Abstract
Neuronal gap junctional hemichannels, composed of pannexin-1 subunits, have been suggested to play a crucial role in epilepsy and brain ischaemia. After a few minutes of anoxia or ischaemia, neurons in brain slices show a rapid depolarization to ∼−20 mV, called the anoxic depolarization. Glutamate receptor blockers can prevent the anoxic depolarization, suggesting that it is produced by a cation influx through glutamate-gated channels. However, in isolated hippocampal pyramidal cells, simulated ischaemia evokes a large inward current and an increase in permeability to large molecules, mediated by the opening of pannexin-1 hemichannels. N-methyl-d-aspartate is also reported to open these hemichannels, suggesting that the activation of N-methyl-d-aspartate receptors, which occurs when glutamate is released in ischaemia, might cause the anoxic depolarization by evoking a secondary ion flux through pannexin-1 hemichannels. We tested the contribution of pannexin hemichannels to the anoxic depolarization in CA1 pyramidal cells in the more physiological environment of hippocampal slices. Three independent inhibitors of hemichannels—carbenoxolone, lanthanum and mefloquine—had no significant effect on the current generating the anoxic depolarization, while a cocktail of glutamate and gamma-aminobutyric acid class A receptor blockers abolished it. We conclude that pannexin hemichannels do not generate the large inward current that underlies the anoxic depolarization. Glutamate receptor channels remain the main candidate for generating the large inward current that produces the anoxic depolarization.
Keywords: ischaemia, glutamate, gap junction, hemichannel, pannexin
Introduction
Loss of the oxygen or blood supply to the brain (anoxia or ischaemia) leads to a cessation of ATP generation in the affected area. The resulting inhibition of the Na+/K+ pump causes a run-down of transmembrane ion gradients that is initially slow, but after a few minutes a rapid rise of [K+]o to ∼60 mM occurs (Hansen, 1985), while [Na+]o falls by about the same amount. At this time glutamate transporters reverse, raising the extracellular glutamate concentration to ∼100 µM (Madl and Burgesser, 1993; Wahl et al., 1994; Roettger and Lipton, 1996; Rossi et al., 2000) and the membrane potential depolarizes to −20 mV (Hansen, 1985).
Recording from neurons in hippocampal and cerebellar slices has shown that this anoxic depolarization is associated with a large glutamate-evoked inward current, which can be blocked by a cocktail of agents blocking N-methyl-d-aspartate (NMDA), α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) and kainate receptors (Ben-Ari, 1990; Rossi et al., 2000; Hamann et al., 2005). Recently however, the notion that this inward current reflects ion entry through glutamate-gated channels has been challenged by the discovery that, in isolated CA1 pyramidal cells, ischaemia evokes a large inward current even in the presence of glutamate receptor blockers (Thompson et al., 2006). This current is inhibited by carbenoxolone and lanthanum (La3+) (Thompson et al., 2006), which block gap junction hemichannels. Hemichannels are made of connexin or pannexin proteins, they function as ion channels in neuronal and glial membranes (John et al., 1999; Contreras et al., 2003; Ripps et al., 2004; Bruzzone et al., 2005; Iglesias et al., 2009) and they are expressed at the post-synaptic densities of hippocampal pyramidal cells (Ray et al., 2005; Vogt et al., 2005; Zoidl et al., 2007).
The activation of NMDA receptors is also reported to evoke a current component mediated by hemichannels, which is blocked by carbenoxolone and by 10PanX (Thompson et al., 2008), i.e. blockers of hemichannels formed by pannexin-1 (Pelegrin and Surprenant, 2006; Ma et al., 2008). Thus, in ischaemia, pannexins might generate a large inward current, producing the anoxic depolarization, either because of a direct activating effect of ischaemia on pannexin hemichannels as seen in isolated pyramidal cells (Thompson et al., 2006), or as a consequence of secondary hemichannel opening produced by ischaemia-evoked glutamate release activating NMDA receptors (Thompson et al., 2008).
To fully understand the deleterious events triggered by stroke and to aid the identification of possible therapeutic targets, it is crucial to know whether the anoxic depolarization is generated largely by current flow through glutamate-gated channels or through pannexin hemichannels. The contribution of glutamate-gated channels may differ between isolated cells and cells in situ, because in situ the extracellular glutamate concentration will rise much more in ischaemia as a result of the restricted nature of the extracellular space. We therefore assessed the relative contribution of pannexins and glutamate-gated channels to generating the anoxic depolarization in CA1 pyramidal cells in hippocampal slices.
