Abstract
Anemia in pregnancy is a worldwide problem, but it is most prevalent in the developing world. This research project was conducted to determine the predictors of anemia in pregnant women in Westmoreland, Jamaica. A cross-sectional study design was conducted and descriptive, bivariate, and multiple logistic regression analyses were used. Body mass index, Mid-upper arm circumference, and the number of antenatal care visits showed a statistically significant association with anemia. Based on the results, we believe that maintaining a healthy body weight, and frequently visiting an antenatal clinic, will help to lower the prevalence of anemia among pregnant women in Westmoreland.
Maternal anemia is a ubiquitous pregnancy complication in developing countries (Zhang, Li, & Ananth, 2009). In Westmoreland, Jamaica anemia in pregnancy has been a major issue because a large percentage (almost 20% in 2006) of pregnant women with documented blood tests are found to be anemic. Anemia is regarded as a major risk factor for unfavorable pregnancy outcomes (Xing et al, 2009). Acute onset of anemia during pregnancy will greatly increase the risk of death because this can lead to rapid cardiac decompensation (Brabin, Hakimi, & Pelletier, 2001). In addition, anemia has been associated with a number of adverse pregnancy outcomes, including preterm birth, restricted fetal growth, and perinatal mortality (Rasmussen, 2001). Our primary objectives were to determine the strongest predictors of anemia in pregnancy within a study population in which published data is lacking, and to address an ongoing health problem for women worldwide, especially Jamaica.
Anemia in pregnancy, defined as having a hemoglobin level less than 11g/dL, is one of the many adverse health conditions that affect women in both developed and developing countries (WHO, 2006). Moreover, anemia that complicates pregnancy threatens the life of both the mother and the fetus (Lone, Qureshi, & Emanuel, 2004). Anemia can result from the alterations in iron metabolism that occur as a response to infectious diseases, such as malaria and hookworm infections (Crawley, 2004). Infections and inflammatory diseases decrease iron absorption in the small intestine, and induce iron sequestration in macrophages, which is the hallmark of anemia of inflammation (Crawley, 2004). Although anemia in pregnancy is a worldwide problem, it is more prevalent in the developing world (Abel, Rajaratnam, Kalaimani, & Kirubakaran, 2000). It is most prevalent in resource poor settings as a result of poor diet, cultural beliefs, lack of education, and little access to efficient health care.
In 2000, maternal mortality was estimated at 110 deaths per 100,000 live births on the island of Jamaica (PAHO, 2005). In the same year, 74% of women attending prenatal clinics at health centers underwent hemoglobin testing, and the prevalence of anemia was 15% (PAHO, 2005). The majority of women attending prenatal care do so only in their late second or third trimesters, making it impossible for them to receive the recommended number of iron tablets in pregnancy (Galloway et al., 2002). Most Ministries of Health in developing countries have policies to give pregnant women either iron by itself or combined with folate in tablet form or in prenatal vitamins (ACC/SCN, 1997). Pregnant women in Jamaica are usually tested for hemoglobin levels at their first prenatal visit during their first trimester, and the overall prevalence of anemia among pregnant women may reflect pre-pregnancy levels (PAHO, 2005). Westmoreland is a rural parish in Jamaica and antenatal care is provided at twenty health centers throughout the parish. The majority of women surveyed in Westmoreland did not present to clinics for antenatal care until their second or third trimester.
In Westmoreland, Jamaica antenatal visits are primarily conducted by midwives in the health center. Women are encouraged to initiate antenatal visits by their second missed period. The Family Health Manual outlines the steps at each visit and focuses on history and examination, blood investigations, and health education. Any abnormal findings results in appropriate referrals to the medical doctor in the health center or the antenatal high risk clinic. Regarding anemia in pregnancy, women are screened at their first visit, and routinely given nutrition education and iron and folic supplements. If women are found to be anemic, a referral is made to the doctor, high risk clinic, and a nutritionist. If necessary, further investigations are done and appropriate treatment is prescribed.
IDA, also called hypochromic microcytic anemia, is the most common form of anemia and is identified by light colored, small red blood cells of inadequate number (Jarrah, Halabi, Bond, & Abegglen, 2007). Initial IDA symptoms include tiredness and lack of interest in external activities (Jarrah, Halabi, Bond, & Abegglen, 2007). As IDA progresses, the person experiences fatigue and lethargy, headaches, depression, and loss of concentration and memory (Jarrah, Halabi, Bond, & Abegglen, 2007). In pregnancy, IDA can result in irreversible damage to normal mental and physical abilities for both the mother and unborn child.
