Abstract
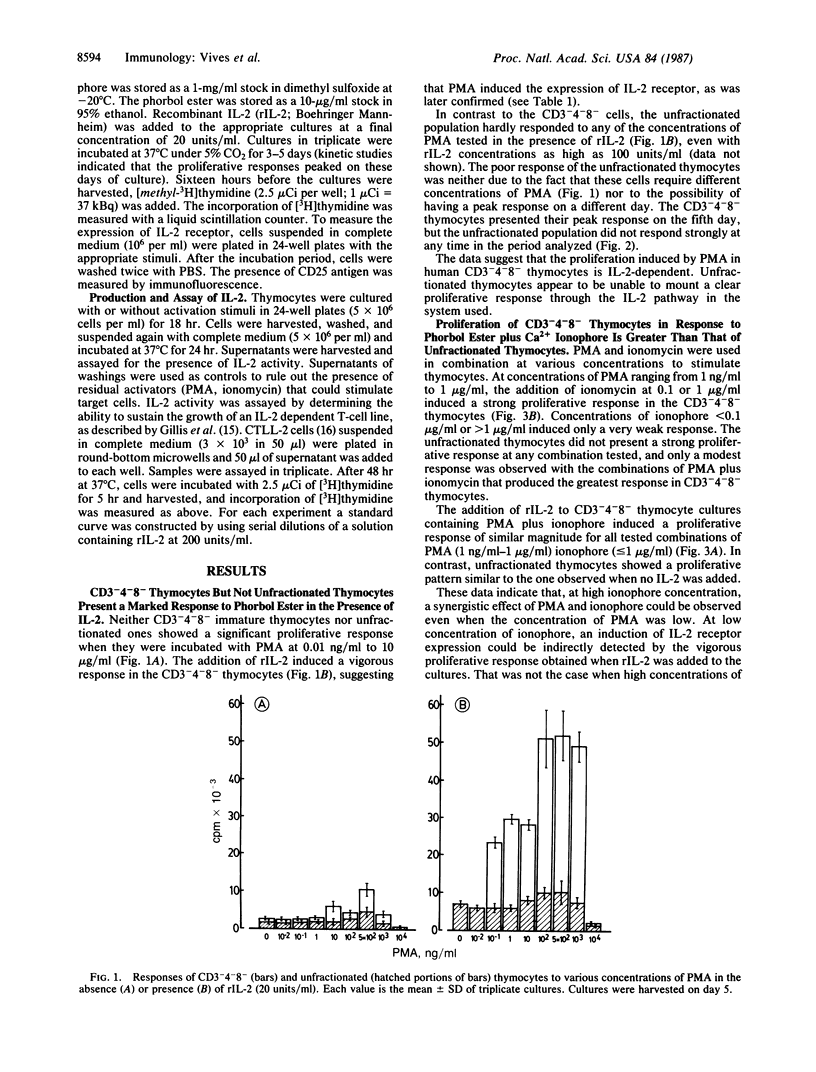
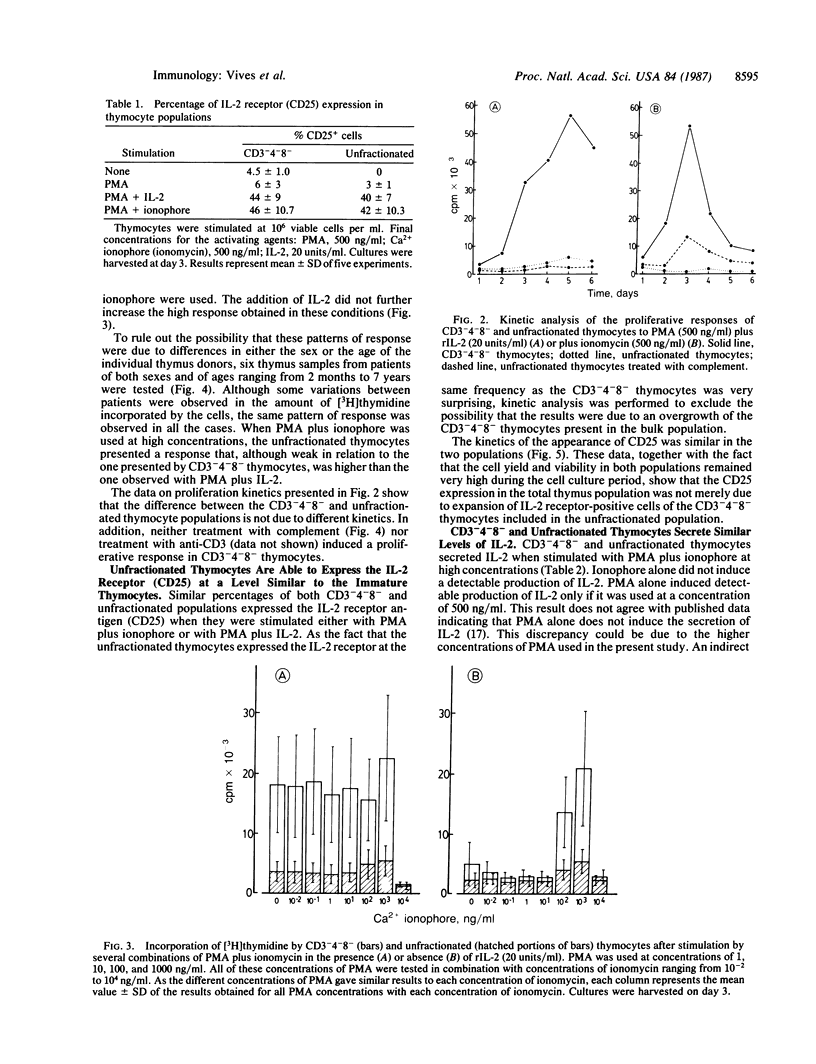
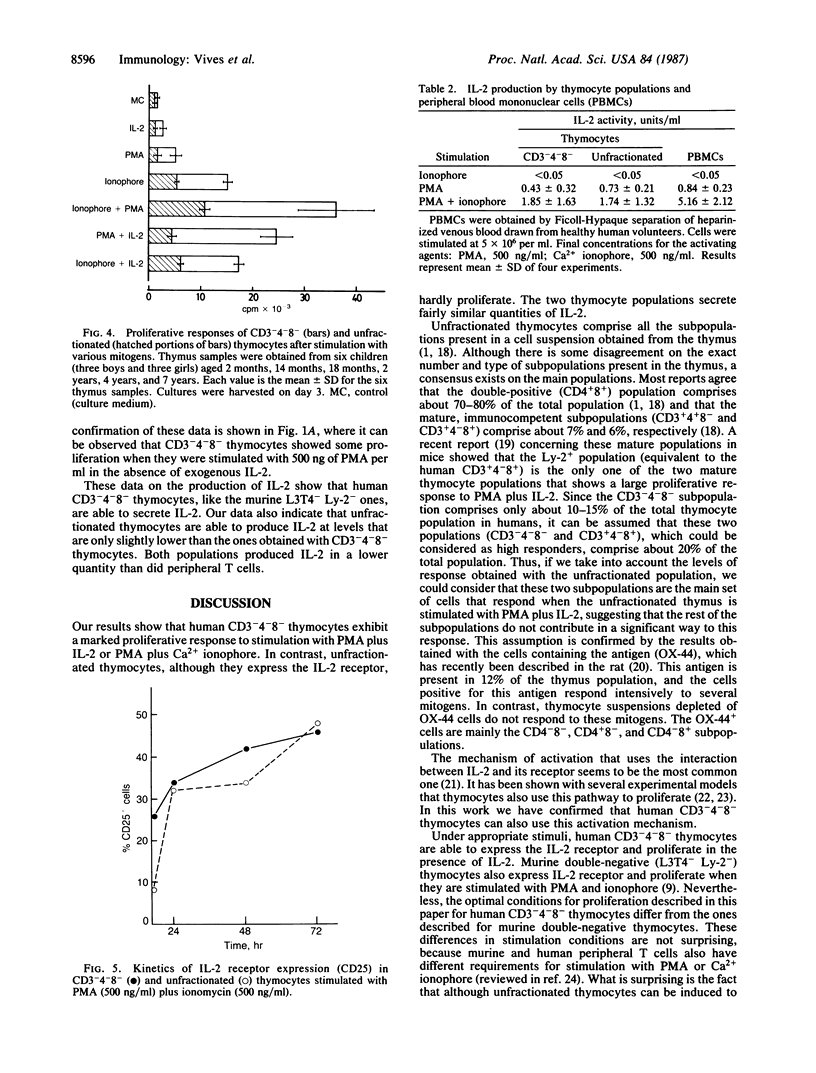
CD3-4-8- and unfractionated thymocytes were compared for their capacity to proliferate, to express interleukin 2 (IL-2) receptor, and to secret IL-2. Phorbol ester and Ca2+ ionophore were used as mitogens. CD3-4-8- thymocytes responded vigorously when stimulated with phorbol ester in the presence of IL-2 or in combination with Ca2+ ionophore. In contrast, unfractionated thymocytes responded weakly when stimulated with either of these mitogens. Surprisingly, however, the stimulation of these populations with either phorbol ester plus IL-2 or phorbol ester plus ionophore induced a high and similar level of IL-2 receptor expression in both thymocyte populations. A similar level of IL-2 secretion in both populations was also obtained when they were stimulated with a combination of phorbol ester plus ionophore. These results suggest that during the maturation process, the majority of thymocytes lose their capacity to be activated by some mitogens, although they maintain their capacity to secrete IL-2 and to express the IL-2 receptor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cantrell D. A., Smith K. A. The interleukin-2 T-cell system: a new cell growth model. Science. 1984 Jun 22;224(4655):1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- Ceredig R. Proliferation in vitro and interleukin production by 14 day fetal and adult Lyt-2-/L3T4- mouse thymocytes. J Immunol. 1986 Oct 1;137(7):2260–2267. [PubMed] [Google Scholar]
- Dupuy d'Angeac A., Monis M., Rème T. Mitogenic effect of PMA + IL 2 on subpopulations of corticoresistant thymocytes. J Immunol. 1986 Dec 1;137(11):3501–3508. [PubMed] [Google Scholar]