Materials and methods
Preparation
Post-natal Day 12 or Day 20 rats were killed by cervical dislocation in accordance with UK regulations. We tested the possible involvement of pannexins in generating the anoxic depolarization at two ages because pannexin expression is highest early in development (Ray et al., 2005; Vogt et al., 2005), yet the brain’s susceptibility to ischaemic damage increases with age. Hippocampal slices (225–300 µm thick) were prepared as described by Allen et al. (2004) and were superfused at 33 ± 1°C with solution containing 124 mM NaCl, 26 mM NaHCO3, 1 mM NaH2PO4, 2.5 mM KCl, 2 mM MgCl2, 2.5 mM CaCl2, 10 mM glucose, bubbled with 95% O2/5% CO2, pH 7.4. To simulate ischaemia we replaced external O2 with N2 and external glucose with 7 mM sucrose, added 2 mM iodoacetate to block glycolysis, and added 25 µM antimycin to block oxidative phosphorylation (Allen et al., 2005). This concentration of iodoacetate was chosen to give a complete block of the glycolytic enzyme glyceraldehyde 3-phosphate dehydrogenase. In intact cells in culture, where the drug has easy access to the cells, the half maximal inhibitory concentration (IC50) for blocking glyceraldehyde 3-phosphate dehydrogenase is 130 µM, and 0.5 mM iodoacetate gives a fairly complete block of glycolysis (while a concentration 1000-fold higher is needed to inhibit enzymes such as lactate dehydrogenase and pyruvate kinase) (Even et al., 1999). We used a 4-fold higher concentration of iodoacetate (2 mM) because of the hindered diffusive access to the deeper cells in the brain slice. This chemical ischaemia mimics that produced by oxygen and glucose deprivation but causes an earlier onset of anoxic depolarization (Allen et al., 2005) because using simple oxygen and glucose deprivation makes it hard to prevent oxygen diffusing from the surface of the solution to the slice.
LaCl3, carbenoxolone (100 µM each) or mefloquine (25 µM) were added to these solutions. La3+, when present, may bind to phosphate in the solution. We therefore performed experiments both in the absence of phosphate to avoid any La3+-phosphate formation and with NaH2PO4 added to mimic the conditions of Thompson et al. (2006), who showed that La3+ blocked pannexin hemichannels under these conditions. Only one cell was studied for each slice to which ischaemia was applied, so the number of cells is equal to the number of slices studied. Typically, 6–16 cells were studied for each ischaemic condition, interleaved with control cells to which no pharmacological agents were applied, taken from three or more animals.
Electrophysiology
Pyramidal cells from area CA1 were whole-cell clamped with thin-walled electrodes of series resistance 4–10 MΩ, before 60–75% compensation. The large amplitude of the anoxic depolarization current (up to 6 nA) implies the occurrence of a significant series resistance voltage error, even after compensation. However, as the series resistance was similar in the various experimental conditions tested, the results are qualitatively comparable and the conclusions derived will be unaffected. Electrode junction potentials were compensated. Cells were held at ∼−30 mV in order to be able to sense ischaemia-evoked currents through pannexin hemichannels and through glutamate-gated channels, including NMDA receptor channels. The clamped cell acts as a sensor of glutamate released from a multitude of neighbouring cells.
The patch pipette solution normally contained 135 mM CsCl, 4 mM NaCl, 0.7 mM CaCl2, 10 mM 4-(2-hydroxyethyl)-1-piperazineeethanesulphonic acid (HEPES), 10 mM 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid (BAPTA), 4 mM Mg-ATP, 0.5 mM Na2-GTP and 10 mM QX-314, at pH 7.2 (adjusted with CsOH). Internal QX-314 and Cs+ block voltage-gated Na+ and K+ currents in the cell being patch-clamped and thus improve voltage uniformity, without affecting the behaviour of the surrounding cells in the slice (as external tetrodotoxin or K+ channel blockers would). No significant difference was observed if QX-314 was not included in the internal solution for the amplitude of the peak anoxic depolarization current (without QX-314: 3950 ± 366 pA, n = 4; with QX-314: 5084 ± 499 pA, n = 7 interleaved cells; P = 0.15; all in P12 animals) or for the latency to the anoxic depolarization (without QX-314: 417 ± 43 s, n = 4; with QX-314: 381 ± 14, n = 7, P = 0.35). In some experiments on slices from P20 animals (Fig. 3), K+ was used instead of Cs+ in the internal solution described above. A comparison of interleaved recordings with K+ and Cs+ inside showed that there was no significant difference in the peak anoxic depolarization current amplitude (with K+: 3941 ± 389 pA, n = 10; with Cs+: 4285 ± 32 pA, n = 3; P = 0.65), the current measured 5 min after the anoxic depolarization (with K+: 2079 ± 384 pA, n = 6; with Cs+: 2013 ± 161 pA, n = 3; P = 0.91) or the anoxic depolarization onset time (with K+: 244 ± 17 s, n = 10; with Cs+: 291 ± 27 s, n = 3; P = 0.2). Lucifer yellow (1 mg/ml) was added to visualize the target neuron after recording. Internal solution lacking ATP and GTP had a corresponding amount of CsCl added to balance the osmolarity.
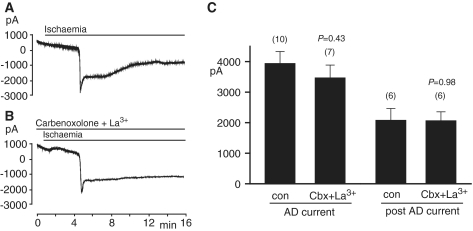
Figure 3.
