985;4(2):87–95. doi: 10.1007/BF02918805. [DOI] [PubMed] [Google Scholar]
- Ceredig R., MacDonald H. R., Jenkinson E. J. Flow microfluorometric analysis of mouse thymus development in vivo and in vitro. Eur J Immunol. 1983 Mar;13(3):185–190. doi: 10.1002/eji.1830130302. [DOI] [PubMed] [Google Scholar]
- Ceredig R. Proliferation in vitro and interleukin production by 14 day fetal and adult Lyt-2-/L3T4- mouse thymocytes. J Immunol. 1986 Oct 1;137(7):2260–2267. [PubMed] [Google Scholar]
- Ceredig R., Sekaly R. P., MacDonald H. R. Differentiation in vitro of Lyt 2+ thymocytes from embryonic Lyt 2- precursors. Nature. 1983 May 19;303(5914):248–250. doi: 10.1038/303248a0. [DOI] [PubMed] [Google Scholar]
- Crispe I. N., Bevan M. J. Expression and functional significance of the J11d marker on mouse thymocytes. J Immunol. 1987 Apr 1;138(7):2013–2018. [PubMed] [Google Scholar]
- Fowlkes B. J., Edison L., Mathieson B. J., Chused T. M. Early T lymphocytes. Differentiation in vivo of adult intrathymic precursor cells. J Exp Med. 1985 Sep 1;162(3):802–822. doi: 10.1084/jem.162.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlkes B. J., Kruisbeek A. M., Ton-That H., Weston M. A., Coligan J. E., Schwartz R. H., Pardoll D. M. A novel population of T-cell receptor alpha beta-bearing thymocytes which predominantly expresses a single V beta gene family. Nature. 1987 Sep 17;329(6136):251–254. doi: 10.1038/329251a0. [DOI] [PubMed] [Google Scholar]
- Guesdon J. L., Ternynck T., Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979 Aug;27(8):1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- Haskins K., Hannum C., White J., Roehm N., Kubo R., Kappler J., Marrack P. The antigen-specific, major histocompatibility complex-restricted receptor on T cells. VI. An antibody to a receptor allotype. J Exp Med. 1984 Aug 1;160(2):452–471. doi: 10.1084/jem.160.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jotereau F. V., Le Douarin N. M. Demonstration of a cyclic renewal of the lymphocyte precursor cells in the quail thymus during embryonic and perinatal life. J Immunol. 1982 Nov;129(5):1869–1877. [PubMed] [Google Scholar]
- Jotereau F., Heuze F., Salomon-Vie V., Gascan H. Cell kinetics in the fetal mouse thymus: precursor cell input, proliferation, and emigration. J Immunol. 1987 Feb 15;138(4):1026–1030. [PubMed] [Google Scholar]
- Lowenthal J. W., Howe R. C., Ceredig R., MacDonald H. R. Functional status of interleukin 2 receptors expressed by immature (Lyt-2-/L3T4-) thymocytes. J Immunol. 1986 Oct 15;137(8):2579–2584. [PubMed] [Google Scholar]
- Lugo J. P., Krishnan S. N., Sailor R. D., Rothenberg E. V. Early precursor thymocytes can produce interleukin 2 upon stimulation with calcium ionophore and phorbol ester. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1862–1866. doi: 10.1073/pnas.83.6.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch F., Chaudhri G., Allan J. E., Doherty P. C., Ceredig R. Expression of Pgp-1 (or Ly24) by subpopulations of mouse thymocytes and activated peripheral T lymphocytes. Eur J Immunol. 1987 Jan;17(1):137–140. doi: 10.1002/eji.1830170123. [DOI] [PubMed] [Google Scholar]
- Macdonald H. R., Engers H. D., Cerottini J. C., Brunner K. T. Generation of cytotoxic T lymphocytes in vitro. II. Effect of repeated exposure to alloantigens on the cytotoxic activity of long-term mixed leukocyte cultures. J Exp Med. 1974 Sep 1;140(3):718–730. doi: 10.1084/jem.140.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieson B. J., Fowlkes B. J. Cell surface antigen expression on thymocytes: development and phenotypic differentiation of intrathymic subsets. Immunol Rev. 1984 Dec;82:141–173. doi: 10.1111/j.1600-065x.1984.tb01121.x. [DOI] [PubMed] [Google Scholar]
- McKinnon D., Ceredig R. Changes in the expression of potassium channels during mouse T cell development. J Exp Med. 1986 Dec 1;164(6):1846–1861. doi: 10.1084/jem.164.6.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Von Boehmer H. Requirements for growth of immature thymocytes from fetal and adult mice in vitro. Eur J Immunol. 1986 Jan;16(1):12–19. doi: 10.1002/eji.1830160104. [DOI] [PubMed] [Google Scholar]
- Pardoll D. M., Fowlkes B. J., Bluestone J. A., Kruisbeek A., Maloy W. L., Coligan J. E., Schwartz R. H. Differential expression of two distinct T-cell receptors during thymocyte development. Nature. 1987 Mar 5;326(6108):79–81. doi: 10.1038/326079a0. [DOI] [PubMed] [Google Scholar]
- Raulet D. H. Expression and function of interleukin-2 receptors on immature thymocytes. Nature. 1985 Mar 7;314(6006):101–103. doi: 10.1038/314101a0. [DOI] [PubMed] [Google Scholar]
- Raulet D. H., Garman R. D., Saito H., Tonegawa S. Developmental regulation of T-cell receptor gene expression. Nature. 1985 Mar 7;314(6006):103–107. doi: 10.1038/314103a0. [DOI] [PubMed] [Google Scholar]
- Roehm N. W., Carbone A., Kushnir E., Taylor B. A., Riblet R. J., Marrack P., Kappler J. W. The major histocompatibility complex-restricted antigen receptor on T cells: the genetics of expression of an allotype. J Immunol. 1985 Sep;135(3):2176–2182. [PubMed] [Google Scholar]
- Scollay R., Bartlett P., Shortman K. T cell development in the adult murine thymus: changes in the expression of the surface antigens Ly2, L3T4 and B2A2 during development from early precursor cells to emigrants. Immunol Rev. 1984 Dec;82:79–103. doi: 10.1111/j.1600-065x.1984.tb01118.x. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfrè G., Secher D. S., Milstein C. Monoclonal xenogeneic antibodies to murine cell surface antigens: identification of novel leukocyte differentiation antigens. Eur J Immunol. 1978 Aug;8(8):539–551. doi: 10.1002/eji.1830080802. [DOI] [PubMed] [Google Scholar]
- Staerz U. D., Rammensee H. G., Benedetto J. D., Bevan M. J. Characterization of a murine monoclonal antibody specific for an allotypic determinant on T cell antigen receptor. J Immunol. 1985 Jun;134(6):3994–4000. [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Regulation of the assembly and expression of variable-region genes. Annu Rev Immunol. 1986;4:339–368. doi: 10.1146/annurev.iy.04.040186.002011. [DOI] [PubMed] [Google Scholar]