In developing countries, the cause of anemia during pregnancy is multi-factorial and includes nutritional deficiencies of iron, folate, vitamin B12 and parasitic diseases, such as malaria and hookworm infections (VanderJagt, Brock, Melah, El-Nafaty, Crossey, & Glew, 2007). The relative contribution of each of these factors to anemia during pregnancy varies greatly by geographical location, season, and dietary practice (VanderJagt et al., 2007). The requirements of iron during pregnancy are high, and it is difficult to meet the requirements through diet alone (VanderJagt et al., 2007). Therefore, maintaining iron balance during pregnancy is dependent on maternal iron stores (VanderJagt et al., 2007). Evidence is accumulating to show that iron supplementation of iron-deficient pregnant women improves both maternal and infant iron stores postpartum (Preziosi, Prual, Galan, Daouda, Boureima, & Hercberg, 1997; Allen, 2000), but the functional consequences of this improvement have not been documented adequately (Allen, 2000; Grantham-McGregor & Ani, 2001).
Ultimately, all pregnant women in areas of high prevalence of malnutrition should routinely receive iron and folate supplements, together with appropriate dietary advice, to prevent anemia (WHO, 2006). Where the prevalence of anemia in pregnant women is less than 40%, a dose of 60 mg iron and 400 μg folic acid daily for 6 months is considered to meet the physiological requirements for iron in pregnancy (WHO, 2006). Where the prevalence of anemia in pregnant women is high (40% or more), supplementation should continue for three months in the postpartum period (WHO, 2006).
Because of the importance of iron stores in maintaining iron balance during pregnancy, efforts should also be directed to improving the iron stores of young women prior to pregnancy (VanderJagt et al., 2007). Once women become pregnant, they should be counseled regarding the risks of adverse outcomes with anemia, such as premature birth and low birth weight (LBW) (Lone, Qureshi, & Emanuel, 2004). Furthermore, such counseling should provide accurate information about the need for iron supplements to meet the physiological demands for iron during pregnancy (Galloway et al., 2002).
In the health care sector of Jamaica two areas of concern are anemia and malnutrition. The Jamaican Ministry of Health clinic data for 1984–1991 indicate that, on average, some 28.9% of pregnant women who were tested were anemic (PAHO, 1998). In addition, the 1985 National Health Survey estimated that 25% of children under age 5 years were anemic, with the peak incidence being in the age group 6–11 months old (PAHO, 1998). The Westmoreland Health department estimates that there are at least 2,800 births per year in the Parish. In 2006, there were 1,730 pregnant women seeking antenatal care in the Westmoreland Health Department, where care is delivered in the communities from 20 health centers representing an average of 144 new mothers every month. Of these 1,730 women, blood test results for anemia were documented for 1,314 (76%), and 244 (18.6%) were found to have hemoglobin levels less than 10g/dl. With 14.8% of the Jamaican population below the poverty line, and with an economy heavily reliant on a wavering tourism industry, cheap and sustainable efforts by local communities to combat anemia will be most effective (Kukoyi et al., unpublished).
This research project was conducted to determine the predictors of anemia among pregnant women in the parish of Westmoreland, and to provide data that can be used to design interventions to lower the incidence of anemia in pregnancy in the parish.
METHODS
Study site and population
The study was conducted in the parish of Westmoreland, Jamaica (see figure 1a), which is located on the west end of the island of Jamaica. The study population consisted of pregnant women who were seen in the Public primary care antenatal clinics in the parish.
Figure 1a.
Map of Jamaica, West Indies with Westmoreland outlined in black
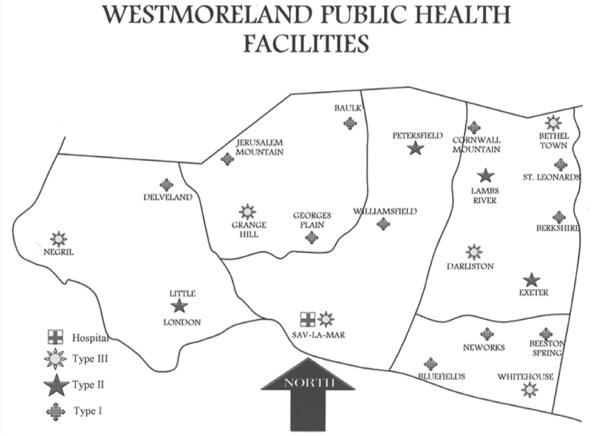
The Parish has 21 health centers (see figure 1b), which are spread out across five health districts (Grange Hill, Savanna-la-mar, Negril, Whitehouse, and Darliston/Bethel Town). These are classified from Type 1 –3 depending on the population served and the services offered (see Table 4). Of the 21 health centers, 20 are in operation and 12 (type 1, 2 and 3 centers offering antenatal clinics) comprised the sampling units for the study. These health centers included Grange Hill, Negril, Little London, Savanna-la-mar, Petersfield, Whitehouse, Darliston, Beeston Spring, Williamsfield, Georges Plain, Baulk, and Bluefields.