The effect of blockers of pannexin hemichannels on the response to ischaemia at −33mV in cells in slices from P20 rats (recording with ATP but no QX-314 in the K+-based patch pipette solution). (A) Normal response to ischaemia. (B) As in A, but with the pannexin hemichannel blockers La3+ and carbenoxolone (100 µM each) in the external solution. (C) Quantification of the effect of hemichannel blockers on the maximum inward current and inward current measured 5 min after the anoxic depolarization (AD). Number of cells studied is indicated above each bar. P-values are for comparison without blockers. con = control; Cbx = carbenoxolone.
For experiments blocking pannexins with carbenoxolone, La3+ or mefloquine, the slices were superfused with these agents for ≥30 min to ensure penetration into the slice before switching to the ischaemia solution. In addition, for carbenoxolone, the same concentration of the drug was also added to the patch pipette solution.
Imaging of dye uptake by pyramidal cells
To monitor hemichannel-mediated permeability to large molecules in ischaemia, propidium iodide (5 µg/ml) was included in the external solution and slices were superfused with this for ≥30 min at 33 ± 1°C before switching to ischaemia for 90 min. The solution either contained or lacked the hemichannel blockers carbenoxolone (100 µM) or mefloquine (25 µM) throughout the whole experiment. Imaging of the propidium in the CA1 pyramidal cell layer, where its fluorescence is increased when it enters cells and binds to DNA/RNA, was carried out with a ×20 lens and a charge-coupled device imaging system. Propidium fluorescence was excited at 545 nm and light emitted at 610 nm was collected (using a rhodamine filter block). Images were acquired once every 5 min, and the average propidium fluorescence was calculated across a window centred on the CA1 pyramidal cell layer. For each condition, fluorescent images were taken from 5–8 P12 hippocampal slices.
Data analysis and statistics
Ischaemia-evoked currents were measured from the pre-ischaemic holding current. Data are shown as mean ± SEM. Numbers (n) of cells recorded from, or of slices imaged, are always given so that, if desired, standard deviation can be calculated as SEM × √N. P-values are from two-tailed t-tests.
Results
The normal response to ischaemia
When ischaemia solution was applied without blockers of hemichannels or glutamate receptors, the membrane current of pyramidal cells slowly became more inward (Fig. 1A) and showed a superimposed noise increase produced by an increase in the frequency of spontaneous glutamatergic and GABAergic synaptic currents (Rossi et al., 2000; Allen et al., 2004). After several minutes, a much larger inward current, the anoxic depolarization current, developed rapidly and was followed by a sag back to a slowly decaying plateau current (Fig. 1A).
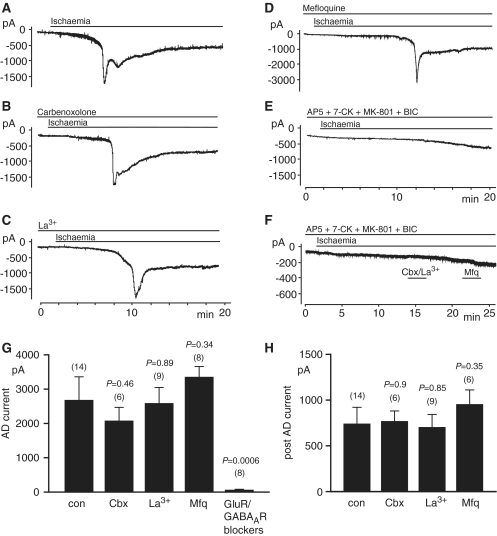
Figure 1.
The effect of blockers of pannexin hemichannels and of glutamate and GABA receptors on the anoxic depolarization current with 4 mM ATP in the pipette solution (slices from P12 rats). (A) Response of a CA1 pyramidal cell at −33mV to simulated ischaemia. After 5–7 min of ischaemia, a large inward current develops. (B) As A, but with the pannexin hemichannel blocker carbenoxolone (100 µM) in the external and pipette solutions. (C) As A, but with the pannexin hemichannel blocker La3+ (100 µM) in the external solution. (D) As A, but with the pannexin hemichannel blocker mefloquine (25 µM) in the external solution. (E) As A, but with NMDA, AMPA, kainate and GABAA receptor blockers [50 µM D-AP5, 50 µM MK-801, 25 µM 2,3-dihydroxy-6-nitro-7-sulphamoyl-benzo[f]quinoxaline-2,3-dione (NBQX), 100 µM 7-chlorokynurenate (7-CK) and 100 µM bicuculline (BIC)] present from the onset of ischaemia. (F) As E, but with application of carbenoxolone (Cbx) + La3+ and of mefloquine (Mfq), to test whether the small inward current occurring in the presence of the transmitter receptor blockers reflects pannexin activation (note different time scale). (G and H) Quantification of the effect of hemichannel and receptor blockers on the current evoked by ischaemia, for experiments as in panels A–E. (G) Maximum inward current, relative to the current level before ischaemia [i.e. the peak of the anoxic depolarization (AD) current, except in the presence of glutamate/GABAA receptor blockers when, because no anoxic depolarization occurred, the maximum inward current reached within 15 min after the start of ischaemia was measured]. (H) Inward current (relative to the current level before ischaemia) measured 5 min after the anoxic depolarization (not shown for the presence of glutamate/GABAA receptor blockers as no anoxic depolarization occurred). Number of cells studied is indicated above each bar. P-values are for comparison without blockers. con = control; GluR = glutamate receptor; GABAAR = GABAA receptor.