- Fox D. A., Hussey R. E., Fitzgerald K. A., Bensussan A., Daley J. F., Schlossman S. F., Reinherz E. L. Activation of human thymocytes via the 50KD T11 sheep erythrocyte binding protein induces the expression of interleukin 2 receptors on both T3+ and T3- populations. J Immunol. 1985 Jan;134(1):330–335. [PubMed] [Google Scholar]
- Gelin C., Boumsell L., Dausset J., Bernard A. The heterogeneity and functional capacities of human thymocyte subpopulations. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4912–4916. doi: 10.1073/pnas.81.15.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Jerne N. K. The somatic generation of immune recognition. Eur J Immunol. 1971 Jan;1(1):1–9. doi: 10.1002/eji.1830010102. [DOI] [PubMed] [Google Scholar]
- Kingston R., Jenkinson E. J., Owen J. J. A single stem cell can recolonize an embryonic thymus, producing phenotypically distinct T-cell populations. 1985 Oct 31-Nov 6Nature. 317(6040):811–813. doi: 10.1038/317811a0. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Allison J. P., Phillips J. H. Correlation of cell surface antigen expression on human thymocytes by multi-color flow cytometric analysis: implications for differentiation. J Immunol. 1986 Oct 15;137(8):2501–2507. [PubMed] [Google Scholar]
- Lugo J. P., Krishnan S. N., Sailor R. D., Rothenberg E. V. Early precursor thymocytes can produce interleukin 2 upon stimulation with calcium ionophore and phorbol ester. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1862–1866. doi: 10.1073/pnas.83.6.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan I. C., Gray D. Antigen-driven selection of virgin and memory B cells. Immunol Rev. 1986 Jun;91:61–85. doi: 10.1111/j.1600-065x.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- Mathieson B. J., Sharrow S. O., Rosenberg Y., Hämmerling U. Lyt 1+23- cells appear in the thymus before Lyt 123+ cells. Nature. 1981 Jan 15;289(5794):179–181. doi: 10.1038/289179a0. [DOI] [PubMed] [Google Scholar]
