Preface
I was honored to deliver the 2nd Stanley Korsmeyer memorial Lecture on May 9th, 2007 in Padova, Italy. Stan will always occupy a very special place in my heart: I admired him greatly not only for his magnificent and original science but also for his integrity and his grace. This review, which summarizes my laboratory's contribution to cell and cancer biology in the last 30 years, is dedicated to Stan's memory, and to Elaine Fuchs, one of my most cherished friends without whose support this work would not have gained the degree of recognition it enjoys today. My thanks also to the Pezcoller Foundation for making that week in May, 2007 one of the most memorable in my scientific life.
I-Introduction
One of the fundamental questions in cell and developmental biology is how do different tissues emerge from a single cell and how do they maintain their organ- and tissue-specificity? All the many billion cells within our bodies, e.g. in our brain and liver, have the same DNA sequence and the same genetic information. The central question is what determines the stability and the homeostasis of tissues. Why do different organs `remember' how to carry on their multitude of specialized functions with relatively few mistakes? If we thought deeply about the number of organs in our bodies and the integration of function that has to occur at any given moment as we continue our journey from birth to old age, this becomes an astonishing feat of evolution. The answer to these questions is not only fundamental to determining how normal organs remain functional and disease-free, but also will shine light on how tissues may become cancerous.
Tumors by definition are made of cells that have `forgotten' to act correctly within an organ: they behave aberrantly, pile up and go elsewhere as they metastasize. The current conventional wisdom is that there are single or multiple mutations within the cancer cells that make them autonomous. As a result these cells will not respond to any normal signals which tells tissue-specific genes to act cooperatively within organs, but why are tumors of different organs still organ-specific i.e. why a brain tumor is different from a liver tumor? Should normal and tumor cells be treated as if they are totally unrelated species with cancer cells not obeying any of the normal regulatory signals? Is the only way to deal with tumors is to kill the cancer cells once they have been formed?
This brief chapter will outline the progress we have made in the last three decades in search of some answers to these fundamental questions. I describe some of the rationale for why we have chosen the paths we took. A number of recent reviews from my laboratory describe some of our findings in more detail as well as the techniques and assays we have developed to study the readers are referred to those for further information (1–5)
II-The Importance of Context and Tissue Microenvironment
Over the past three decades our laboratory has pursued these fundamental questions using two model systems: 1-Rous sarcoma virus (RSV), the first oncogenic virus isolated by Rous (6), and chick embryo fibroblasts (CEF) and 2-the mammary glands of both mouse and humans concentrating on what defines `normal' in vivo and how do we define a cancer cell in culture (7). We have made a number of important observations that has pointed us towards a paradigm shift on how we view tissue-specificity and cancer. We now believe there is much proof for the model of `dynamic reciprocity' (8, see below) between the different cell types within an organ and between the tumor cells and their surrounding stroma, i.e. the context is crucial in regulation of tissue-specificity and in how cells become malignant (7, 9,10).
This concept is dramatically demonstrated in our papers on RSV in the 1980s: When CEF's are infected in culture, they form a transformed monolayer that loses contact inhibition, and when RSV is injected into chickens it produces massive local tumors that eventually kill the bird. However, when this same virus preparation is injected into the chick embryos stage 26, the embryos remain viable and do not form tumors until much later despite the fact that the virus integrates and expresses pp60src (11). Indeed if we attached pp60src to a reporter gene such as LacZ, the blue cells within the feathers of the chick could each have been a tumor if the context was not important, but they were not. (Fig.1: 12, 13).
Fig.1.
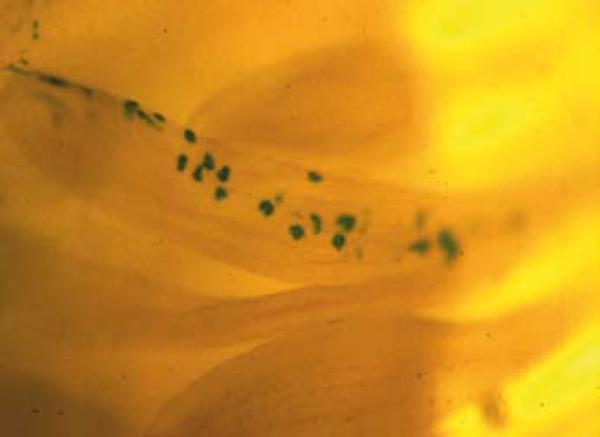
Contribution of v-Src-infected cells to normal tissue structures during chick embryo development. Chick limb buds were infected at day 4 in ovo (embryonic stage 24) with a virus encoding v-Src and a genetic marker, beta-galactosidase. The contribution of v-Src-infected cells to normal tissues (in this case a day 14 feather filament) is revealed by X-gal staining of embryo whole mounts (unpublished picture: A. Stoker and M.J.Bissell, unpublished photomicrograph: see (12). Reproduced also in (13).
On the other hand, if the embryos were dissociated and the cells plated on tissue culture plastic, there was mass transformation (11,14,15). To answer the question of why the virus can form tumors in hatched chickens and not in the embryos, is that even in the chickens, a single oncogene is not sufficient to form tumors because the injection-induced `wounding' may itself be a co-carcinogen. Injecting the virus in one wing and then wounding the wing on the other side was sufficient to cause tumors at the site of the wound (16). Interestingly, we were able to show that the molecule responsible for the wounding effect was TGFβ which at the time was thought to work essentially by preventing the growth of epithelial cells (17).