Pannexin hemichannel blockers do not alter the response to ischaemia
We then included carbenoxolone (100 µM) in the superfusion and pipette solutions to block hemichannels (Ripps et al., 2004; Bruzzone et al., 2005). The half maximal inhibitory concentration for carbenoxolone blocking pannexin hemichannels is ∼5 µM (D’hondt et al., 2009). Numerous studies (e.g. Rekling et al., 2000; Sharifullina et al., 2005; Elsen et al., 2008) have shown that 100 µM carbenoxolone blocks gap junctions in brain slices. Carbenoxolone did not affect the occurrence of the anoxic depolarization current (Fig. 1B). The amplitude of the anoxic depolarization current and the amplitude of the inward current 5 min after the anoxic depolarization (which was measured in case the activation of pannexin hemichannels took some time to occur) (Thompson et al., 2008), were not significantly affected by the presence of carbenoxolone (Fig. 1G and H), and the latency to the anoxic depolarization was also not significantly affected (without carbenoxolone: 383 ± 30 s, n = 13; with carbenoxolone: 342 ± 22 s, n = 6, P = 0.4).
La3+ (100 µM) is commonly used to block hemichannels (Contreras et al., 2002; Ye et al., 2003), including pannexin hemichannels [Thompson et al. (2006), but note that Pelegrin and Surprenant (2006) reported no effect on pannexin-1 channels]. La3+ also did not affect the amplitude of the anoxic depolarization current (Fig. 1C and G) or of the inward current measured 5 min after the anoxic depolarization (Fig. 1H) and did not significantly affect the latency to anoxic depolarization (without La3+: 383 ± 30 s, n = 13; with La3+: 352 ± 28 s, n = 8, P = 0.49).
Mefloquine (25 µM) is another gap junction blocker that inhibits pannexins at lower concentrations than connexins (half maximal inhibitory concentration ∼100 nM for pannexins versus 5–34 µM for connexins; D’hondt et al., 2009) and has been used at this concentration to block neuronal gap junctions in brain slices (Cruikshank et al., 2004; Gee et al., 2010). Mefloquine also did not affect the amplitude of the anoxic depolarization (Fig. 1D and G) or the inward current measured 5 min after the anoxic depolarization (Fig. 1D and H), but prolonged the latency to the anoxic depolarization (with mefloquine: 544 ± 24 s, n = 8; without mefloquine: 382 ± 13 s, n = 12, P = 0.00013). The effect on the latency may reflect blockade of K+ channels by this agent (Gribble et al., 2000; Traebert et al., 2004), delaying the K+ efflux that initiates the anoxic depolarization (Rossi et al., 2000).
In contrast, as previously reported (Rossi et al., 2000), a cocktail of blockers of glutamate and gamma-aminobutyric acid class A (GABAA) receptors abolished the anoxic depolarization (a GABAA receptor blocker was included as GABA release contributes to the membrane conductance increase in ischaemia; Allen et al., 2004), leaving only a slowly developing inward current that did not achieve the magnitude of the normal rapid anoxic depolarization current, even after >15 min of ischaemia (Fig. 1E and G). This small and slowly developing current was only seen in four out of eight cells tested, and its mean value in all eight cells was 48 ± 24 pA (measured 15 min after the onset of ischaemia). This current was not blocked by a cocktail of the pannexin blockers carbenoxolone (100 µM) and LaCl3 (100 µM), nor by mefloquine (25 µM), as shown in Fig. 1F. Thus, pannexin-1 hemichannels do not contribute to the small and slow inward current, ruling out the possibility that, when glutamate receptors are blocked, there is a significant activation of pannexin-1 hemichannels in the first 25 min of ischaemia.