Figure 1b.
Detailed map of Type 1, 2, and 3 health centers in Westmoreland, Jamaica
Table 4.
Health center type, population served, and services offered
| Heath Centre Type | Population served | Services offered |
|---|---|---|
| Type 1 | 4,000 |
|
| Type 2 | 12,000 |
|
| Type 3 | 20,000 |
|
Design and procedure
A multi-center cross-sectional design was used. All women presenting at selected health centers for antenatal care (ANC) independent of their stage of pregnancy were asked to participate in the study if they met the inclusion criteria. The inclusion criteria were being pregnant, having a prior antenatal care visit, and having a prior hemoglobin test. The purpose of the study was explained to the women and they were asked if they would like to participate. Upon agreement to participate, the informed consent was obtained and a signature was required as proof of consent.
Data was collected over an eight week period from June – August 2008 with the aid of two interviewers and 1 filed supervisor. Information on the most recent hemoglobin level and Sickle cell test (usually tested at first antenatal care visit) was obtained from the women's medical records. Information on all medications prescribed was also collected from the participant's medical records. An interviewer-administered questionnaire was completed collecting data on demographic characteristics (age, education, socioeconomic status, residence, etc.), gestational age, obstetric history, prescribed and over-the-counter medications taken during pregnancy, and adherence to iron, folic acid, and multivitamin supplements which are provided as part of routine ANC. The questionnaire consisted of thirty questions, which were mainly closed-ended. A total of 204 women were included in the study.
Mid Upper Arm Circumference (MUAC) was used as a proxy for body weight, since it is largely independent of gestational age (Krasovec & Anderson, 1991). MUAC was measured to the nearest 0.5cm, and values below 25.0cm were used in the analyses as an indicator of low body weight. According to Jamaican standards, pregnant women who are less than 28 weeks and have a hemoglobin level <10g/dl are considered anemic. In addition, pregnant women who have a gestational age of 28 weeks or higher with a hemoglobin level <11g/dl, are considered to be anemic. However, for the purposes of this study, the World Health Organization standard (Hb <11g/dl) was used to determine anemia status in pregnancy based on hemoglobin levels (WHO, 2006).
Participation in the study was voluntary and no incentives were provided. The Institutional Review Board (IRB) of the University of Alabama at Birmingham, the Advisory Panel of Ethics and Medico-Legal Affairs in the Jamaican Ministry of Health, and the Western Regional Health Authority approved the study protocol prior to its implementation.
Data Analysis
Descriptive analysis was performed to summarize the demographic characteristics, obstetric history, antenatal care, and diagnoses of the participants. Differences in means were compared using the Student's t test, and differences in proportions were analyzed using the chi-square test. Frequencies and percentages of variables were calculated by anemia status. The relationship between anemia, demographic characteristics, obstetric history, antenatal care, and diagnoses was investigated through bivariate analysis, and stratified by anemia status. This analysis was done to determine significant associations between certain variables and anemia status. The associations between anemia and its determinants (variables with a p-value < or = 0.05) were examined by multiple logistic regression analysis and expressed as crude odds ratios (COR) and adjusted odds ratios (AOR).
The OR was regarded as an approximation for relative risk. SAS system for Windows, version 9.1 was used for analyses. For all statistical tests a two-sided P-value <0.05 was considered significant.
RESULTS
The overall prevalence of anemia among the 204 pregnant women surveyed was 34.8% (Table 1). Following the inclusion criteria, each participant had a prior antenatal clinic visit, received at least one hemoglobin test, and answered questions voluntarily at recruitment.
Table 1.