I had been struck already by the fact that we did not have a clear definition of what is `normal' and what is `malignant' in a plastic culture dish. In a review mentioned above I outlined the data from the vast literature of cell culture that had accumulated over the years, and our own work:
“If there is one generalization that can be made from all the issue and cell culture studies with regard to the differentiated state, it is this; since most, if not all, functions are changed in culture, quantitatively and/ or qualitatively, there is little or no “constitutive” regulation in higher organisms; i.e., the differentiated state of normal cells is unstable and the environment regulates gene expression”(7)
The above summary simply states what we now have renamed `plasticity': `The differentiated state is plastic, and the (micro) environment regulates gene expression'. This was shown to be true also for malignant cells as discussed in our experiments with RSV, and as elegantly shown in a land mark paper by the pioneering work of Beatrice Mintz and her colleagues (18). They showed that the malignant potential of teratocarcinoma cells could be constrained during embryogenesis in mice by placing these cells in the blastocyst of a surrogate mother. Astonishingly, whereas the cells injected in the mouse flank formed tumors, those placed in blastocysts and put inside a surrogate mother, were tumor-free. Tissues derived from the teratocarcinoma cells which could be identified because they were derived from mice with black hair, were normal despite the fact that the mice were the offspring of a normal mouse and malignant cells! The assumption at the time was that if the teratomcarcinoma cells did not form tumors, they could not (should not) contain oncogenic mutations. This finding in the height of the discovery of oncogenes and tumor suppressor mutations did not seem possible. Thus despite its landmark nature, the work is not widely known and is not included in the current textbooks (see also George Klein, 19). Our work with RSV and our later findings in the mammary gland indicate that abundant mutations could be present and oncogene activity could be on, yet the cells so infected could still be nonmalignant because of the constraints exerted by the microenvironment.
These findings led to the following broad and ambitious research goals in my laboratory:
How is tissue-specificity maintained?
How could one study the problem in mammals?
How are these processes go awry in cancer and aging? And,
How can one use this information for therapy?
III-Mammary Gland as a Model to Study Tissue-Specificity
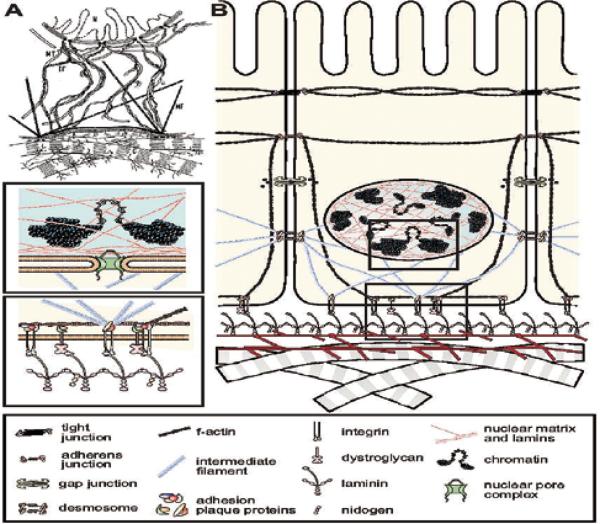
To address the first 3 questions, we concentrated on mammary gland as an experimental system. This organ, unlike many others, changes during the adult life of the organism and the gland undergoes cyclical changes both with estrus and pregnancy cycles. Previously, Emerman and Pitelka (20) had shown that whereas normal mammary cells lost both milk protein expression and morphological traits on tissue culture plastic, the same cells could reorganize and retain some milk proteins in collagen1 gels that had been allowed to float, as Michalopoulos and Pitot had shown previously for hepatocytes (21). Why the floating but not the attached gels would do this or whether the retention of some of the milk protein indicated less milk degradation or endogenous synthesis of milk proteins was not clear. Using radioactive tracers, we subsequently showed that indeed the cells were induced to synthesize new milk protein when they were allowed to float (22, 23). Since mammary cells in vivo, do not sit on top of collagen 1 and do not float, we argued that perhaps it is the basement membrane (BM) surrounding the epithelial cells that allows them to function. In a theoretical paper, written with two of my postdoctoral fellows, we argued that the unit of function in higher organisms was the cell plus its extracellular matrix, that the latter molecules were not simply scaffolds, but had information and could signal through ECM receptors, via the cytoskeleton which in turn were connected to the nuclear matrix and chromatin. We referred to this model of ECM/chromatin connection as the model of dynamic reciprocity (8) (Fig.2)
Fig.2.
(A) Two versions of the model of dynamic reciprocity. The original model of dynamic reciprocity, or “the minimum required unit for tissue-specific functions”. N=nucleus; MT=microtubules; IF=intermediate filaments; MF=microfilaments; C=collagen. Reproduced with permission from (8) (B) An updated view of dynamic reciprocity. Reproduced with permission from (3).
We then expanded the concept of the unit of function to the organ itself (9). Subsequently we developed a 3D ECM assay using a reconstituted BM gel prepared from a BM-producing tumor in mice (24-later referred to as Matrigel) and showed that both primary mammary cells as well as mammary cell lines from mice could reorganize and form hollow acini which could produce copious amount of milk proteins (Fig 3 and 4; 25; for review see 26)
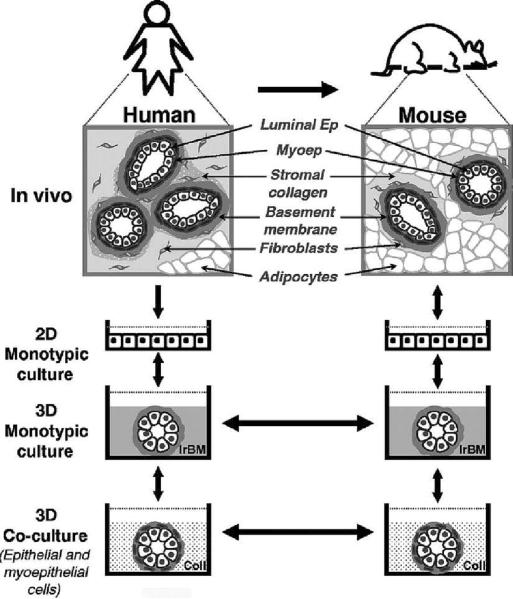
Fig.3.