The anoxic depolarization current is independent of the availability of intracellular adenosine triphosphate in the clamped cell
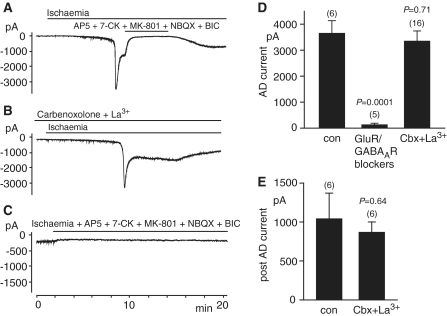
The results described above were performed with 4 mM ATP in the patch pipette solution, to keep the clamped cell in a physiological state optimal for sensing the rise of extracellular glutamate concentration in ischaemia. This might, however, attenuate the inward current produced in ischaemic conditions, since Thompson et al. (2008) have proposed that pannexin hemichannel activation is promoted by ATP depletion. We therefore performed the same set of experiments in the absence of intracellular ATP. However, the anoxic depolarization current amplitude (P = 0.24), the current measured 5 min after the anoxic depolarization (P = 0.31) and the anoxic depolarization onset time (397 ± 37 s, n = 6; P = 0.78), were not different compared to recordings obtained with ATP in the internal solution (Fig. 2A, D and E). Consistent with previous studies (Rossi et al., 2000) the ischaemic steady state current was reversibly almost completely abolished by antagonists of ionotropic glutamate and GABAA receptors (Fig. 2A) and no anoxic depolarization was observed when these drugs were present from the beginning of ischaemia (Fig. 2C and D). As for experiments with ATP in the internal solution, the hemichannel blockers carbenoxolone and La3+, even applied in combination, did not prevent the anoxic depolarization (Fig. 2B, D and E). Furthermore, excluding phosphate (which is known to bind La3+) from the perfusion solution, and thus raising the concentration of free La3+, had no effect on the ischaemia-evoked inward current [the peak inward current was 3354 ± 582 pA (n = 11) in phosphate-containing solution and 2672 ± 427 pA (n = 5) in solution containing La3+ and no phosphate; P = 0.26].
Figure 2.
The effect of blockers of pannexin hemichannels and of glutamate and GABA receptors, when recording at −33 mV without ATP in the patch pipette solution (in slices from P12 rats). (A) Normal response to ischaemia as in Fig. 1A. No difference to the condition with internal ATP was detected. The ischaemic steady state current is reversibly blocked by antagonists of NMDA, AMPA, kainate and GABAA receptors. (B) As A, but with the pannexin hemichannel blockers La3+ and carbenoxolone (100 µM each) in the external solution and with 100 µM carbenoxolone also added to the pipette solution. (C) As in A, but with NMDA, AMPA, kainate and GABAA receptor blockers present from the onset of ischaemia. (D and E) Quantification of the effect on the maximum inward current (D) and inward current measured 5 min after the anoxic depolarization (AD) (E) of hemichannel and receptor blockers, for experiments as in (A–C). Number of cells studied is indicated above each bar. P-values are for comparison without blockers. con = control; Cbx = carbenoxolone; GABAAR = GABAA receptor; GluR = glutamate receptor.
In summary, the magnitude and time course of the anoxic depolarization current do not depend on the availability of intracellular ATP and, whether or not ATP is present, they are not affected by hemichannel blockers.
Pannexin blockers do not affect the anoxic depolarization in older animals
Ion channel expression alters during development. The experiments above were done on slices from P12 animals because pannexin-1 expression is highest around this age (Ray et al., 2005; Vogt et al., 2005) and this ought to facilitate detection of a contribution of pannexins to the ischaemia-evoked current. However the experiments of Thompson et al. (2006) were on cells from P15–20 animals and there could conceivably be a developmental upregulation of the signalling pathways that lead to pannexin activation. We therefore repeated the experiments above on slices from P20 animals.
We found that in P20 animals (Fig. 3A), the peak anoxic depolarization current was not altered compared to the P12 condition (P20: 3941 ± 389 pA, n = 10; P12: 4280 ± 369 pA, n = 7; P = 0.55; both sets of cells were studied with K+-based internal medium, containing ATP and lacking QX-314), while the current 5 min after the anoxic depolarization was significantly larger (P20: 2079 ± 384 pA, n = 6; P12: 983 ± 201 pA, n = 7; P = 0.024) and the anoxic depolarization showed a shorter time to onset than in P12 animals (P20: 244 ± 17 s, n = 10; P12: 374 ± 35 s, n = 7; P = 0.002). The larger current at later times and the shorter time to the anoxic depolarization may correlate with the greater vulnerability of the older brain towards ischaemic damage, and reflect an increased number of neurotransmitter transporters (Danbolt, 2001) that reverse and release glutamate in ischaemia (Rossi et al., 2000) and a greater number of receptors to respond to that glutamate (Monyer et al., 1994).
Importantly however, consistent with our previous results at P12, the hemichannel blockers carbenoxolone and La3+ (applied in combination) did not affect the anoxic depolarization current in the older animals. Compared to recordings without the pannexin blockers, there was no significant change in the peak anoxic depolarization current (Fig. 3B–C; P = 0.43), the current measured 5 min after the anoxic depolarization (Fig. 3B–C; P = 0.98) or the anoxic depolarization onset time (with pannexin blockers: 255 ± 13 s, n = 7; without pannexin blockers: 244 ± 17 s, n = 10; P = 0.63). Thus, even in older animals, pannexin channel activation does not contribute to the anoxic depolarization.
Pannexins do not mediate a significant membrane permeability to large molecules early in ischaemia
To test whether ischaemia evoked an increased permeability to large molecules mediated by pannexin hemichannels, as seen in isolated pyramidal cells (Thompson et al., 2006), we measured the fluorescence of propidium iodide as it entered cells and bound to DNA and RNA during ischaemia (Fig. 4A and B). Before ischaemia, the fluorescence (averaged over the CA1 area) showed a slow rise as the slice loaded with propidium. When ischaemia solution was applied, there was an initial decrease in the rate of rise of fluorescence, which we attribute to increased light scattering produced by the slice swelling in ischaemia. This was then followed by a later acceleration in the rate of fluorescence increase.