Socio-demographic characteristics and anthropometric measurements of women surveyed (N=204) by anemia status
| Variables | Anemia: No [N=133 (65.2%)] | Anemia: Yes [N=71 (34.8%)] | p-value |
|---|---|---|---|
| Age | |||
| < 20 | 18 (13.5) | 13 (18.3) | 0.37 |
| ≥ 20 | 115 (86.5) | 58 (81.7) | |
| Race | |||
| Black | 105 (79.0) | 51 (71.8) | 0.25 |
| Other | 28 (21.0) | 20 (28.2) | |
| Education | |||
| Primary education or less | 43 (32.3) | 24 (33.8) | 0.76 |
| Secondary education | 68 (51.1) | 38 (53.5) | |
| College or University | 22 (16.5) | 9 (12.7) | |
| Religious affiliation | |||
| Christian | 120 (90.2) | 65 (91.6) | 0.76 |
| Other | 13 (9.8) | 6 (8.4) | |
| Marital status | |||
| Single, divorced or widowed | 65 (48.9) | 29 (40.9) | 0.27 |
| Married or living in union | 68 (51.1) | 42 (59.2) | |
| Work category | |||
| Employed or self-employed | 73 (54.9) | 40 (56.3) | 0.84 |
| Unemployed | 60 (45.1) | 31 (43.7) | |
| Number of children in household | |||
| 0–2 | 91 (68.4) | 43 (60.6) | 0.26 |
| ≥ 3 | 42 (31.6) | 28 (39.4) | |
| Weekly income | |||
| J$0–2500 | 15 (20.3) | 10 (25.0) | 0.26 |
| J$2501–3800 | 16 (21.6) | 13 (32.5) | |
| > J$3800 | 43 (58.1) | 17 (42.5) | |
| Smoke | |||
| Yes | 14 (14.9) | 9 (18.0) | 0.62 |
| No | 80 (85.1) | 41 (82.0) | |
| Drink | |||
| Yes | 18 (19.2) | 6 (12.0) | 0.27 |
| No | 76 (80.8) | 44 (88.0) | |
| BMI | |||
| < 25 | 37 (28.2) | 37 (52.1) | < 0.01 |
| 25–29 | 49 (37.4) | 15 (21.1) | |
| ≥ 30 | 45 (34.4) | 19 (26.8) | |
| Mid Upper Arm Circumference | |||
| < 25 | 9 (6.8) | 13 (18.6) | 0.03 |
| 25–30 | 62 (47.0) | 32 (45.7) | |
| > 30 | 61 (46.2) | 25 (35.7) |
Bold- Statistically significant; MUAC (cm); US $1 = J$70
Sum of N for some variables may not equal total N due to missing data
Most of the women surveyed were age 20 years or older (85%), had secondary education (52%), were married or living in union with a partner (54%), and earned more than minimum wage [$3800 Jamaican dollars (JMD)] per week (29%). Bivariate analysis showed that pregnant women with a Body Mass Index (BMI) below 25 and a Mid-Upper Arm Circumference (MUAC) less than 25 cm were more likely to be anemic. More specifically, 52% of anemic women had a BMI < 25 compared to 28.2% of non-anemic women, and 18.6% of anemic women had a MUAC < 25 cm compared with 6.8% of non-anemic women.
Table 2 outlines the obstetric history of the women surveyed by anemia. Based on our bivariate analysis, only the number of antenatal care visits was significantly associated with anemia (p-value < 0.01). Almost 61% of anemic women had less than four visits to an antenatal clinic during their pregnancy compared with 39% of non-anemic women. Therefore, a lower number of clinic visits seemed to increase the chances of being anemic during pregnancy within this population.
Table 2.
Obstetric history of pregnant women (N=204) by anemia status
| Variables | Anemia: No [N=133 (%)] | Anemia: Yes [N=71(%)] | p-value |
|---|---|---|---|
| Trimester at recruitment | |||
| 1 | 1 (0.8) | 2 (2.8) | 0.47 |
| 2 | 44 (33.1) | 25 (36.2) | |
| 3 | 88 (66.2) | 44 (62.0) | |
| First pregnancy | |||
| Yes | 39 (29.3) | 21 (29.6) | 0.97 |
| No | 94 (70.7) | 50 (70.4) | |
| Birth interval | |||
| < 20 months | 9 (9.6) | 3 (6.0) | 0.46 |
| ≥ 20 months | 85 (90.4) | 47 (94.0) | |
| Number of previous pregnancies | |||
| 0 | 39 (29.3) | 21 (29.6) | 0.61 |
| 1 | 28 (21.1) | 11 (15.5) | |
| ≥ 2 | 66 (49.6) | 39 (54.9) | |
| History of miscarriage | |||
| Yes | 31 (33.0) | 14 (28.0) | 0.54 |
| No | 63 (67.0) | 36 (72.0) | |
| History of abortion | |||
| Yes | 6 (6.4) | 1 (2.0) | 0.24 |
| No | 88 (93.6) | 49 (98.0) | |
| Cravings for non-food items | |||
| Yes | 38 (40.4) | 26 (52.0) | 0.18 |
| No | 56 (59.6) | 24 (48.0) | |
| Severe or unusual illness | |||
| Yes | 21 (15.8) | 10 (14.1) | 0.75 |
| No | 112 (84.2) | 61 (85.9) | |
| Chronic illness | |||
| Yes | 9 (42.9) | 6 (60.0) | 0.37 |
| No | 12 (57.1) | 4 (40.0) | |
| Number of Antenatal care visits | |||
| < 4 | 52 (39.1) | 43 (60.6) | < 0.01 |
| ≥ 4 | 81 (60.9) | 28 (39.4) | |
| Iron supplements given | |||
| Yes | 69 (53.1) | 34 (47.9) | 0.48 |
| No | 61 (46.9) | 37 (52.1) | |
| Folic acid supplements given | |||
| Yes | 69 (53.1) | 34 (47.9) | 0.48 |
| No | 61 (46.9) | 37 (52.1) | |
| Sickle cell anemia | |||
| Yes | 4 (3.0) | 3 (4.2) | 0.65 |
| No | 129 (97.0) | 68 (95.8) |
Bold- Statistically significant
Sum of N for some variables may not equal total N due to missing data
Table 3 outlines the crude and adjusted odds ratios of the association between selected variables and anemia. To produce this final table, all of the statistically significant variables (p-value < 0.05) from the bivariate analysis were entered as independent variables in a logistic regression model with anemia as the dependant variable. The variables that appeared to be confounders in the initial analysis were also included in the regression model. In the final model, pregnant women with a BMI of 25–29 were 60% (p-value = 0.02; OR = 0.4; CI = 0.1–0.9) less likely to be anemic than those with a BMI less than 25 (table 3). Women with a MUAC between 25 and 30 cm were 70% (p-value = 0.03; OR = 0.3; CI = 0.1–0.9) less likely to be anemic than women who had a MUAC less than 25 cm (table 3). The Crude Odds Ratio for BMI was significant for women with BMI greater than or equal to 30, but the Adjusted Odds Ratio for this category was not significant. The Crude Odds Ratio for women with MUAC greater than 30 cm was significant, but the Adjusted Odds Ratio was not significant. Pregnant women who had four or more antenatal care visits were 30% (p-value < 0.01; OR = 0.7; CI = 0.3–0.9) less likely to be anemic than women who had less than four antenatal visits. For each of these significant variables, data-based confounding does not appear to be present as there are not meaningful differences between the crude and adjusted odds ratios.
Table 3.
Crude and adjusted ratios of the association between selected variables and anemia
| Variables | Crude Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) | P-Value |
|---|---|---|---|
| Age | |||
| < 20 | Ref. | Ref. | |
| ≥ 20 | 0.7 (0.3–1.5) | 0.7 (0.1–4.0) | 0.71 |
| Weekly income | |||
| J$0–2500 | Ref. | Ref. | |
| J$2501–3800 | 1.2 (0.4–3.6) | 1.2 (0.3–4.4) | 0.63 |
| > J$3800 | 0.6 (0.2–1.6) | 0.8 (0.2–2.7) | 0.55 |
| Number of children in household | |||
| 0–2 | Ref. | Ref. | |
| ≥ 3 | 1.4 (0.8–2.6) | 0.9 (0.3–2.3) | 0.77 |
| BMI | |||
| < 25 | Ref. | Ref. | |
| 25–29 | 0.3 (0.1–0.6) | 0.4 (0.1–0.9) | 0.02 |
| ≥ 30 | 0.4 (0.2–0.9) | 0.6 (0.1–1.5) | 0.23 |
| Mid Upper Arm Circumference | |||
| < 25 | Ref. | Ref. | |
| 25–30 | 0.4 (0.2–0.9) | 0.3 (0.1–0.9) | 0.03 |
| > 30 | 0.3 (0.1–0.7) | 0.3 (0.1–1.9) | 0.29 |
| First pregnancy | |||
| Yes | Ref. | Ref. | 0.86 |
| No | 1.0 (0.6–2.1) | 4.0 (0.4–36.0) | |
| Number of previous pregnancies | |||
| 0 | Ref. | Ref. | |
| 1 | 0.7 (0.3–1.8) | 0.5 (0.1–6.7) | 0.62 |
| ≥ 2 | 1.1 (0.6–2.1) | 1.3 (0.3–3.2) | 0.58 |
| Number of Antenatal care visits | |||
| < 4 | Ref. | Ref. | |
| ≥ 4 | 0.4 (0.2–0.8) | 0.7 (0.3–0.9) | < 0.01 |
| Iron supplements given | |||
| Yes | Ref. | Ref. | |
| No | 1.2 (0.7–2.2) | 0.3 (0.1–7.3) | 0.47 |
| Folic acid supplements given | |||
| Yes | Ref. | Ref. | |
| No | 1.2 (0.7–2.2) | 3.5 (0.2–18.9) | 0.43 |
| Sickle cell anemia | |||
| Yes | Ref. | Ref. | |
| No | 0.7 (0.2–3.2) | 0.2 (0.1–2.7) | 0.22 |
Bold-Statistically Significant; MUAC (cm)
Note: All missing observations were excluded from the analysis.
CONCLUSIONS and DISCUSSION
Based on the data presented in this study, we believe that anemia is a major health issue for pregnant women in Westmoreland, Jamaica. Compared to the data that was reported by the Westmoreland Health Department in 2006, there appears to be an approximate 16% increase of anemia among pregnant women in that region of the island (18.6% to 34.8%). In looking at this comparison, it must be considered that the Jamaican standards for anemia vary according to gestational weeks, while the WHO anemia standards for hemoglobin levels during pregnancy (Hb < 11g/dL) do not vary according to gestational weeks. This variation in anemia definition standards is one of the limitations to this study. The variables BMI, MUAC, and number of antenatal care visits seem to be strong predictors of anemia among pregnant women in Westmoreland. This indicates that maintaining healthy body weight and frequently attending antenatal clinics would help to lower the incidence and overall prevalence of anemia among pregnant women.
Women of reproductive age (WRA) are at risk of developing iron deficiency, which if continued for several months can progress to anemia (Veteri & Berger, 2005). Adolescence, like pregnancy, increases iron requirements in girls to accommodate the demands of growth and iron losses due to the onset of menstruation (Iannotti et al., 2005). According to the Jamaican Survey of Living Conditions (JSLC), in 1994, 16% of the 10–14-year-old age group of adolescents surveyed were anemic, with hemoglobin levels below the accepted standard of 12 g/dl for males and 15 g/dl for females (JSLC, 1994; PAHO, 1998). This suggests that the reproductive years of women is the best time to increase the body's uptake of iron by maintaining a balanced diet, and taking vitamin supplements.
Obstetricians have an important role to play by making women aware of the iron content in a balanced diet, especially in green leafy vegetables (Lone, Qureshi, & Emanuel, 2004). In the Parish of Westmoreland, this practice would be essential, because it would lower the risk of the women having low body weight when they begin to have children, thus making them less likely to become anemic. Studies performed in developing countries, where the prevalence of anemia is high, have also shown a positive impact of iron supplementation during pregnancy on fetal growth (Rioux & LeBlanc, 2007). The World Health Organization (WHO) estimates that 43% of all non-pregnant women 15 to 59 years of age who live in the developing world have anemia; during pregnancy, this prevalence increases to 56% (Viteri & Berger, 2005). Such an alarming prevalence is evidence that continued effort needs to be directed towards controlling anemia before, during, and after pregnancy.
Many of the women in this study did not present for antenatal care until late in their second trimester of pregnancy. Approximately 48% of the women reported having their first antenatal care visit during their second trimester, resulting in a decreased number of antenatal visits. According to the results, we believe that women who visited antenatal clinics four or more times during their pregnancy were less likely to become anemic. In addition, we believe that pregnant women in Westmoreland, Jamaica who frequently visit the antenatal clinic are less likely to develop anemia during their pregnancy because of anemia screening at the first visit, routine nutrition education, iron and folic acid supplementation, and referrals to the medical doctor or “high risk” clinic if there are any abnormal findings. Since the number of antenatal care visits showed a strong association with anemia in this population, it would be beneficial to stress the importance of seeking care earlier in pregnancy. Maintaining a healthy body weight, and making early and timely visits to the antenatal clinic would help to lower the prevalence of anemia among pregnant women in Westmoreland. In addition, the marketing of healthy diets and iron supplementation in all women of reproductive age within this population may prove to be beneficial.