Hierarchical modeling of human breast function. Similarities between the organization of human and mouse mammary glands have enabled our observations in one tissue to be transferred to the other(keeping in mind that they are not identical in all aspects). This dynamic exchange of information has led to the gradual development of mammary gland models that now represent a continuum of organotypic systems ranging in complexity from monotypic 3D cultures to multicellular co-cultures to in vivo xenograft models. Each of the 3D models depicted here represents a physiologically relevant assay in its own right. However, when engineered with common cellular components and used in series, these models become invaluable tools for the identification and verification of disease-related molecules as well as for the design and translation of effective drug therapies. Future models that are more faithful to the human mammary microenvironment may be achieved by adding other cell types that interact within the mammary gland: fibroblasts, endothelial and fat cells as well as immune cells such as mast cells. Ep, epithelial cell; Myoep, myoepithelial cell. Adapted from previous publications and produced with permission from (53–55).
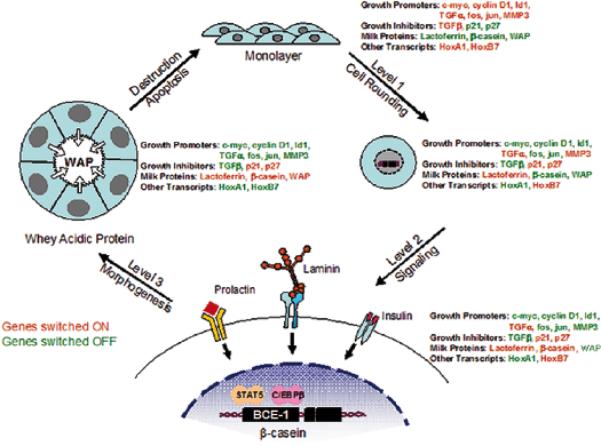
Fig. 4.
An illustration of the different levels through which ECM controls gene expression and tissue function. As cells transition from a 2D monolayer to a 3D environment, they undergo changes in cell shape that influence the expression of certain genes. Exposure to ECM engages specific cell surface receptors and initiates the transduction of biochemical and mechanical signals through the cell to the nucleus, where they further influence gene expression. As the duration of exposure time to ECM increases, cells undergo morphogenic events involving the formation of acinar structures, and once again exhibit changes in their gene expression profile. Thus, tissue structure influences gene expression and, therefore, dictates tissue function. (Modified, with permission, from 56,10,2).
We showed not only that it is indeed the laminin1 in the BM that signals to the milk proteins (27), but that the floatation of collagen1 gel allows the cells to polarize and form an endogenous laminin-containing BM (28) which in turn signals for changes in chromatin organization and transcription of milk protein genes (see below). We also showed a hierarchy in production of milk protein genes: different levels of architectural organizations allow increasingly higher levels of differentiations; this is shown schematically in (Fig.4.)
IV-Discovery of ECM-response element and chromatin reorganization
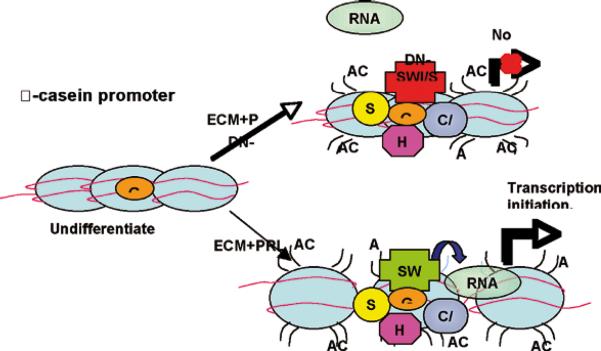
Experimental proof for the above model, i.e. the existence of an ECM/chromatin signaling axis was provided when we discovered an ECM-response element in the promoter of the bovine β-casein gene which we refer to as bovine casein element-1 (BCE-1; 29, 30). More recent work has indicated that chromatin reorganization (acetylation, methylation, etc) is required for allowing the signaling to bring the appropriate transcription factors and chromatin remodeling enzymes to the site of ECM response element to allow transcription of the gene (31, 32) (Fig.5)
Fig.5.
Model displaying how exposure of mammary epithelial cells to ECM and prolactin may induce the recruitment of transcription factors and chromatin remodeling enzymes to the -casein promoter, and how aberrations in SWI/SNF function interfere with RNA polymerase II recruitment.(Reproduced with permission from 32).
V-Loss of ECM integrity leads to disruption of the differentiated state, apoptosis and formation of mammary tumors
To demonstrate the importance of integrity of BM/ECM in vivo, in a long and productive collaboration with Zena Werb at UCSF we showed that loss of milk proteins after involution of the mammary gland required loss of BM by proteolytic degradation (33) and that inhibition of BM degradation by MMPs(MMP3) prevented involution (34). Alternatively, destruction of BM inappropriately using engineered mice by over-expressing MMP3 during pregnancy- when there is little or no expression of MMPs in normal gland- led to loss of milk protein expression and extensive apoptosis (35) and as animals aged, to mammary tumors with extensive chromosomal abnormalities (36,)
We showed further that attachment to BM was necessary for the homeostasis: if MMPs were conditionally expressed after mammary acini were formed in 3D, the cells cleaved caspases and underwent apoptosis (37). These dramatic results confirmed that BM integrity is crucial for maintenance of organ-specificity, and that continuous loss of tissue organization- even without introduction of oncogenes and loss of tumor suppressor genes-is sufficient to lead to malignancy and/or cell death.