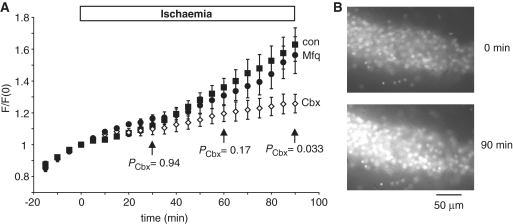
Figure 4.
Effect of hemichannel blockers on ischaemia-evoked permeability of the pyramidal cell membrane to propidium. (A) Propidium fluorescence (F, normalized to the value at start of ischaemia) in area CA1 of hippocampal slices to which ischaemia solution was applied, with and without pannexin blockers [eight slices without blockers (con), five with carbenoxolone (Cbx) 100 µM and six with mefloquine (Mfq) 25 µM]. When used, the blockers were present throughout the whole experiment. PCbx-values compare the carbenoxolone data with the control condition. (B) Specimen images from one slice at the start of ischaemia (t = 0) and after 90 min ischaemia.
Two pannexin blockers, mefloquine (25 µM) and carbenoxolone (100 µM), present in the solution throughout the whole experiment (i.e. also in the control solution before ischaemia solution was applied), had different effects on the ischaemia-evoked fluorescence changes. Mefloquine had no significant effect on the sequence of fluorescence changes (Fig. 4A), while carbenoxolone reduced the rate of rise of fluorescence in ischaemia, resulting in the fluorescence being significantly lower after >1 h of ischaemia. Both of these drugs block pannexin hemichannels but have different side effects, with carbenoxolone also blocking calcium currents and NMDA receptors (Vessey et al., 2004; Chepkova et al., 2008) while mefloquine blocks K+ channels (Gribble et al., 2000; Traebert et al., 2004). The reduction of the rate of rise of fluorescence in ischaemia by carbenoxolone implies that a pathway is activated that is permeable to propidium (and blocked by carbenoxolone), but the fact that mefloquine has no effect on the dye uptake suggests that this pathway is not mediated by pannexins. The suppressive effect of carbenoxolone on the dye uptake may reflect its actions on NMDA receptors or calcium channels (the activity of which is assumed to activate the dye uptake pathway).
Discussion
The anoxic depolarization is an early event triggering neuronal damage in brain ischaemia. It is associated with a massive release of glutamate into the extracellular space and has been attributed to inward current flowing through glutamate receptor channels. Recent work on isolated neurons (Thompson et al., 2006) has suggested however, that the anoxic depolarization could reflect inward current flowing through pannexin hemichannels. If correct, this would focus attention on therapeutic approaches targeting hemichannels and would suggest a series of time-consuming experiments developing agents that specifically block pannexin hemichannels, investigating whether they protect against neuronal death when given after ischaemia (as would be needed for human therapy) and determining whether they are effective in a series of animal and then human tests.
Here we present three lines of data indicating that, for neurons in situ, pannexin hemichannels do not contribute significantly to the generation of anoxic depolarization and do not mediate dye uptake at later times in ischaemia, suggesting that blocking pannexin hemichannels may not be a useful therapeutic approach.
Firstly, with glutamate receptors blocked, the amplitude of the ischaemia-evoked inward current mediated by pannexin hemichannels in isolated pyramidal cells is ∼1.4 nA at −60 mV [from the mean current density in Thompson et al. (2006) using a mean capacitance of 20 pF: B.A. MacVicar personal communication]. For a reversal potential of 0 mV this implies a current of ∼0.7 nA at −30 mV (as we used) and presumably the current would be significantly larger if the cells’ dendritic and axonal arborizations had not been truncated in the cell isolation procedure used by Thompson et al. (2006). This is much larger than the ischaemia-evoked current that we observe in neurons in situ with glutamate receptors blocked (Figs 1E, F, G and 2C, D). This discrepancy between the magnitude of the ischaemia-evoked current produced (with glutamate receptors blocked) in isolated pyramidal cells and in the same cells in hippocampal slices in situ, demonstrates that the degree of pannexin activation occurring in isolated cells (Thompson et al., 2006) does not occur in situ.
Secondly, pannexins have been reported to be activated by NMDA receptor activity (Thompson et al., 2008), and so might be expected to contribute to the ischaemia-evoked current in conditions where glutamate receptors are not blocked. However, in hippocampal slices, in the absence of glutamate receptor blockers, neither the peak anoxic depolarization current nor the current 5 min after the anoxic depolarization were significantly affected by three widely used pannexin hemichannel blockers (Figs 1G, H, and 2D, E), and this was also true when experiments were done in older (P20) animals, with K+ and without QX-314 in the pipette (Fig. 3C). We cannot rule out the possibility that pannexin activation contributes in a minor way to the peak of the anoxic depolarization current because the predicted amplitude of this contribution (∼0.7 nA, see above) is comparable to the variability in the peak current (Figs 1G, 2D and 3C). However, the slow activation of pannexins by NMDA reported by Thompson et al. (2008; Fig. 2C) would suggest that a larger contribution should be expected for the steady state current reached 5 min after the anoxic depolarization, yet this current is also unaffected by pannexin blockers (Figs 1F, 1H, 2E and 3C). Thus, if pannexins do contribute to ischaemia-evoked currents in situ, their contribution is small compared with that of glutamate-gated channels.