- Matsuyama M., Wiadrowski M. N., Metcalf D. Autoradiographic analysis of lymphopoiesis and lymphocyte migration in mice bearing multiple thymus grafts. J Exp Med. 1966 Mar 1;123(3):559–576. doi: 10.1084/jem.123.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie M., Born W., Marrack P., Kappler J. The role of the T-cell receptor in thymocyte maturation: effects in vivo of anti-receptor antibody. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8728–8732. doi: 10.1073/pnas.83.22.8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Von Boehmer H. Requirements for growth of immature thymocytes from fetal and adult mice in vitro. Eur J Immunol. 1986 Jan;16(1):12–19. doi: 10.1002/eji.1830160104. [DOI] [PubMed] [Google Scholar]
- Paterson D. J., Green J. R., Jefferies W. A., Puklavec M., Williams A. F. The MRC OX-44 antigen marks a functionally relevant subset among rat thymocytes. J Exp Med. 1987 Jan 1;165(1):1–13. doi: 10.1084/jem.165.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penit C. In vivo thymocyte maturation. BUdR labeling of cycling thymocytes and phenotypic analysis of their progeny support the single lineage model. J Immunol. 1986 Oct 1;137(7):2115–2121. [PubMed] [Google Scholar]
- Reem G. H., Davis K. L., Carding S. Induction of interleukin 2 receptors on immature human thymocytes and co-expression of T3 and T6 antigens. Clin Exp Immunol. 1986 Nov;66(2):358–364. [PMC free article] [PubMed] [Google Scholar]
- Reem G. H., Yeh N. H. Regulation by interleukin 2 of interleukin 2 receptors and gamma-interferon synthesis by human thymocytes: augmentation of interleukin 2 receptors by interleukin 2. J Immunol. 1985 Feb;134(2):953–958. [PubMed] [Google Scholar]