VI-MMPs, Epithelial to Mesenchymal Transition/Transformation (EMT), Genomic Instability and Malignancy
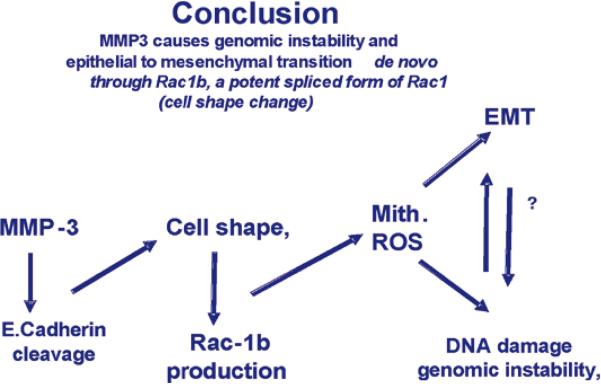
To understand the molecular mechanisms of why and how the mice developed mammary tumors, we designed a cell culture model where transfected MMP3 could be turned on and off a tetrocyclin system. We discovered that E. Cadherin is a substrate for MMP3 and that cells underwent an epithelial to mesenchymal transition (38, and Fig.5b), a process that occurs in embryogenesis (39), but also has been demonstrated to occur as tumor cells become more aggressive during invasion and metastasis (40). Astonishingly, we showed also that destruction of BM in mouse mammary cell lines that were mutated for p53, led to rapid formation of reactive oxygen species and genomic instability (Fig 7) set into motion via changes in the cytoskeleton and differential splicing of RAC to form RAC1b. (41; Scheme 1).
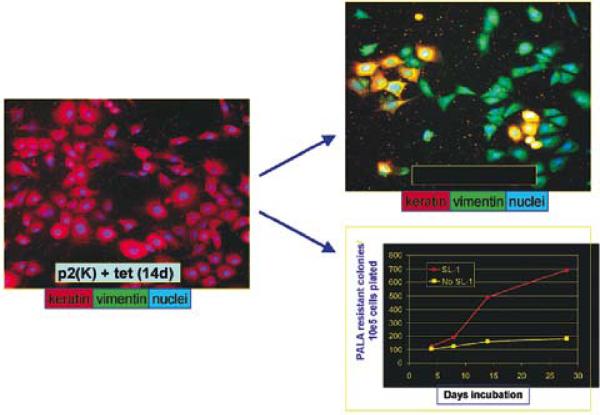
Fig. 7.
MMP3 causes EMT and genomic instability in ScP2 cells: Mouse mammary epithelial cells (mutant for p53) were transfected with a tet repressible MMP3 (the same mutated MMP3 used in Fig.6; 35). Removal of tet led to expression of MMP3, changes in the cytoskeleton, EMT and genomic instability measured by amplification of the CAD locus. Adapted from (39, 41).
Scheme 1.
Flow chart of the steps postulated to give rise to genomic instability and EMT as a result of MMP3 activation, Adapted from previous publication (41).
The latter is a spliced form of RAC that has been reported recently to be expressed in many tumors (see 41, and the references within). These data illustrate the fundamental integration of form and function in maintenance of tissue-specificity and provide additional support for how the concept of `dynamic reciprocity' (Fig 2) operates at multiple levels.
VII-Phenotype is Dominant Over Genotype in 3D: Reversion of the Malignant Phenotype
The concepts and data presented above should be applicable not only to animal models but also to human cells in 3D cultures (and presumably to human tissues in vivo. Together with the laboratory of Ole Petersen in Copenhagen, we adapted the laminin-rich 3D gel assay we had already developed for mouse cells (25) also for human cells (42, Fig.8) to distinguish non malignant and malignant cells -both primary cells and cell lines-from each other rapidly and reproducibly. This assay and some of its reiterations are being used by increasing number of investigators and have been instrumental in establishing that signaling in 2D and 3D are fundamentally different [for review see (1)]. Using a number of signaling inhibitors, we have shown that malignant cells with a frankly malignant genome can be reverted to a phenotype that resembles non malignant cells (43). We were surprised to see that we can revert even metastatic cells such as MDA-MB-231, and that reversion using a given pathway inhibitor in 3D could correct the level of signaling in a number of other crucial pathways (for reviews see 1 and 4). We thus hypothesize that there are a number of central `nodes' that connect and integrate signaling in 3D. It is noteworthy that these connections do not seem to operate in 2D (44; 1)
Fig. 8.
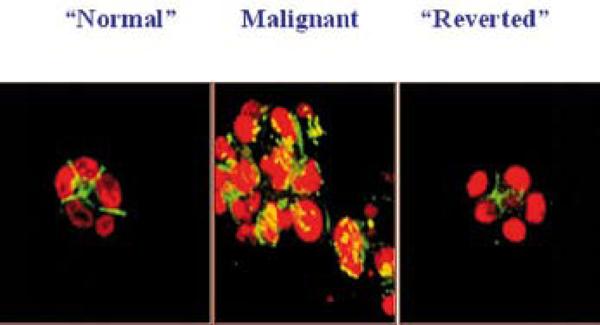
1-inhibitory antibody treatment of tumor cells leads to the formation of reverted acini. Confocal fluorescence microscopy images of F actin: Both the S-1 (left) and T4-2 - reverted acini (right) showed basally localized nuclei (propidium iodide) and organized filamentous F-actin (FITC), while T4-2 mock-treated colonies (T4-2 IgG, middle) had disorganized, hatched bundles of actin and pleiomorphic nuclei. Adapted from previous publication (Reproduced with permission from 43).
VIII-The Implications of 3D Assays and Tissue Polarity for Breast Cancer Therapy
It should be clear from above that these concepts and findings would have implication for response to chemotherapy as well as useful for therapeutic discovery, i.e. the 3D models would be more accurate predictors of how normal and malignant cells would respond to therapy in vivo. Accordingly our more recent data have shown both of these statement to be true. In a complex series of experiments, we showed that response of normal and malignant human breast epithelial cells to six different apoptotic agents used in the clinic were profoundly influenced by whether or not they were in 3D cultures and as such whether or not they formed a polar structure in 3D (46; Fig.9).
Fig. 9.