The magnitude of the pannexin-mediated current seen in isolated cells by Thompson et al. (2006) raises the question of how the pannexin hemichannel current can fail to contribute significantly to the anoxic depolarization current in cells in situ in brain slices. To explain this, we hypothesize that the process of isolating the pyramidal cells makes their hemichannels more susceptible to being activated by ischaemia. Supporting this is the fact that pannexin-1 hemichannel opening is increased by mechanical stretch (Bao et al., 2004); conceivably the isolated cells, which may have their cytoskeleton disrupted and are not physically restrained by surrounding cells, are more easily distended by the ion movements that cause swelling in ischaemia. In addition, cell isolation may lead to an increased level of intracellular Ca2+, which has been reported (Locovei et al., 2006) to activate pannexin hemichannels [although Thompson et al. (2008) found that Ca2+ did not activate the channels in hippocampal neurons], or may lead to a modification of pannexin’s sensitivity to [Ca2+]i or other activators.
Thirdly, although we could not detect a pannexin contribution to ischaemia-evoked currents, we confirmed the demonstration of Thompson et al. (2008) that ischaemia activates a pathway allowing transmembrane dye movements (Fig. 4). However, these movements were not blocked by the pannexin blocker mefloquine, although they were blocked by carbenoxolone (presumably as a result of non-specific actions of this agent for example on voltage-gated calcium channels) (Vessey et al., 2004; Chepkova et al., 2008). It is worth noting that measurements of the movements of large charged dye molecules across the membrane cannot distinguish movement through a physiologically operating mechanism, like a pannexin channel, from movement through a ruptured membrane as occurs in cell death. Whichever occurs here, the permeability pathway activated by ischaemia might serve as an important route for the loss from neurons of molecules that may initiate downstream damaging processes in ischaemia. However, our data suggest that pannexins are not the molecular substrate for this pathway.
The experiments we report are on brain slices and therefore we cannot rule out the possibility that pannexins may have some effect on the response to stroke in vivo. Our simulated ischaemia conditions with blocking of glycolysis and oxidative phosphorylation, like the experiments of Thompson et al. (2006) that had no glucose or oxygen present, mimic those occurring in the core of an ischaemic region. It is conceivable, for example, that in vivo pannexins might be activated and contribute to cell damage in the milder conditions of the penumbra of an ischaemic region where some oxygen and glucose are received through unblocked blood vessels. Studying animals with pannexins selectively knocked out in neurons would be one approach to investigating this in future.
Funding
ERC, Fondation Leducq and Wellcome Trust.
Acknowledgements
The authors thank David Rossi for comments on the article.
Glossary
Abbreviations
- AMPA
α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
- GABA
gamma-aminobutyric acid
- GABAA
gamma-aminobutyric acid class A
- NMDA
N-methyl-d-aspartate
References
- Allen NJ, Rossi DJ, Attwell D. Sequential release of GABA by exocytosis and reversed uptake leads to neuronal swelling in simulated ischemia of hippocampal slices. J Neurosci. 2004;24:3837–49. doi: 10.1523/JNEUROSCI.5539-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NJ, Káradóttir R, Attwell D. A preferential role for glycolysis in preventing the anoxic depolarization of rat hippocampal area CA1 pyramidal cells. J Neurosci. 2005;25:848–59. doi: 10.1523/JNEUROSCI.4157-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–8. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Galanin and glibenclamide modulate the anoxic release of glutamate in rat CA3 hippocampal neurons. Eur J Neurosci. 1990;2:62–8. doi: 10.1111/j.1460-9568.1990.tb00381.x. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–43. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- Chepkova AN, Sergeeva OA, Haas HL. Carbenoxolone impairs LTP and blocks NMDA receptors in murine hippocampus. Neuropharmacol. 2008;55:139–47. doi: 10.1016/j.neuropharm.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Contreras JE, Saez JC, Bukauskas FF, Bennett MV. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci USA. 2003;100:11388–93. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras JE, Sánchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, et al. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci USA. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Hopperstad M, Younger M, Connors BW, Spray DC, Srinivas M. Potent block of Cx36 and Cx50 gap junction channels by mefloquine. Proc Natl Acad Sci USA. 2004;101:12364–9. doi: 10.1073/pnas.0402044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- D’hondt C, Ponsaerts R, De Smedt H, Bultynck G, Himpens B. Pannexins, distant relatives of the connexin family with specific cellular functions? Bioessays. 2009;31:953–74. doi: 10.1002/bies.200800236. [DOI] [PubMed] [Google Scholar]