- Reem G. H., Yeh N. H., Urdal D. L., Kilian P. L., Farrar J. J. Induction and upregulation by interleukin 2 of high-affinity interleukin 2 receptors on thymocytes and T cells. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8663–8666. doi: 10.1073/pnas.82.24.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer H. D., Acuto O., Fabbi M., Tizard R., Ramachandran K., Smart J. E., Reinherz E. L. Genes encoding the Ti beta subunit of the antigen/MHC receptor undergo rearrangement during intrathymic ontogeny prior to surface T3-Ti expression. Cell. 1984 Dec;39(2 Pt 1):261–266. doi: 10.1016/0092-8674(84)90003-5. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Cantrell D. A. Interleukin 2 regulates its own receptors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):864–868. doi: 10.1073/pnas.82.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A. T-cell growth factor. Immunol Rev. 1980;51:337–357. doi: 10.1111/j.1600-065x.1980.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Stutman O. Intrathymic and extrathymic T cell maturation. Immunol Rev. 1978;42:138–184. doi: 10.1111/j.1600-065x.1978.tb00261.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Ohmura T., Fujimoto K., Onoue K. Interleukin 2 mRNA induction in human lymphocytes: analysis of the synergistic effect of a calcium ionophore A23187 and a phorbol ester. Eur J Immunol. 1985 Dec;15(12):1204–1208. doi: 10.1002/eji.1830151212. [DOI] [PubMed] [Google Scholar]
- de Vries J. E., Vyth-Dreese F. A., Figdor C. G., Spits H., Leemans J. M., Bont W. S. Induction of phenotypic differentiation, interleukin 2 production, and PHA responsiveness of "immature" human thymocytes by interleukin 1 and phorbol ester. J Immunol. 1983 Jul;131(1):201–206. [PubMed] [Google Scholar]