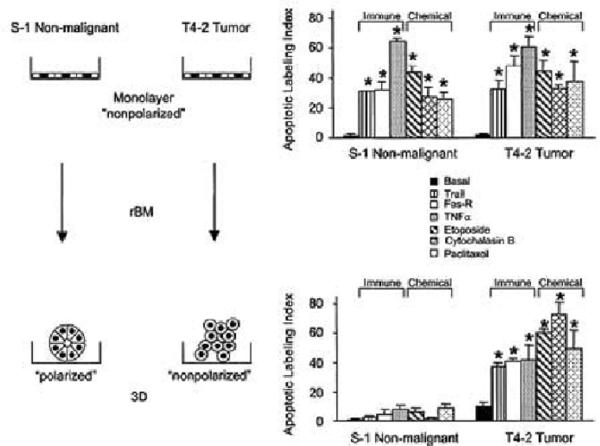
Schematic presentation of data obtained on the importance of 3D structure and tissue polarity in response of non malignant and malignant breast cancer cell lines to chemotherapy Only nonmalignant cells within an organized and polarmammary acini are resistant to apoptosis. But when T4-2 cells are `reverted' to an organized structure in 3D (see section VII), they too become resistant, These findings have profound implication for dormancy in breast cancer. Apoptotic labeling indices calculated for S-1 and T4-2 cells treated with Trai. Modified from (46).
Furthermore, the agents discovered to revert in 3D culture were usually effective in reducing tumor burden invivo (47, and references therein).Using the `wisdom of the acinus' (the polar structures formed in 3D), we have discovered much useful information for both markers for breast cancer and possible therapeutic targets as well as means of testing new and combination drugs on human tumor cells in 3D (48, 49). (Fig.10)
Fig. 10.
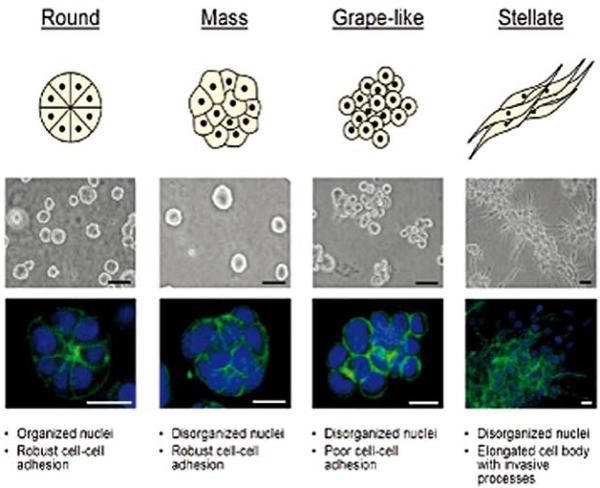
Breast cell line colony morphologies in 3D culture fall into four distinct groups. A panel of twenty-five breast cell lines were cultured in three-dimensions and grouped into four distinct morphologies. A schematic and key descriptors of each morphology is shown on top in addition to phase contrast (middle) and F-actin and nuclear fluorescence images(bottom) of representative cell lines of each morphology. Scale bars: phase contrast, 50 μm; fluorescence, 20 μm. Reproduced with permission from (49)
In our most recent studies, we utilized 25 breast cancer cell lines used previously by RNA arrays on cells grown in 2D and classified into major breast cancer subgroups (62). We showed that when these cells are grown on top of gels in 3D lrECM, for 4 days and then arrayed, they could be sub-grouped into 4 morphological categories allowing us to do a finer classification of the type of breast cancer they were derived from (Fig.10).
IX-Architecture determines the site of Branching and invasion in the Mammary Gland
Having put the concept of three-dimensionality to good use for tumor therapy as indicated from the brief summary above, it has been gratifying to see the acceptance and popularization of some of these ideas and findings in the broader scientific community (e.g. see commentaries in (50).
More recently therefore we have turned our attention to developing novel assays for understanding how normal tissues such as the mammary gland invade into the stromal fat pad. This is because we (as well as many others before us, believe that tumors usurp normal pathways and use them to their own ends (for review see 61).
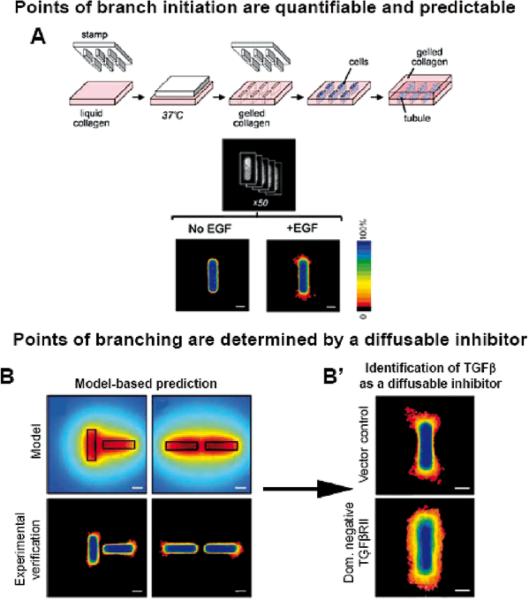
Using micropatterns and genetic engineering as well as some older results from our published scientific repertoire (62), we now have developed a technique for studying branching morphogenesis (51). We show that the architecture of the gel into which we seed the cells determines the pattern of branching and further that this pattern is dependent on a gradient of inhibitory morphogene(s) which includes TGFβ (Fig.11).
Fig. 11.
(A) Schematic of 3D microfabrication method to engineer tubules. The position of cells was quantified by stacking images of nuclei from 50 tubules to generate a frequency map before induction of branching. F frequency map of tubules 24 hours after adding EGF to induce branching. B. Position of branching can be predicted by calculated concentration profile. Calculated profiles of diffusible inhibitors in tubules oriented perpendicular and parallel to each other. Frequency maps 24 hours after induction of branching confirm that branching is inhibited in regions predicted to be surrounded by a high concentration of inhibitors in perpendicular and parallel tubules. Scale bars, 50 mm. B' Positional control of branching is disrupted by blocking signaling of endogenous TGF 1. Shown are frequency maps 24 hours after induction of branching in tubules of vector control cells and (E) cells overexpressing dominant negative TGFb receptor type II (HA-DNTbRII). Scale bars, 50 mm. Reproduced with permission from (51).