- Elsen FP, Shields EJ, Roe MT, Vandam RJ, Kelty JD. Carbenoxolone induced depression of rhythmogenesis in the pre-Bötzinger Complex. BMC Neurosci. 2008;23:46. doi: 10.1186/1471-2202-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even S, Garrigues C, Loubiere P, Lindley ND, Cocaign-Bousquet M. Pyruvate metabolism in Lactococcus lactis is dependent upon glyceraldehyde-3-phosphate dehydrogenase activity. Metab Eng. 1999;1:198–205. doi: 10.1006/mben.1999.0120. [DOI] [PubMed] [Google Scholar]
- Gee CE, Benquet P, Demont-Guignard S, Wendling F, Gerber U. Energy deprivation transiently enhances rhythmic inhibitory events in the CA3 hippocampal network in vitro. Neuroscience. 2010;168:605–12. doi: 10.1016/j.neuroscience.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Gribble FM, Davis TM, Higham CE, Clark A, Ashcroft FM. The antimalarial agent mefloquine inhibits ATP-sensitive K-channels. Br J Pharmacol. 2000;131:756–60. doi: 10.1038/sj.bjp.0703638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Mohr C, Andrade AL, Attwell D. The electrical response of cerebellar Purkinje neurons to simulated ischaemia. Brain. 2005;128:2408–20. doi: 10.1093/brain/awh619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AJ. Effect of anoxia on ion distribution in the brain. Physiol Rev. 1985;65:101–48. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci. 2009;29:7092–7. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John SA, Kondo R, Wang SY, Goldhaber JI, Weiss JN. Connexin-43 hemichannels opened by metabolic inhibition. J Biol Chem. 1999;274:236–40. doi: 10.1074/jbc.274.1.236. [DOI] [PubMed] [Google Scholar]
- Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–44. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Ma W, Hui H, Pelegrin P, Surprenant A. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther. 2009;328:409–18. doi: 10.1124/jpet.108.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madl JE, Burgesser K. Adenosine triphosphate depletion reverses sodium-dependent, neuronal uptake of glutamate in rat hippocampal slices. J Neurosci. 1993;13:4429–44. doi: 10.1523/JNEUROSCI.13-10-04429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer C, Nurnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–40. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–82. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Zoidl G, Weickert S, Wahle P, Dermietzel R. Site-specific and developmental expression of pannexin1 in the mouse nervous system. Eur J Neurosci. 2005;21:3277–90. doi: 10.1111/j.1460-9568.2005.04139.x. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Shao XM, Feldman JL. Electrical coupling and excitatory synaptic transmission between rhythmogenic respiratory neurons in the preBötzinger complex. J Neurosci. 2000;20:RC113. doi: 10.1523/JNEUROSCI.20-23-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripps H, Qian H, Zakevicius J. Properties of connexin26 hemichannels expressed in Xenopus oocytes. Cell Mol Neurobiol. 2004;24:647–65. doi: 10.1023/B:CEMN.0000036403.43484.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roettger V, Lipton P. Mechanism of glutamate release from rat hippocampal slices during in vitro ischemia. Neuroscience. 1996;75:677–85. doi: 10.1016/0306-4522(96)00314-4. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–21. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- Sharifullina E, Ostroumov K, Nistri A. Metabotropic glutamate receptor activity induces a novel oscillatory pattern in neonatal rat hypoglossal motoneurones. J Physiol. 2005;563:139–59. doi: 10.1113/jphysiol.2004.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006;312:924–27. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, Beazely MA, et al. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science. 2008;322:1555–9. doi: 10.1126/science.1165209. [DOI] [PubMed] [Google Scholar]
- Traebert M, Dumotier B, Meister L, Hoffmann P, Dominguez-Estevez M, Suter W. Inhibition of hERG K+ currents by antimalarial drugs in stably transfected HEK293 cells. Eur J Pharmacol. 2004;484:41–8. doi: 10.1016/j.ejphar.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Vessey JP, Lalonde MR, Mizan HA, Welch NC, Kelly ME, Barnes S. Carbenoxolone inhibition of voltage-gated Ca channels and synaptic transmission in the retina. J Neurophysiol. 2004;92:1252–6. doi: 10.1152/jn.00148.2004. [DOI] [PubMed] [Google Scholar]
- Vogt A, Hormuzdi SG, Monyer H. Pannexin1 and pannexin2 expression in the developing and mature rat brain. Brain Res Mol Brain Res. 2005;141:113–20. doi: 10.1016/j.molbrainres.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Wahl F, Obrenovitch TP, Hardy AM, Plotkine M, Boulu R, Symon L. Extracellular glutamate during focal cerebral ischaemia in rats: time course and calcium dependency. J Neurochem. 1994;63:1003–11. doi: 10.1046/j.1471-4159.1994.63031003.x. [DOI] [PubMed] [Google Scholar]
- Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–96. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoidl G, Petrasch-Parwez E, Ray A, Meier C, Bunse S, Habbes HW, et al. Localization of the pannexin1 protein at postsynaptic sites in the cerebral cortex and hippocampus. Neuroscience. 2007;146:9–16. doi: 10.1016/j.neuroscience.2007.01.061. [DOI] [PubMed] [Google Scholar]