Prior data on malnutrition and anemia prevalence in Jamaica indicate that further research needs to be done to assess the ongoing factors that contribute to anemia in pregnancy on the island. More specifically, a closer look at the associated factors and underlying causes of IDA among pregnant women in Westmoreland is necessary to develop a true understanding of the nutritional deficiency in that population. In addition, more research on anemia in pregnancy in Jamaica needs to be documented as there is a lack of published data on the condition. Increased documentation of research can help to determine what kinds of interventions would be effective among pregnant women.
This study indicates that an intervention that targets women early in pregnancy, and women of reproductive age (before pregnancy), would increase the effectiveness. In addition, iron supplementation may have other important benefits that are not yet generally recognized (Allen, 2002). These should be investigated further and, if confirmed, used as additional support of the importance of iron intervention programs (Allen, 2002). In this study, iron supplementation did not show statistical significance in lowering anemia risk. We believe that this may have been due to adherence to the recommended iron supplementation, but our study did not assess this factor. Overall, with an increased understanding of the core factors, efforts can be directed toward mitigating and eventually ameliorating IDA among pregnant women in Westmoreland.
The cross-sectional design and small sample size are limitations of this study. The fact that no incentives were given and participation was strictly voluntary probably contributed to a smaller sample size. It is possible that a larger sample size would have produced more statistically significant associations between other variables and anemia. This study cannot be generalized to the entire population of Jamaica since it was only conducted in the Parish of Westmoreland. However, future studies can be conducted with a sample that is more representative of all of the parishes to assess predictors of anemia in pregnancy across the island.
Acknowledgments
This research was supported by the University of Alabama at Birmingham (UAB) Minority Health International Training Program, the UAB Sparkman Center – Framework Program for Global Health, the Westmoreland Health Department (Savanna-la-mar, Jamaica), and the Jamaican Ministry of Health.
REFERENCES
- Abel R, Rajaratnam J, Kalaimani A, Kirubakaran S. Can iron status be improved in each of the three trimesters? A community based study. European Journal of Clinical Nutrition. 2000;54:490–493. doi: 10.1038/sj.ejcn.1601044. [DOI] [PubMed] [Google Scholar]
- ACC/SCN . Third report on the world nutrition situation. Administrative Committee on Coordination/Subcommittee on Nutrition; United Nations: 1997. [Google Scholar]
- Allen LH. Iron Supplements: Scientific Issues Concerning Efficacy and Implications for Research and Programs. The Journal of Nutrition. 2002;132:813S–819S. doi: 10.1093/jn/132.4.813S. [DOI] [PubMed] [Google Scholar]
- Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am. J. Clin. Nutr. 2000;71:1280S–1284S. doi: 10.1093/ajcn/71.5.1280s. [DOI] [PubMed] [Google Scholar]
- Brabin B, Hakimi M, Pelletier D. Iron-Deficiency Anemia: Reexamining the Nature and Magnitude of the Public Health Problem, an Analysis of Anemia and Pregnancy-Related Maternal Mortality. The Journal of Nutrition. 2001;31:604S–615S. doi: 10.1093/jn/131.2.604S. [DOI] [PubMed] [Google Scholar]
- Crawley J. Reducing the Burden of Anemia in Infants and Young Children in Malaria-Endemic Countries of Africa: From Evidence to Action. Am. J. Trop. Med. Hyg. 2004;71:25–34. [PubMed] [Google Scholar]
- Galloway R, Dusch E, Elder L, Achadi E, Grajeda R, Hurtado E, et al. Women's perceptions of iron deficiency and anemia prevention and control in eight developing countries. Social Science & Medicine. 2002;55:529–544. doi: 10.1016/s0277-9536(01)00185-x. [DOI] [PubMed] [Google Scholar]
- Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J. Nutr. 2001;131:649S–666S. doi: 10.1093/jn/131.2.649S. [DOI] [PubMed] [Google Scholar]
- Iannotti LL, O'Brien KO, Chang S-C, Mancini J, Schulman-Nathanson M, Liu S, et al. Iron Deficiency Anemia and Depleted Body Iron Reserves Are Prevalent among Pregnant African-American Adolescents. The Journal of Nutrition. 2005;135:2572–2577. doi: 10.1093/jn/135.11.2572. [DOI] [PubMed] [Google Scholar]
- Jamaica Survey of Living Conditions (JSLC) Joint publication of the Planning Institute of Jamaica and the Statistical Institute of Jamaica. World Bank, Dutch Government; 1994. Funded by Government of Jamaica. [Google Scholar]
- Jarrah SS, Halabi JO, Bond AE, Abegglen J. Iron Deficiency Anemia (IDA) Perceptions and Dietary Iron Intake Among Young Women and Pregnant Women in Jordan. Journal of Transcultural Nursing. 2007;18:19–27. doi: 10.1177/1043659606294193. [DOI] [PubMed] [Google Scholar]
- Krasovec K, Anderson M, editors. Maternal nutrition and pregnancy outcomes: anthropometric assessment. Pan American Health Organization (PAHO); Washington, D.C.: 1991. p. ix.p. 214. Scientific Publication No. 529. [Google Scholar]
- Kukoyi OY, Nguyen N, Yatich N, Ncube N, Bessler P, Jolly PE. Risk factors for anemia among women of childbearing age in Westmoreland, Jamaica. 2008 November; Submitted to Women & Health. [Google Scholar]
- Lone FW, Qureshi RN, Emmanuel F. Maternal anaemia and its impact on perinatal outcome. Tropical Medicine and International Health. 2004;9:486–490. doi: 10.1111/j.1365-3156.2004.01222.x. [DOI] [PubMed] [Google Scholar]
- Pan American Health Organization Health Situation Analysis and Trends Summary: Country Chapter Summary from Health in the Americas, Jamaica. 1998 Accessed 12/4/2008. Available from: www.paho.org.
- Pan American Health Organization Promoting Health in the Americas Basic Health Indicator Data Base & Country Health Profile. 2005 Accessed 03/30/2008. Available from: www.paho.org.
- Preziosi P, Prual A, Galan P, Daouda H, Boureima H, Hercberg S. Effect of iron supplementation on the iron status of pregnant women: consequences for newborns. Am. J. Clin. Nutr. 1997;66:1178–1182. doi: 10.1093/ajcn/66.5.1178. [DOI] [PubMed] [Google Scholar]
- Rasmussen K. Is there a causal relationship between iron deficiency or iron-deficiency anemia and weight at birth, length of gestation and perinatal mortality? Journal of Nutrition. 2001;131:590S–603S. doi: 10.1093/jn/131.2.590S. [DOI] [PubMed] [Google Scholar]
- Rioux FM, LeBlanc CP. Iron supplementation during pregnancy: what are the risks and benefits of current practices? Appl. Physiol. Nutr. Metab. 2007;32:282–288. doi: 10.1139/H07-012. [DOI] [PubMed] [Google Scholar]
- Steketee RW. Pregnancy, Nutrition, and Parasitic Diseases. The Journal of Nutrition. 2003;133:1661–1667. doi: 10.1093/jn/133.5.1661S. [DOI] [PubMed] [Google Scholar]
- VanderJagt DJ, Brock HS, Melah GS, El-Nafaty AU, Crossey MJ, Glew RH. Nutritional Factors Associated with Anaemia in Pregnant Women in Northern Nigeria. J Health Popul Nutr. 2007;25:75–81. [PMC free article] [PubMed] [Google Scholar]
- Viteri FE, Berger J. Importance of Pre-Pregnancy and Pregnancy Iron Status: Can Long-Term Weekly Preventive Iron and Folic Acid Supplementation Achieve Desirable and Safe Status? Nutrition Reviews. 2005;63(12):S65–S76. doi: 10.1301/nr.2005.dec.s65-s76. [DOI] [PubMed] [Google Scholar]
- World Health Organization Iron and folate supplementation: Integrated management of pregnancy and childbirth. 2006 Available from: www.who.int/entity/making_pregnancy_safer/publications/Standards1.8N.pdf.
- Xing Y, Yan H, Dang S, Zhuoma B, Zhou X, Wang D. Hemoglobin levels and anemia evaluation during pregnancy in the highlands of Tibet: a hospital-based study. BMC Public Health. 2009;9:336. doi: 10.1186/1471-2458-9-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Li Z, Ananth CV. Prevalence and risk factors for anaemia in pregnant women: a population-based prospective cohort study in China. Pediatric and Perinatal Epidemiology. 2009;23:282–291. doi: 10.1111/j.1365-3016.2009.01031.x. [DOI] [PubMed] [Google Scholar]