X- The Shape of Things to Come
I firmly believe that we are in a position now to more fully understand how tissue-specificity is maintained, and why cancer most probably is both a problem in the genes as well as the microenvironment. I also believe that whereas we know so much about the alphabet and the language of the genes, only now we can begin to learn something about the language of the `form'. To do so more fully we need to produce physiologically accurate models of other organs and tissues as well as a more complete 3 D models of the mammary gland. As such and in collaboration with a number of colleagues we have embarked on a new journey to produce as complete a 3D models of the mammary gland and breast cancer as is possible using epithelial, myoepithelial, stromal, endothelial and immune cells together in a variety of scaffolds and combinations. The mammary stem cells, as well as `breast cancer stem cells'- if they exist- will have to play important roles in this endeavor. However at this point, we have only a few results to report (63–65) and much to do. But these will be the shape of things to come (Fig.12; 52).
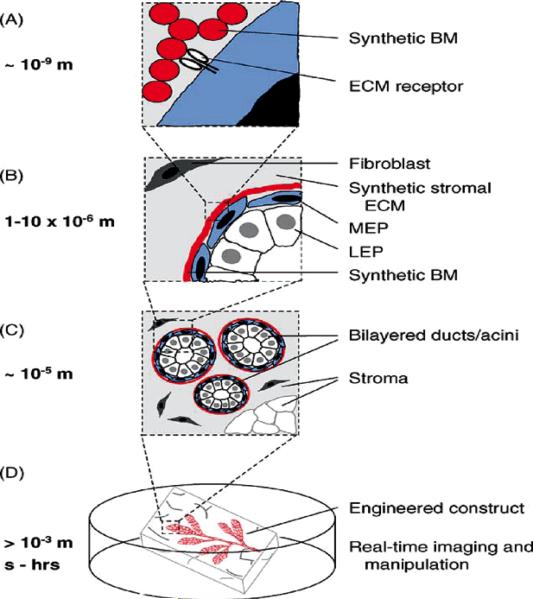
Fig. 12.
The tissue-engineered breast. New strategies should enable the control of the microenvironment at the nano-, micro-, and macro-scales with temporal precision. (A) Synthetic and recombinant ECM polymers impart cues sensed directly by cell-surface receptors. (B–C) Microfabricated constructs control the positions of multiple cell types with respect to each other and the ECM with micrometer precision across large areas of tissue. (D) Engineered breast tissues that can be visualized and manipulated in real time. Adapted from previous publication (Reproduced with permission from 52).
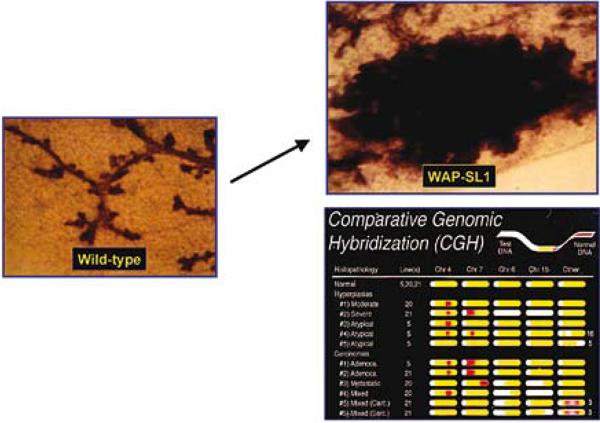
Fig.6.
MMP 3 overexpression leads to formation of mammary tumors as mice age: A mutated form of MMP3 (SL-1) was attached to the promoter of the whey acidic protein gene to engineer the mouse. This milk protein begins to be expressed essentially on midpregnancy and this constructs effectively delivers a large amount of MMP3 essentially to the mammary gland of pregnant mice. Animals develop mammary tumors as they age and the tumors are shown to have substantial genomic defects measured by Comparative Genomic Hybridization (CGH). Adopted from (36).
Acknowledgements
I thank Mrs. Negest Williams for indispensable help in preparation of this manuscript, and my many students and postdoctoral fellows over the years who have contributed so well and so much to the success of our program.
The studies from my laboratory were supported by grants from the OBER (Office of Biological and Environmental Research) of the U.S. DOE (Department of Energy), the U.S. NCI (National Cancer Institute) and the U.S. DOD (Department of Defense) BCRP (Breast Cancer Research Program) as well as an Innovator Award from the U.S. DOD BCRP. I am a recipient of a Distinguished Fellowship in Biological Sciences from the DOE, (OBER). A number of my studnets and postdoctoral fellows have been supported by fellowships from American Cancer Society, US DOD, Komen Foundation and the Breast Cancer Research Program of the University of California (UCBCRP), as well as by European Organizations.
Abbreviations
- (BCE-1)
Bovine casein element-1
- (BM)
Basement membrane
- (CEF)
Chick embryo fibroblasts
- (DNA)
Deoxyribonucleic acid
- (ECM)
Extracellular matrix
- (EMT)
Epithelial-mesenchymal transition/transformation/conversion
- (LacZ)
E. coli gene encoding beta-galactosidase
- (MMP3)
Stromelysin 1
- (MMPs)
Matrix metalloproteinases
- (pp60src)
The phosphoprotein (60 kD) encoded by the src oncogene
- (RAC)
A small GTPase in the ras superfamily
- (RAC1b)
A tumor-specific splice variant of the Rac1 GTPase
- (RSV)
Rous sarcoma virus
- (v-Src)
Viral gene of RSV
- (TGFβ)
Transforming growth factor β
References
- 1.Bissell MJ, Kenny PA, Radisky D. Microenvironmental regulators of tissue structure and function also regulate tumor induction and progression: the role of extracellular matrix and its degrading enzymes. Cold Spring Harbor Symposia on Quantitative Biology, Symposium. 2005;70:343–356. doi: 10.1101/sqb.2005.70.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spencer VA, Xu R, Bissell MJ. Extracellular Matrix, Nuclear and Chromatin Structure and Gene Expression in Normal Tissues and Malignant Tumors: A Work in Progress. Advances in Cancer Research. 2007;97:275–94. doi: 10.1016/S0065-230X(06)97012-2. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: Tissue architecture regulates development, homeostasis, and cancer. Annual Review of Cell and Developmental Biology – Review Article. 2006 Sep 27;22 doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bissell MJ, Rizki A, Mian IS. Tissue architecture: the ultimate regulator of breast epithelial function. Curr Opin Cell Biol. 2003;(6):753–62. doi: 10.1016/j.ceb.2003.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alcaraz J, Nelson CM, Bissell MJ. Biomechanical approaches for studying integration of tissue structure and function in mammary epithelia. J Mammary Gland Biol Neoplasia. 2004 Oct;9(4):361–74. doi: 10.1007/s10911-004-1406-8. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rous P. A sarcoma of the fowl transmissible by an agent separable from the tumor cells. J. Exptl. Med. 1911;13:397–411. doi: 10.1084/jem.13.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bissell MJ. The differentiated state of normal and malignant cells or how to define a “normal” cell in culture. Int Rev Cytol. 1981;70:27–100. doi: 10.1016/s0074-7696(08)61130-4. [DOI] [PubMed] [Google Scholar]
- 8.Bissell MJ, Hall HG, Parry G. How does extracellular matrix direct gene expression? J Theor Biol. 1982;99(1):31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 9.Hall HG, Bissell MJ. Characterization of the intermediate filament proteins of murine mammary gland epithelial cells. Response to collagen substratum. Exp Cell Res. 1986;162(2):379–389. doi: 10.1016/0014-4827(86)90343-5. [DOI] [PubMed] [Google Scholar]
- 10.Roskelley CD, Bissell MJ. Dynamic reciprocity revisited: a continuous, bidirectional flow of information between cells and the extracellular matrix regulates mammary epithelial cell function. Biochem. Cell Biology. 1995;73(7–8):391–397. doi: 10.1139/o95-046. [DOI] [PubMed] [Google Scholar]
- 11.Dolberg DS, Bissell MJ. Inability of Rous sarcoma virus to cause sarcomas in the avian embryo. Nature. 1984;309(5968):552–556. doi: 10.1038/309552a0. [DOI] [PubMed] [Google Scholar]
- 12.Stoker AW, Hatier C, Bissell MJ. The embryonic environment strongly attenuates v-src oncogenesis in mesenchymal and epithelial tissues, but not in endothelia. J Cell Biol. 1990;111(1):217–228. doi: 10.1083/jcb.111.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenny PA, Bissell MJ. Tumor reversion: Correction of malignant behavior by microenvironmental cues. Int J Cancer Review. 2003;107(5):588–695. doi: 10.1002/ijc.11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boudreau N, Reddy ST, Stoker AW, Fairman C, Bissell MJ. The embryonic environment and the extracellular matrix suppress oncogenic transformation by Rous sarcoma virus in the chick embryo. Mol Cell Differ. 1995;3:261–274. [Google Scholar]
- 15.Carter VC, Howlett AR, Martin GS, Bissell MJ. The tyrosine phosphorylation substrate p36 is developmentally regulated in embryonic avian limb and is induced in cell culture. J Cell Biol. 1986;103(5):2017–2024. doi: 10.1083/jcb.103.5.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolberg DS, Hollingsworth R, Hertle M, Bissell MJ. Wounding and its role in RSV-mediated tumor formation. Science. 1985;230(4726):676–678. doi: 10.1126/science.2996144. [DOI] [PubMed] [Google Scholar]
- 17.Sieweke MH, Thompson NL, Sporn MB, Bissell MJ. Mediation of wound-related Rous sarcoma virus tumorigenesis by TGF-ß. Science. 1990;248(4963):1656–1660. doi: 10.1126/science.2163544. [DOI] [PubMed] [Google Scholar]
- 18.Mintz B, Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci USA. 1975;72:3585–9. doi: 10.1073/pnas.72.9.3585. 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klien George. Book Review. BIOMEDICINE: A Cellular and Molecular Foundation for Understanding Cancer. Science. 2007 August 11;313:762–763. 2006. [Google Scholar]
- 20.Emerman JT, Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977;13:316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- 21.Michalopoulas G, Pitot HC. Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations. Cell Res. 1975;94:70–78. doi: 10.1016/0014-4827(75)90532-7. [DOI] [PubMed] [Google Scholar]
- 22.Lee EY, Parry G, Bissell MJ. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol. 1984;98(1):146–155. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee EYH, Lee WH, Kaetzel CS, Parry G, Bissell MJ. Interaction of mouse mammary epithelial cells with collagen substrata: regulation of casein gene expression and secretion. Proc Natl Acad Sci USA. 1985;82(5):1419–1423. doi: 10.1073/pnas.82.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, Martin GR. Basement membrane complexes with biological activity. Biochemistry. 1986 Jan 28;25(2):312–8. doi: 10.1021/bi00350a005. 1986. [DOI] [PubMed] [Google Scholar]
- 25.Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989 Feb;105(2):223–35. doi: 10.1242/dev.105.2.223. 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roskelley CD, Srebrow A, Bissell MJ. A hierarchy of ECM-mediated signalling regulates tissue-specific gene expression. Roskelley CD, Current Opinion in Cell Biol. 1995;7(5):736–747. doi: 10.1016/0955-0674(95)80117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Streuli CH, Bissell MJ. Mammary epithelial cells, extracellular matrix, and gene expression. In: Lippman M, Dickson R, editors. Regulatory Mechanisms in Breast Cancer. Kluwer Academic Publishers; Norwell, MA: 1991. pp. 365–381. [DOI] [PubMed] [Google Scholar]
- 28.Streuli CH, Bissell MJ. Expression of extracellular matrix components is regulated by substratum. J Cell Biol. 1990;110(4):1405–1415. doi: 10.1083/jcb.110.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidhauser C, Bissell MJ, Myers CA, Casperson GF. Extracellular matrix and hormones transcriptionally regulate bovine ß-casein 5' sequences in stably transfected mouse mammary cells. Proc Natl Acad Sci USA. 1990;87:9118–9122. doi: 10.1073/pnas.87.23.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidhauser C, Casperon GF, Myers CA, Sanzo KT, Bolten S, Bissell MJ. A novel transcriptional enhancer is involved in the prolactin- and extracellular matrix-dependent regulation of ß-casein gene expression. Mol Cell Biol. 1992;3(6):699–709. doi: 10.1091/mbc.3.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myers CA, Schmidhauser C, Mellentin-Michelotti J, Fragoso G, Roskelley CD, Casperson G, Mossi R, Pujuguet P, Hager G, Bissell MJ. Characterization of BCE-1, a transcriptional enhancer regulated by prolactin and extracellular matrix and modulated by the state of histone acetylation. Mol Cell Biol. 1998;18(4):2184–2195. doi: 10.1128/mcb.18.4.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu R, Spencer VA, Bissell MJ. Extracellular Matrix-Regulated Gene Expression Requires Cooperation of SWI SWI/SNF and Transcription Factors. J Biol Chem. 2007 Mar 26; doi: 10.1074/jbc.M610316200. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talhouk RS, Chin JS, Unemori EN, Werb Z, Bissell MJ. Proteinases of the mammary gland: developmental regulation in vivo and vectorial secretion in culture. Development. 1991;112(2):439–449. doi: 10.1242/dev.112.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talhouk RS, Bissell MJ, Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J Cell Biol. 1992;118(5):1271–1282. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, Werb Z. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J Cell Biol. 1994;125(3):681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98(2):137–46. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267(5199):891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol. 1997;139(7):1861–1872. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anatomica. 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 40.Gilles C, Thompson EW. The epithelial to mesenchymal transition and metastatic progression in carcinoma. The Breast Journal. 1996;2:83–96. [Google Scholar]
- 41.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z, Bissell MJ. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436(7047):123–7. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petersen OW, Rønnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA. 1992;89(19):9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137(1):231–246. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, Lupu R, Bissell MJ. Reciprocal interactions between ß1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci USA. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weaver VM, Lelièvre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, Werb Z, Bissell MJ. β4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2(3):205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park CC, Zhang H, Pallavicini M, Gray JW, Baehner F, Park CJ, Bissell MJ. 1 Integrin Inhibitory Antibody Induces Apoptosis of Breast Cancer Cells, Inhibits Growth, and Distinguishes Malignant from Normal Phenotype in Three Dimensional Cultures and In vivo. Cancer Research. 2006 Feb 1;66(3):1526–35. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fournier MV, Martin KJ, Kenny PA, Xhaja K, Bosch I, Yaswen P, Bissell MJ. Gene expression signature in organized and growth-arrested mammary acini predicts good outcome in breast cancer. Cancer Research. 2006 Jul 15;66(14):7095–102. doi: 10.1158/0008-5472.CAN-06-0515. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kenny PA, Bissell MJ. Targeting TACE-dependent EGFR ligand shedding in breast cancer. Journal Clinical Investigation. 2007;117(2):337–345. doi: 10.1172/JCI29518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacks T, Weinberg RA. Taking the study of cancer cell survival to a new dimension. Cell. 2002 Dec 27;111(7):923–5. doi: 10.1016/s0092-8674(02)01229-1. 2002. Review. [DOI] [PubMed] [Google Scholar]
- 51.Nelson CM, van Duijn M, Inman JL, Fletcher DA, Bissell MJ. Tissue Geometry Determines Sites of Branching Morphogenesis in Organotypic Cultures. Science. 2006 Oct 13;314(5797):298–300. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nelson CM, Bissell MJ. Modeling dynamic reciprocity: Engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin Cancer Biol. 2005;15(5):342–52. doi: 10.1016/j.semcancer.2005.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rønnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Revs. 1996;76(1):69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- 54.Schmeichel KL, Weaver VM, Bissell MJ. Structural cues from the tissue microenvironment are essential determinants of the human mammary epithelial cell phenotype. J Mammary Gland Biol Neo. 1998;3(2):201–213. doi: 10.1023/a:1018751124382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmeichel KL, Bissell MJ. Modeling tissue-specific signaling and organ function in three dimensions. J Cell Sci. 2003;116:2377–2388. doi: 10.1242/jcs.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bissell MJ, Nelson JW, editors. Curr Opin Cell Biol. Vol. 11. 1999. Cell-to-cell contact and extracellular matrix integration of form and function: The central role of adhesion molecules; pp. 537–539. [Google Scholar]
- 57.Kenny PA, Lee GY, Bissell MJ. Targeting the Tumor microenvironment. Frontiers in Bioscience. 2007 May 1;12:3468–74. doi: 10.2741/2327. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bissell MJ, Radisky D. Putting tumours in context. Nature Reviews (Cancer) 2001;1(1):46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwarz RI, Bissell MJ. Dependence of the differentiated state on the cellular environment: Modulation of collagen synthesis in tendon cells. Proc Natl Acad Sci USA. 1977;74(10):4453–4457. doi: 10.1073/pnas.74.10.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gudjonsson T, Adriance MC, Sternlicht MD, Petersen OW, Bissell MJ. Myoepithelial cells: their origin and function in breast morphogenesis and neoplasia. J Mammary Gland Biol Neoplasia. 2005 Jul;10(3):261–72. doi: 10.1007/s10911-005-9586-4. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Villadsen R, Fridriksdottir AJ, Rønnov-Jessen L, Gudjonsson T, Rank F, Labarge MA, Bissell MJ, Petersen OW. Evidence for a Stem Cell Hierarchy in the Adult Human Breast. The Journal of Cell Biology. 2007 Apr 9;177(1):87–101. doi: 10.1083/jcb.200611114. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW.