Abstract
Environments in which the pharmacological effects of alcohol have been experienced become potent triggers for relapse in abstinent humans. Animal models developed to study the effect of environmental contexts on relapse to alcohol-seeking behavior demonstrate that alcohol-seeking is renewed by exposure to an alcohol-associated context, following the extinction of alcohol-seeking in a different context. Hence, contexts in which alcohol conditioning and extinction learning occur can be critical determinants for whether or not alcohol-seeking behavior is observed. This review summarizes preclinical research to date examining the role of alcohol contexts on the reinstatement of extinguished responding for alcohol. Behavioral studies have elucidated factors that are important for eliciting context-dependent relapse, and have uncovered novel interactions between alcohol-seeking driven by discrete alcohol cues in different contexts. Neuropharmacological studies provide substantial evidence for a role of dopaminergic systems in context-dependent reinstatement, and growing support for opioidergic mechanisms as well. Several key limbic brain regions have been identified in the modulation of alcohol-seeking by context, supporting a proposed neural circuit that includes the hippocampus, nucleus accumbens, basolateral amygdala, lateral hypothalamus, and the paraventricular thalamus.
Keywords: Context, cue, reinstatement, relapse, extinction, ethanol, addiction
INTRODUCTION
The development of a drug or alcohol addiction is dependent upon learned behavior. For example, one learns that the pharmacological effects of alcohol occur following certain actions, like drinking, and are preceded by or concomitant with certain sensory cues, such as the sight, smell or taste of the alcoholic beverage. This learning occurs in a location (or set of locations) known as the environmental context, simply defined as the multimodal constellation of stimuli that characterize a specific place. The importance of the context in which alcohol ingestion occurs has been noted for quite some time, but is now being increasingly appreciated. Researchers and clinicians have observed that the environmental context in which drugs of abuse are used can influence subsequent pharmacological effects of drugs, as well as susceptibility to relapse to drug taking. For example, environmental stimuli can precipitate drug-taking behavior in abstinent patients [1]. These and other observations have led to the idea that exposure to environments previously associated with drinking can contribute to relapse in abstinent individuals.
This idea may have relevance to behavioral techniques used to reduce relapse, particularly to cue-reactivity treatment, in which conditioned responding to drug cues during treatment is extinguished through repeated presentations of the cue(s) without the drug present, with the goal of reducing relapse upon exposure to similar cues post-treatment [2–7]. Although positive effects of cue reactivity treatment have been reported [6, 8, 9] there is some indication that, overall, this method may have only limited success, particularly for alcoholics [10, 11]. One possible reason for this limited success is the strong influence on conditioned behavior of the context in which that conditioning occurs. In cue-reactivity treatment, conditioned responding to alcohol cues is extinguished in a single, potentially unrealistic, laboratory setting, one that is very different from the environments in which alcohol may have been previously experienced. It has been proposed that the extinction of reactivity to alcohol cues during treatment may be specific to the treatment context, and may not generalize to similar cues experienced outside the treatment context [12, 13]. Hence, exposure to the same or similar alcohol cues outside the treatment context – particularly in contexts in which those cues have been previously associated with the drug or alcohol – may induce conditioned responses that contribute to relapse. Relapse to alcoholism therefore may be facilitated by the environmental context when discrete alcohol cues are encountered in an alcohol-associated setting.
This theory is in agreement with current conceptions of extinction learning in which the extinction of a given behavioral response does not erase the original learned association; rather, extinction results in the acquisition of a new memory that competes with the original excitatory conditioning. Thus, the original association is not eliminated, but is still maintained in memory along with a newer inhibitory association [13–15]. The difference in conditioned responding in the extinction and conditioning contexts shows that the environmental context can bias which association is retrieved from memory, the original association or that acquired during extinction. Therefore, therapies that seek to reduce conditioned behavioral responses through extinction techniques are faced with a difficult challenge that arises from the natural properties of learning systems.
When thinking about alcohol addiction, this capacity of context to influence behavior may manifest as an inability to successfully abstain from drinking in environments that are associated with drinking behavior and/or pharmacological effects of alcohol. Further, behavioral treatments that seek to reduce the relapse-inducing influence of discrete cues (such as the visual sight of the bottle or the smell of alcohol) by extinguishing conditioned responses to those cues in the clinic may fail because the alcohol-associated context experienced outside the clinic may provide the retrieval signal for the original learned association between cue and alcohol, rather than the newly-learned associations of cue-no alcohol. In one study of social drinkers, a significant renewal of responses to alcohol cues that had been extinguished in a distinct environment was observed when subjects experienced those cues in a environment different from the extinction environment [16]. However, this context-dependent renewal effect was not observed in two further studies of human heavy drinkers [17] and alcohol-dependent outpatients [18]. In the latter two cases, it may be that renewal would have been observed in environments previously associated with alcohol’s pharmacological effects, something not tested in the referenced studies, i.e., the test context was neither the extinction context nor an alcohol-associated context.
ANIMAL MODEL OF CONTEXT-DEPENDENT RELAPSE TO ALCOHOL-SEEKING
Animal models have been developed to better understand the behavioral and neural regulation of relapse to alcohol-seeking by extinction and conditioning contexts. Initial studies were based on the reinstatement procedure, in which the ability of conditioned and unconditioned stimuli to induce the reinstatement of an instrumental response is provoked in extinguished animal subjects; this increase in behavior after extinction is taken as a measure of drug-seeking [19–21]. Stimuli that increase, or reinstate, instrumental responding above extinction levels include non-contingent presentation of the drug reinforcer, foot shock stress, and exposure to environmental stimuli that were previously associated with drug-reinforced instrumental responding (for review, see [22–26]). Recently, this extinction/reinstatement relapse model was adapted to address the effects of context on relapse. Crombag and Shaham [27] trained rats to lever-press for infusions of a heroin-cocaine combination in a distinctive context. Behavior was then extinguished in a different context by withholding drug. Following extinction, rats were placed back into the original context and responses on the lever that previously delivered drug reinforcement were monitored. The authors found that the return to the initial drug context increased lever-pressing above response levels achieved in the extinction context, indicative of context-dependent relapse.
Several researchers have adapted this procedure to investigate context-dependent reinstatement of instrumental responding for alcohol [28–37]) (Fig. 1). In the first study, published by our laboratory, rats were trained to lever press in 1-hr sessions for small aliquots of 10% alcohol delivered to a reward magazine adjacent to the lever [30]. Training occurred in operant conditioning chambers made distinctive by visual, olfactory and tactile cues; this multimodal collection of stimuli was termed Context A. After 20 sessions of alcohol self-administration, rats received extinction training for 6 daily 1-hr sessions during which lever-pressing was not reinforced by alcohol delivery. Importantly, extinction training occurred in a distinct context – that is, the visual, olfactory, and tactile cues were different than during self-administration training – termed Context B. The day after the final extinction session, subjects were placed back into the original testing context, Context A, and responding on the lever was measured, although no alcohol was delivered at test. In this procedure increased lever-pressing at test would reflect the retrieval of the original instrumental learning and not new learning which could be initiated if responding were reinforced. As predicted, placement into Context A led to elevated lever-pressing relative to extinction levels (compare Extinction responding with responding during Reinstatement Test Day 1 in Fig. 2A). Other laboratories have similarly demonstrated the potent effect of the alcohol self-administration context on instrumental response reinstatement [28, 29, 32–37]. For example, Hamlin and colleagues [36] reported that placement into the original training context reinstates responding at a nose-poke operandum that was previously reinforced by aliquots of a 4% beer solution, following extinction in a different context. Many of these studies have differed in the specifics of the training parameters, in the operant response required, and in the percentage of alcohol in the reinforcer solution. However, the clear pattern of instrumental response reinstatement observed following placement back into an alcohol-associated context indicates that this is a reliable phenomenon, well-suited for the investigation of the influence of the drinking context on alcohol-seeking behavior.
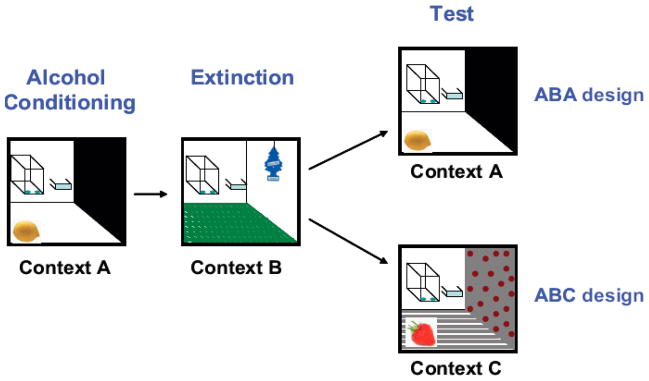
Fig. (1). Context-dependent reinstatement of alcohol-seeking.
This schematic illustrates the use of distinct sensory features (floor texture, odors, distinctive visual features on the walls) used to create distinct training, extinction, and testing environments, or contexts. Training is conducted in Context A, and responding is extinguished in Context B. The reinstatement of responding can be tested in the the original training context (the ABA design) or in a new, novel context (the ABC design).
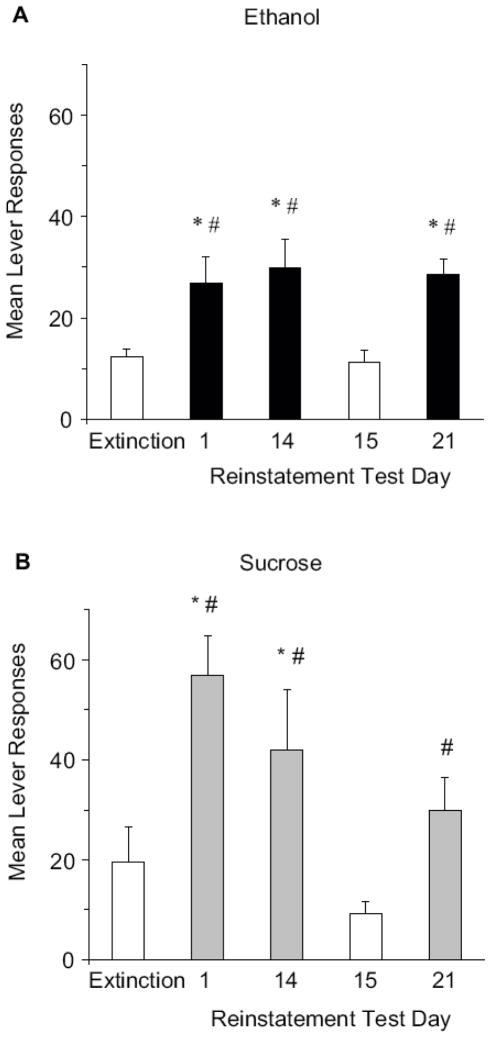
Fig. (2). The alcohol training context reinstates alcohol-seeking after extinction in a different context.
A) Rats (N=8) were trained to lever press for alcohol in Context A (black bars) and extinguished in Context B (white bars). Twenty-four hours after the last extinction session (Extinction), subjects underwent the first reinstatement test in Context A (Reinstatement Test Day 1). Two and three weeks later, subjects were tested again for reinstatement of the operant response in Context A (Reinstatement Test Days 14 and 21), and in Context B 15 days after the first reinstatement test (Day 15). No reward was delivered for any of the tests. Depicted are mean alcohol lever presses +/− S.E.M. B) A separate group of rats (N=6) was trained to lever press for sucrose in Context A (gray bars) and extinguished in Context B (white bars). Reinstatement was tested as described in (A). Depicted are mean sucrose lever presses +/− S.E.M. * P < 0.01 as compared to extinction and # P < 0.01 as compared to Reinstatement Test Day 15. Adapted from [30] with permission.
We have observed consistent context-dependent reinstatement of alcohol-seeking across repeated tests, suggesting that the memory of an alcohol context can influence alcohol-seeking long after the last episode of alcohol consumption in that context (Fig. 2A). In rats trained to respond for sucrose, re-exposure to the training context also reinstates sucrose-seeking that has been extinguished in a different context (Fig. 2B). However, context-dependent reinstatement of sucrose-seeking is reduced with repeated testing as shown by the decrease in the magnitude of reinstatement across repeated tests (Fig. 2B). Thus, mechanisms underlying the modulation by context of sucrose-seeking may not be as long-lasting as they are for alcohol [30].
When subjects are trained and tested in the same context, and extinguished in a different context, the experimental design is referred to as ABA (Fig. 1). To determine if context-dependent reinstatement results simply from the removal of inhibitory control over behavior that is established in the extinction context, testing may also occur in a third, unique, novel context using an ABC design (Fig. 1). When tested in the ABC design, we found that placement into a novel context reinstated responding for sucrose, but not for alcohol [30, 31]. Hence, mechanisms underlying the contextual control over responding for pharmacological agents, such as alcohol, may be different than for natural rewards, like sucrose. This conclusion is supported by the lack of significant reinstatement in a novel context for rats trained to self-administer other drugs, such as intravenous heroin (c.f., [38]).
In our initial efforts to develop a model of context-dependent reinstatement of alcohol-seeking, the daily water consumption of rats was restricted to motivate high intakes of alcohol. In follow up studies, we and others found that the self-administration context can reinstate instrumental responding for alcohol in rats who were not water restricted, indicating that it is the reinforcing property of alcohol itself, rather than thirst, that motivates instrumental responding [31].
Because rodents are often trained to drink alcohol using mixtures of alcohol and a sweetener, such as sucrose, it is relevant to ask in such cases whether rats are responding at test for alcohol or for the sweetener. Notably, the majority of studies on context-dependent reinstatement of alcohol-seeking have not used adulteration of the alcohol with sucrose or another sweetener during the operant conditioning [28–30, 39, 40]. In a few cases in the rat where sucrose was used, it was only in the home cage and instrumental training itself was initiated using 10% ethanol without sucrose [31, 32, 34]. In one study in the mouse, however, sucrose adulteration in the operant chamber was used to train subjects to lever press [33]. Overall, the evidence in the rat strongly supports the notion that alcohol seeking triggered by the context is a consequence of prior exposure to alcohol in that particular environment, and not to a memory of sucrose. This remains to be demonstrated in the mouse. A number of studies have used alcoholic beer as the reinforcer [35–37]. Inclusion of a non-alcoholic beer control group would strengthen the hypothesis that the pharmacological properties of the alcoholic beer were integral to the reinforcing effect of the alcoholic beer, and thus to the eventual seeking behavior driven by the self-administration context.
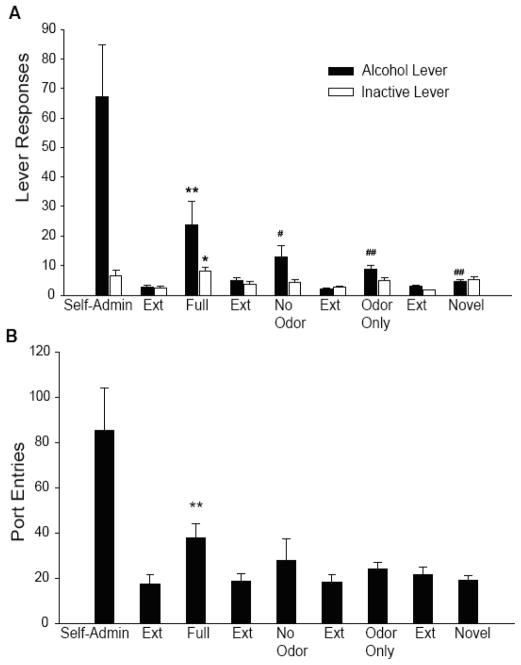
A combination of different sensory cues is typically used to create distinct contexts for training and extinction, prompting the question of whether rats process this combination as a whole or attend to especially salient single cues. Olfactory stimuli, which may receive heightened attention by rodents, are prominent features of the contexts we use; however, olfactory cues are potent discriminative stimuli for reinstatement of alcohol-seeking in their own right (c.f., [41–44]). To determine if the reinstatement observed using the ABA model requires the presence of the full configuration of cues, or just the olfactory cue alone, we tested rats in the presence of the full context or in a novel context with the odor that formed part of Context A. This experiment revealed that response reinstatement is not supported by the olfactory cue alone when subjects have been trained within a multimodal context. As seen in Fig. (3A), responding is greater when subjects are exposed to the Full Context after extinction in a different context, than when exposed to the olfactory cue alone, presented in a novel context. Interestingly, port-checking behavior also increased selectively in response to the Full Context (Fig. 3B). The converse experiment, removing the olfactory cue from the context and testing for reinstatement also revealed a diminished reinstatement, seen in Fig. (3) as the No Odor condition compared with Full Context. Together, these findings indicate that the total configuration of contextual stimuli and not a single stimulus alone is used to distinguish between the self-administration and extinction environments. Of course, when an olfactory cue is used alone as a discriminative stimulus to define the conditions under which alcohol is and is not available, as in the studies by Weiss and colleagues, that olfactory cue can act as a reliable trigger for reinstatement of alcohol-seeking (c.f., [41–44]).
Fig. (3). Context-dependent reinstatement of alcohol-seeking depends upon the full configuration of stimuli that comprise the context.
A) Mean (+/− S.E.M.) alcohol lever and inactive lever presses during the final 5 days of self-administration (Self-Admin) and during four different reinstatement tests including the full configuration of stimuli of the ethanol context (Full Context), the alcohol context without the olfactory stimulus (No Odor), the olfactory stimulus alone presented in a novel context (Odor Only), and the same Novel context without the olfactory stimulus. The mean responding in the extinction context the last day of extinction before each test is also depicted. No alcohol was delivered during the reinstatement tests, and the order of reinstatement tests was counter-balanced across subjects. N=6. **P<0.001, *P<0.05 as compared to Extinction responding; ##P<0.005, #P<0.05 as compared to reinstatement in the full context. B) Mean (+/−) S.E.M. entries into the alcohol port by the same subjects as in Fig. (4A). Interestingly, there is a significant increase in port entries only during a reinstatement test with the Full Context. **P<0.005, as compared to extinction responding. Adapted from [31] with permission.
In summary, context-dependent reinstatement of alcohol-seeking can be reliably induced in rodents. Our research suggests that contexts can reinstate behavior across repeated tests sessions. Failure to observe reinstatement in a novel context (Context C) suggests that memory of the alcohol context plays an important role in reinstatement. In addition, the complete configuration of the prior alcohol training context appears to be the most potent signal for relapse. These observations made using animal models may have translational applicability for use in clinical research on human alcoholics and alcohol-dependent individuals. Further preclinical research on the neurobiology underlying context-dependent relapse to alcohol-seeking can be well-informed by studies conducted using animal models of context-dependent reinstatement for other drugs of abuse, for which important advances have been made [21, 38].
CONTEXTUAL MODULATION OF CUE-INDUCED REINSTATEMENT OF INSTRUMENTAL RESPONDING
Reinstatement is also triggered by discrete alcohol-associated cues, as has been determined in studies that do not alter the context during extinction [41, 42, 44–61]. However, the cue-alcohol pairing is experienced in an environmental context, raising the question of whether reinstatement triggered by a drug-paired cue is modulated by that context. Indeed, many studies on context-dependent reinstatement for alcohol or other drugs of abuse include a response-contingent cue that is concomitant with or immediately preceding drug delivery [27–29, 34, 38, 62–64]; in these studies, the response-contingent cue is presented in both the alcohol and the extinction contexts, as well as at test in the alcohol context.
The assumption is that at test the context modulates the instrumental responding supported by the conditioned reinforcing properties of the cue. We investigated this hypothesis in C57Bl/6 mice, by manipulating the presence of the alcohol-associated discrete cue at test. In this study, a brief response-contingent tone-light cue was presented along with the alcohol reinforcer following lever-press responses. This self-administration training occurred in a distinctive context. Extinction was conducted in a second distinctive context, during which both the cue and the alcohol were withheld. Mice were then tested for response reinstatement using three different procedures: placement into the alcohol training context, placement into the alcohol context with response-contingent cue presentation, and placement into the extinction context with response-contingent cue presentation. The results showed that placement into the alcohol training context triggered response reinstatement even if the cue was not presented. There was also a strong trend (p=0.06) for cue presentation in the extinction context to trigger response reinstatement. But the largest reinstatement effect (i.e., most lever presses) was obtained when the cue was presented in the alcohol context; responding in the context + cue condition was almost double that seen in either of the other two conditions. These findings suggest that the most effective trigger for relapse may not be the conditioned discrete cue per se, but the experience of that cue in a relevant context [33].
We further explored the interaction between context and cues using a procedure in which priming aliquots of alcohol were delivered to extinguished rats in either the extinction or the alcohol training context. In this study, rats were trained to lever-press for alcohol in one context and extinguished in a different context; response-contingent cues were not part of the training. We then tested whether an alcohol prime (a few oral reinforcers delivered at the beginning of the test session) would reinstate responding at the alcohol lever, and whether that reinstatement was greater in the context in which alcohol had been experienced. Interestingly, the cuing power of a few drops of alcohol was apparent in the alcohol context, but there was no significant reinstatement produced by the alcohol prime when delivered in the extinction context [32]. This result may represent another example of the modulation of a response to an alcohol-predictive cue by the context; in this case, the sight, smell and taste of alcohol all may serve as predictive stimuli for the resultant pharmacological effects of alcohol. This model may be interesting to explore further since alcoholics often relapse after consumption of “the first drink” [65].
ANIMAL MODELS OF CONTEXT-DEPENDENT ALCOHOL-SEEKING: THE NEED FOR MORE SPECIFIC MODELS
The findings discussed above suggest that responding supported by an alcohol-associated cue is strongly modulated by the context in which that cue is experienced. However, it appears that performance of the operant response itself can also be modulated by the context. Thus, it is difficult to ascertain which conditioned association is modulated by the subsequent return to the alcohol self-administration context since multiple associations can influence behavior at test (response-outcome, stimulus-outcome, etc.). Alcohol operant self-administration, perhaps even more than intravenous drug self-administration models, is dependent upon multiple behavioral responses as rats must perform the operant response and then locomote to the reward delivery port and consume the alcohol; alcohol-paired cues in such a procedure could impact multiple behaviors, including acting as a conditioned reinforcer for the operant response and stimulating the port-entry response to collect reward. Hence, investigations into the neural underpinnings of context-dependent alcohol-seeking in the operant self-administration procedure are not straightforward.
To more directly study the effect of the alcohol context on alcohol-seeking triggered by an alcohol-predictive cue, we developed a behavioral model termed Pavlovian cue-induced alcohol-seeking, in which rats are trained in a distinctive context to discriminate between one auditory stimulus that is consistently paired with oral alcohol delivery (conditioned stimulus; CS+) and a second stimulus that is never paired with alcohol (CS−). Conditioning is measured by heightened entries during the CS+ into the alcohol delivery port. Conditioned responding to the cues is then extinguished following placement into a second unique context. Finally, to test the modulation of alcohol-seeking by context, rats are exposed to the CS+ and CS− without alcohol in the prior training context, which causes renewal of port-entries to the CS+ [39]. In this procedure, paired presentations of the stimulus and drug occur in a response-independent manner. Although rats must approach the fluid port to consume alcohol, the delivery of alcohol is never dependent upon port-entry responses. By eliminating the response-contingent relationship between behavior and cue/drug delivery this model enables us to investigate the impact of context explicitly on conditioned behavioral responding to an alcohol cue. Further, the addition of a CS− provides an important within-subject control for behavioral and neural manipulations of alcohol-seeking.
Using this model we have found that conducting extinction in multiple contexts, rather than just a single distinct extinction context, greatly reduced the renewal (reinstatement) of responding to the alcohol cue after placement back into the alcohol training context [39]. Identification of behavioral mechanisms, such as this one, that may reduce the strong effects of context on alcohol-seeking could provide new avenues of treatment for humans, potentially resulting in enhanced efficacy of cue-reactivity treatment in humans.
NEURAL BASIS FOR CONTEXT-DEPENDENT ALCOHOL-SEEKING
In addition to exploring new behavioral mechanisms that may be applicable to the treatment of humans, investigation of the underlying neurobiological mechanisms is also of great interest. Understanding the biology may further assist treatment efforts; for example, by providing new ideas for pharmacological treatments. Below, we summarize the published findings to date, focusing primarily on data obtained using models of context-dependent reinstatement of alcohol-seeking.
Opioidergic Transmission
The opioid antagonist, naltrexone, is one of the few approved drugs for use in humans to reduce drinking. More patients remain abstinent when taking naltrexone than those taking placebo [66], and naltrexone reduces reported urge to drink following exposure to alcohol-related cues [67]. Therefore studies of its behavioral and neural mechanisms are of interest. We tested the effects of systemic naltrexone administration on context-dependent reinstatement and found an attenuation of responding when rats were placed into the alcohol context after extinction in a different context [31]. Using a similar procedure, Marinelli et al. [29] also found that systemic administration of naltrexone reduced context-dependent reinstatement of alcohol-seeking, as well as reducing neural activation of the basolateral amygdala and the CA3 region of the hippocampus, as indicated by a reduction in c-fos mRNA. Naltrexone acts at multiple opioid receptor subtypes. In a subsequent study, the effects of the mu opioid receptor selective antagonist, D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP), and the delta opioid receptor antagonist, naltrindole, were also found to reduce context-dependent reinstatement of alcohol-seeking [28]. The studies overall suggest that some aspect of the test process, perhaps the placement into the alcohol-associated context, induces the release of endogenous opioids that act upon delta and mu opioid receptors to facilitate context-dependent reinstatement. The location(s) of these receptors has yet to be determined. In recent unpublished experiments, we have found that local infusion of naltrexone into the ventral tegmental area in the midbrain attenuates context-dependent reinstatement; the same effect was observed after local down-regulation of the expression of the mu opioid receptor using viral-mediated RNA interference (Gill and Janak, unpublished findings). These findings suggest that mu opioid receptors in the ventral tegmental area may regulate context-dependent reinstatement. Interestingly, a likely effect of activation of mu opiod receptors in the ventral tegmental area is an increase in dopamine release at some afferent sites, including the nucleus accumbens (NAc), a ventral forebrain structure that has been widely implicated in relapse [68–70]. Recent evidence confirms a role for NAc dopamine in context-dependent reinstatement, as described below.
Dopaminergic Transmission
The NAc receives a strong dopaminergic projection from the ventral tegmental area, and dopamine released from these projections acts upon dopamine receptors of the D1 and D2 families located on postsynaptic targets (D1 and D2) or on dopaminergic (D2) or glutamatergic presynaptic terminals (D2)[71]. Interestingly, conditioned increases in NAc dopamine in animals trained to self-administer alcohol have been reported. Microdialysis studies have demonstrated that placement in the alcohol self-administration chamber increases dopamine levels in the NAc [72]. This finding suggests that anticipation of the opportunity to self-administer alcohol, signaled by placement within the alcohol-associated environment, activates dopamine systems. Interestingly, these increases in dopamine have been observed in the NAc shell and core subregions, and are higher prior to self-administration of an alcohol-sucrose combination than prior to self-administration of sucrose alone [73]. The pre-session increases in NAc dopamine levels are normalized during reinstatement elicited by a discriminative stimulus that signals alcohol availability [41], revealing a possible functional relationship between dopamine release and reinstatement behavior. Both D1 and D2 antagonists block reinstatement of responding for alcohol that is induced by a discriminative stimulus [43]. Like the context itself, presentation of this discriminative stimulus is constant throughout the alcohol self-administration sessions. D1 and D2 antagonists also block context-dependent reinstatement of responding for cocaine [62], and infusion of a D1 receptor antagonist into the NAc shell blocks context-dependent reinstatement of responding for heroin [74]. Hamlin and colleagues [36] showed that systemic administration of the D1 antagonist, SCH 23390, blocks context-dependent reinstatement of responding for alcohol. Taken together, these results suggest that blockade of dopamine transmission within the NAc should inhibit context-dependent reinstatement of alcohol-seeking.
We recently found that dopamine neurotransmission in both the core and shell is required for context-dependent reinstatement of responding for alcohol [34] (Fig. 4). In this study a response-contingent cue was present throughout training and extinction and testing. One interpretation of our findings, consistent with the literature, is that the impairment observed after D1 antagonist infusion into the NAc core results from inhibition of cue-supported conditioned responding, whereas the impairment observed after D1 antagonist infusion into the NAc shell reflects a specific role of the shell in modulating contextual influences on conditioned responding [34, 74]. These hypotheses await confirmation following further experimentation.
Fig. (4). Dopamine in the nucleus accumbens in required for context-dependent alcohol-seeking.

Panels depict Mean (± S.E.M) responses on the active lever at the end of extinction (white bar) and after microinfusion of the D1 dopamine receptor antagonist, SCH 23390, into the nucleus accumbens (NAC; filled bars). Comparison of responding during extinction and responding after vehicle (0) reveals the context-dependent reinstatement effect; comparison of the different doses from 0 to 0.6 reveals the dose-dependent effect of SCH 23390 on this reinstatement. The top panel depicts combined data from the core and shell subregions as there were no statistical differences in the effects of SCH 23390 after infusion into the subregions (Overall NAC). Data from each subregion are shown in the smaller figures, below. At test, active lever responding produced the light-noise stimulus without alcohol. ^^ p<0.01, ^ p<0.05 significantly different from saline. Adapted from [34].
Nucleus Accumbens (NAc)
The expression of context-dependent reinstatement depends on the recognition of the alcohol-associated context (perhaps mediated by the hippocampus, see below), and then the production of alcohol-seeking behavior. The NAc is described as a limbic-motor interface [75] meaning that the NAc allows for signals from limbic regions, such as the hippocampus, to affect ongoing behavioral output. Hence the NAc is well situated to translate context-stimulated drive into alcohol-seeking. The NAc is implicated in numerous conditioned effects of appetitive stimuli (c.f., [76, 77]), and is a critical component of the brain circuitry that underlies the rewarding and reinforcing effects of drugs, including alcohol [78–82]. The NAc is divided into two subregions, the core and the shell, that have distinct afferent and efferent connections. The NAc core is required for reinstatement of responding for cocaine and other intravenously-delivered drugs [69, 83–85] and for cue- and alcohol-induced reinstatement of responding for alcohol [86]. Recently, Fuchs and colleagues [87] reported that reversible inactivation of both the NAc shell and core attenuated context-dependent reinstatement of responding for cocaine. Using the context-dependent reinstatement of instrumental responding model with an oral alcohol prime, described above, we found that NAc core inactivation blocked the increased responding seen at test, while NAc shell inactivation prevented the normal extinction of instrumental responding observed at test [32]. Thus, as with other triggers for reinstatement, such as cues and priming injections, the ability of contexts to promote reinstatement of extinguished operant responses for alcohol may require the NAc core, probably due to its role in initiating the alcohol-seeking behavioral response.
Other studies have implicated the NAc shell. Context-dependent reinstatement of operant responding for a beer solution is associated with increased c-Fos protein in the NAc shell, especially its more ventral aspect [35, 36]. Interestingly, the NAc shell neurons that are involved may depend upon their efferent target: a pathway from the NAc shell to the lateral hypothalamus was activated by context-dependent reinstatement, as identified using a double labeling technique that looked for the intersection of neurons labeled with c-Fos after context-dependent reinstatement that were also labeled by a retrograde tracer from the lateral hypothalamus to the NAc shell [37]. In addition, using the same technique, a pathway from the medial NAc shell to the lateral hypothalamus was activated by placement into the extinction context [37]. Hence there appear to be distinct roles for NAc shell regions in inhibiting and reinstating responding, which may make the interpretation of drug microinjection studies more difficult given the potential difficulty in selectively affecting the medial or ventral shell after microinfusion of a drug solution. This observation may contribute to the lack of reinstatement impairment we observed after shell inactivation in our previous study [32]. Alternatively, the effect of shell inactivation may differ following delivery of alcohol primes, a more likely explanation given that we have seen significant impairment in context dependent reinstatement for alcohol following the administration of dopamine D1 receptor antagonists into either the core or shell [34]. In addition, a recent study using the Pavlovian cue-induced alcohol-seeking model found that inactivation of both the NAc core and shell blocked context-dependent renewal of responding to an alcohol predictive cue, whereas only NAc core inactivation blocked cue-induced alcohol-seeking when the effect of the cue was tested in the extinction context [40]. These findings are consistent with a general role for the core in conditioned responding and of the shell in effects of context.
Hippocampus
The expression of context-dependent reinstatement depends on the recognition of the alcohol-associated context, and hence on retrieval of information about that context from memory. That the hippocampus provides contextual information is supported by the demonstration of location-specific firing by hippocampus CA1 neurons, called ‘place cells’ [88]. Different neurons are selective for different locations within a given environment. Location-specific firing is acquired by rats shortly after placement in a new environment, is specific for a given environment, and is maintained over time, providing a possible neural readout for the creation of a memory of a specific place. Hippocampal CA1 pyramidal neurons project to the neighboring subicular region of the hippocampus [89]; these cells also process location-specific information [90, 91]. A majority of subicular cells recorded in the behaving rat show location-specific firing during performance of a spatial task reinforced by rewarding medial forebrain bundle stimulation [92].
Other studies support the notion that the hippocampus is necessary for contextual memory. Reliance of context-dependent conditioning on the hippocampus has been demonstrated within the fear-conditioning model [93–96]. Further, rats fear-conditioned in one environment and extinguished in a different environment show robust reinstatement of freezing to the tone conditioned stimulus when returned to the training context, and this effect is blocked by pre-test inactivation of the hippocampus [97, 98]. These studies are analogous to the context-dependent reinstatement model used in the present studies. In summary, the hippocampus is known to encode contextual information (place cells) and is required for retrieval of contextual information in several conditioning paradigms [97–99]. Therefore, it is likely that the hippocampus is required for expression of context-dependent reinstatement of responding for alcohol.
Of relevance to the present topic, context-specific c-Fos expression is induced in the hippocampal CA3 region by placement in a behavioral chamber in which rats received beer for 21 days [100]. The CA3 region of the dorsal hippocampus also is reported to be activated by the alcohol context at test within a context-dependent reinstatement procedure, as indicated by c-Fos protein [36] and mRNA [29]. In agreement with this idea, reversible inactivation of the dorsal hippocampus attenuates context-dependent reinstatement of responding for cocaine [101]. These findings suggest that activation of the hippocampus is dependent on the subject’s association between the rewarding substance and the environment. The dorsal hippocampus projects relatively sparsely to the NAc, and additionally can provide information to the NAc indirectly via its inputs to the prefrontal cortex. The ventral hippocampus projects strongly to the basolateral amygdala [102] and the NAc shell [103, 104], placing it in a prime position to influence NAc output directly via the shell or indirectly via the basolateral amygdala-to-NAc core pathway. In this regard, it is noteworthy that electrical stimulation of the ventral hippocampus reinstates responding for i.v. cocaine [105], In summary, it is of great interest to determine the role of the ventral hippocampus/subiculum in context-dependent reinstatement of alcohol-seeking. This region has a major projection to the NAc, potentially providing direct information about contexts to this motor interface. It also projects to the basolateral amygdala, which may allow it to modulate the recall/retrieval of context-dependent cue-drug associations that then could access the NAc circuitry through the strong excitatory projections from the basolateral amygdala to the NAc.
Basolateral Amygdala
The basolateral complex of the amygdala is important for assigning motivational significance to sensory stimuli, specifically in the association of discrete sensory cues with rewarding or aversive stimuli1 [76, 106, 107]. Lesions and inactivation of the basolateral amygdala, as well as NMDA receptor antagonist infusions into this region, impair learning and performance dependent upon cue-reward associations for both natural rewards, such as food [108–111], and drug rewards, such as cocaine [112]. In addition, neurons within the basolateral amygdala encode the cue within a cue-induced reinstatement model [113], and inactivations/lesions of this region attenuate cue-induced reinstatement for intravenous drugs [112, 114, 115]. Recently, it was reported that inactivation of the basolateral amygdala in mice with muscimol blocked context-dependent renewal of responding to a shock-predictive cue [116]. Taken together, because the BLA is critical for cue-reward learning and cue-induced reinstatement for rewarding stimuli, it is likely that the basolateral amygdala contributes to context-dependent reinstatement of responding by providing information about conditioned stimuli within the alcohol and extinction contexts. Although this hypothesis remains to be tested, increases in neuronal activation in the basolateral amygdala as indexed by c-fos mRNA or protein have been observed upon context-dependent reinstatement for alcohol [29, 36]. In addition, inactivation of the BLA blocks context-dependent reinstatement of responding for intravenous cocaine [101, 117].
Lateral Hypothalamus and Paraventricular Thalamus
The use of immunohistochemical techniques has allowed for a relatively unbiased approach towards identifying neural circuitry involved in context-dependent relapse. Hamlin et al. [35, 36] and Marchant et al. [37] have employed this approach, and in so doing, have identified regions that would have been expected based on previous research (i.e., the NAc, hippocampus, and basolateral amygdala) as well as regions not usually emphasized in studies of the neurobiology of relapse to alcohol or even other drugs of abuse. This research group then went on to confirm a role for these regions using lesions/inactivations. The lateral hypothalamus was one region identified first by its significant c-Fos activation during reinstatement [36]; this finding was confirmed by showing that temporary inactivation of this region by microinfusion of GABAergic agonists blocked context-dependent reinstatement of operant responding for a beer solution [37]. The lateral hypothalamus may be involved in alcohol-seeking behavior due to its demonstrated role in food and water consumption [118, 119]. However, the efferents from the lateral hypothalamus that may be critical in alcohol seeking are unknown at this time; this is an interesting yet challenging task given the diversity of brain regions to which the lateral hypothalamus projects.
Similarly, in a recent paper, a role for the paraventricular thalamus was likewise identified on the basis of a significant increase in cells double labeled with c-Fos and a retrograde tracer from the NAc shell after reinstatement in the alcohol-associated context [35]. Excitotoxic lesions of the paraventricular thalamus were then shown to block context-dependent reinstatement of responding for beer [35]. The role of the paraventricular thalamus in behavior is not entirely clear, but it is sometimes considered to contribute to arousal and attention [120]. Interestingly, the paraventricular thalamus projects to the NAc shell while the NAc shell projects to the lateral hypothalamus, which itself projects to the paraventricular thalamus. Overall, a clever approach, combining retrograde tracers and a measure of neuronal activation, such as the immediate early gene c-Fos, has led to the identification of two regions that to date have received little attention, but that may play a major role in context-dependent reinstatement of responding for alcohol.
PROPOSED NEURAL CIRCUITRY IMPLICATED IN CONTEXT-DEPENDENT REINSTATEMENT OF RESPONDING FOR ALCOHOL
In summary, as depicted in Fig. (5), a circuit with the NAc as a critical integrative node is likely to underlie context-dependent reinstatement for alcohol, with the hippocampus providing requisite information on the discrimination between the alcohol and extinction contexts, either directly through projections to the NAc shell, or indirectly through projections to the basolateral amygdala or, perhaps, other regions such as the prefrontal cortex (not shown in the diagram). Excitatory input to the NAc shell from the paraventricular thalamus may provide an additional signal that combines with the hippocampal input to activate the neurons within the shell. The NAc core is viewed as a critical final common pathway for the alcohol-seeking response, and the influence of conditioned stimuli on alcohol-seeking may arise from projections from the basolateral amygdala to the NAc core. In this conception, the role of the shell is seen as modulating the output of the core, perhaps via projections from the shell to the ventral tegmental area that in turn influence projections back to the core [121, 122]. Presumably, the excitatory input to the NAc from regions such as the hippocampus, thalamus, and basolateral amygdala is modulated by dopaminergic input from the ventral tegmental area such that dopamine release at sites receiving strong excitatory input facilitates that input, resulting in the selection of a specific population of neurons within the NAc that contributes to the production of the alcohol-seeking behavioral response. Recent findings discussed above indicate that the lateral hypothalamus may be a critical component in this circuitry, but it is unclear as of yet how output from the lateral hypothalamus may contribute to alcohol seeking.
Fig. (5). Proposed neural circuitry implicated in context-dependent reinstatement of responding for alcohol.
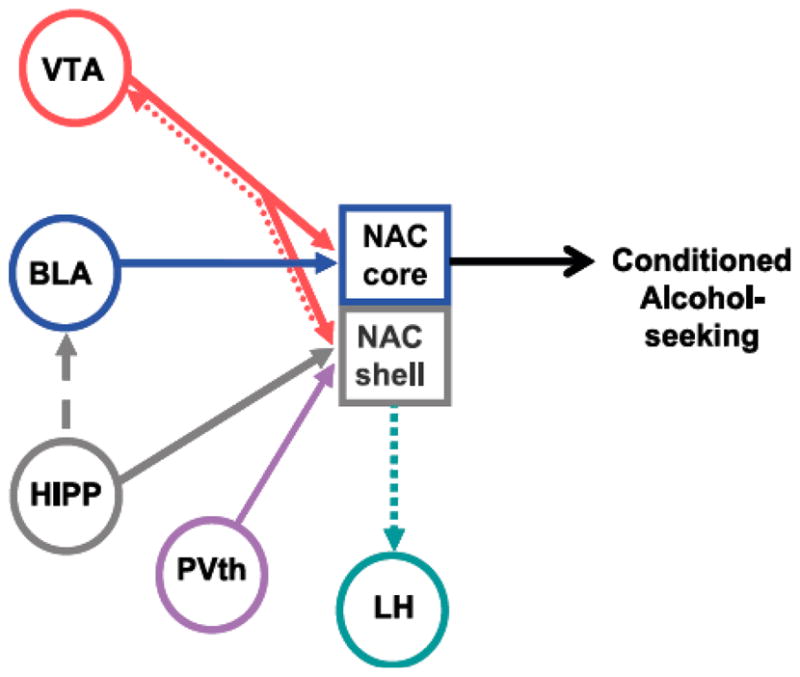
In this highly simplified schematic, the NAc core is proposed as a requisite final common output for alcohol-seeking. The influence of context is proposed to have access to the NAc core through multiple routes: First, through direct projections from the hippocampus (HIPP) to the NAc shell; the NAc shell may then influence the NAc core via projections from the ventral tegmental area (VTA) that influence dopamine projections back to the NAc core. Second, contextual information from the hippocampus (HIPP) may be carried by projections that terminate at the cell body regions of other excitatory inputs to the NAc core, such as the basolateral amygdala (BLA), where this contextual information may modulate the processing of information regarding conditioned stimuli. The information provided by the paraventricular thalamus (PVth) is not clear at the present time. Likewise, the role of NAc shell projections to the lateral hypothalamus (LH) in alcohol-seeking is not defined. See text for relevant research findings and further discussion.
CONCLUSION
Contexts in which alcohol is experienced become potent triggers for relapse, possibly due to their role in the retrieval of learned associations that may influence alcohol-seeking behavior. The ability of contexts to influence relapse may be an especially difficult problem when treating patients since the effects of treatments that seek to extinguish responses to alcohol-associated cues may not transfer to contexts outside of the extinction context. These effects of alcohol and extinction contexts can be reliably modeled in rodents allowing for the exploration of the critical neural and behavioral mechanisms that may contribute to the effect of context on relapse. Thus far, an interconnected circuit of a number of limbic regions, with the NAc as a critical node of convergence for these regions, has tentatively been identified, using a variety of techniques including immunohistochemistry, temporary inactivation, and permanent lesion. A critical role for dopaminergic and opioidergic endogenous neurotransmitter systems is also supported. Not surprisingly, there is considerable overlap in the neural mechanisms identified thus far for context-dependent reinstatement of alcohol-seeking and for context-dependent reinstatement of responding for other drugs of abuse (e.g. [38, 69, 101, 117]). It is expected that the next few years will reveal continued advances in our understanding of the neurobiology underlying context-dependent reinstatement of alcohol-seeking.
Acknowledgments
Supported by NIH grant AA014925 to PHJ.
Footnotes
This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
References
- 1.Wikler A. Conditioning factors in opiate addiction and relapse. In: Wilner DI, Kassenbaum GG, editors. Narcotics. New York: McGraw-Hill; 1965. pp. 85–100. [Google Scholar]
- 2.Laberg JC. What is presented, and what prevented, in cue exposure and response prevention with alcohol dependent subjects? Addict Behav. 1990;15:367–86. doi: 10.1016/0306-4603(90)90046-z. [DOI] [PubMed] [Google Scholar]
- 3.Marlatt GA. Cue exposure and relapse prevention in the treatment of addictive behaviors. Addict Behav. 1990;15:395–9. doi: 10.1016/0306-4603(90)90048-3. [DOI] [PubMed] [Google Scholar]
- 4.Rohsenow DJ, Niaura RS, Childress AR, Abrams DB, Monti PM. Cue reactivity in addictive behaviors: theoretical and treatment implications. Int J Addict. 1990;25:957–93. doi: 10.3109/10826089109071030. [DOI] [PubMed] [Google Scholar]
- 5.Hammersley R. Cue exposure and learning theory. Addict Behav. 1992;17:297–300. doi: 10.1016/0306-4603(92)90035-t. [DOI] [PubMed] [Google Scholar]
- 6.Drummond DC, Glautier S. A controlled trial of cue exposure treatment in alcohol dependence. J Consult Clin Psychol. 1994;62:809–17. doi: 10.1037//0022-006x.62.4.809. [DOI] [PubMed] [Google Scholar]
- 7.Drummond DC. What does cue-reactivity have to offer clinical research? Addiction. 2000;95(Suppl 2):S129–44. doi: 10.1080/09652140050111708. [DOI] [PubMed] [Google Scholar]
- 8.Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–40. [PubMed] [Google Scholar]
- 9.Monti PM, Rohsenow DJ, Rubonis AV, et al. Alcohol cue reactivity: effects of detoxification and extended exposure. J Stud Alcohol. 1993;54:235–45. doi: 10.15288/jsa.1993.54.235. [DOI] [PubMed] [Google Scholar]
- 10.Drummond DC, Cooper T, Glautier SP. Conditioned learning in alcohol dependence: implications for cue exposure treatment. Br J Addict. 1990;85:725–43. doi: 10.1111/j.1360-0443.1990.tb01685.x. [DOI] [PubMed] [Google Scholar]
- 11.Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–67. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- 12.Conklin CA. Environments as cues to smoke: implications for human extinction-based research and treatment. Exp Clin Psychopharmacol. 2006;14:12–9. doi: 10.1037/1064-1297.14.1.12. [DOI] [PubMed] [Google Scholar]
- 13.Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–86. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 14.Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–94. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 15.Pavlov IP. Conditioned Reflexes. London: Oxford University Press; 1927. [Google Scholar]
- 16.Collins BN, Brandon TH. Effects of extinction context and retrieval cues on alcohol cue reactivity among nonalcoholic drinkers. J Consult Clin Psychol. 2002;70:390–7. [PubMed] [Google Scholar]
- 17.MacKillop J, Lisman SA. Effects of a context shift and multiple context extinction on reactivity to alcohol cues. Exp Clin Psychopharmacol. 2008;16:322–31. doi: 10.1037/a0012686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stasiewicz PR, Brandon TH, Bradizza CM. Effects of extinction context and retrieval cues on renewal of alcohol-cue reactivity among alcohol-dependent outpatients. Psychol Addict Behav. 2007;21:244–8. doi: 10.1037/0893-164X.21.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epstein DH, Preston KL. The reinstatement model and relapse prevention: a clinical perspective. Psychopharmacology (Berl) 2003;168:31–41. doi: 10.1007/s00213-003-1470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 22.Weiss F, Ciccocioppo R, Parsons LH, et al. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- 23.See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–29. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- 24.Le AD, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacol Ther. 2002;94:137–56. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- 25.Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Yan Y, Nabeshima T. Mouse model of relapse to the abuse of drugs: procedural considerations and characterizations. Behav Brain Res. 2009;196:1–10. doi: 10.1016/j.bbr.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–73. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- 28.Marinelli PW, Funk D, Harding S, Li Z, Juzytsch W, Le AD. Roles of opioid receptor subtypes in mediating alcohol-seeking induced by discrete cues and context. Eur J Neurosci. 2009;30:671–8. doi: 10.1111/j.1460-9568.2009.06851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marinelli PW, Funk D, Juzytsch W, Li Z, Le AD. Effects of opioid receptor blockade on the renewal of alcohol seeking induced by context: relationship to c-fos mRNA expression. Eur J Neurosci. 2007;26:2815–23. doi: 10.1111/j.1460-9568.2007.05898.x. [DOI] [PubMed] [Google Scholar]
- 30.Zironi I, Burattini C, Aicardi G, Janak PH. Context is a trigger for relapse to alcohol. Behav Brain Res. 2006;167:150–5. doi: 10.1016/j.bbr.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Burattini C, Gill TM, Aicardi G, Janak PH. The ethanol self-administration context as a reinstatement cue: Acute effects of naltrexone. Neuroscience. 2006;139:877–87. doi: 10.1016/j.neuroscience.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Chaudhri N, Sahuque LL, Cone JJ, Janak PH. Reinstated ethanol-seeking in rats is modulated by environmental context and requires the nucleus accumbens core. Eur J Neurosci. 2008;28:2288–98. doi: 10.1111/j.1460-9568.2008.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsiang MT, Janak PH. Alcohol seeking in C57BL/6 mice induced by conditioned cues and contexts in the extinction-reinstatement model. Alcohol. 2006;38:81–8. doi: 10.1016/j.alcohol.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Chaudhri N, Sahuque LL, Janak PH. Ethanol seeking triggered by environmental context is attenuated by blocking dopamine D1 receptors in the nucleus accumbens core and shell in rats. Psychopharmacology (Berl) 2009;207:303–14. doi: 10.1007/s00213-009-1657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamlin AS, Clemens KJ, Choi EA, McNally GP. Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur J Neurosci. 2009;29:802–12. doi: 10.1111/j.1460-9568.2009.06623.x. [DOI] [PubMed] [Google Scholar]
- 36.Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146:525–36. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 37.Marchant NJ, Hamlin AS, McNally GP. Lateral hypothalamus is required for context-induced reinstatement of extinguished reward seeking. J Neurosci. 2009;29:1331–42. doi: 10.1523/JNEUROSCI.5194-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–43. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaudhri N, Sahuque LL, Janak PH. Context-induced relapse of conditioned behavioral responding to ethanol cues in rats. Biol Psychiatry. 2008;64:203–10. doi: 10.1016/j.biopsych.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaudhri N, Sahuque LL, Schairer WW, Janak PH. Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology. 2010;35:783–91. doi: 10.1038/npp.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katner SN, Weiss F. Ethanol-associated olfactory stimuli reinstate ethanol-seeking behavior after extinction and modify extracellular dopamine levels in the nucleus accumbens. Alcohol Clin Exp Res. 1999;23:1751–60. [PubMed] [Google Scholar]
- 42.Katner SN, Magalong JG, Weiss F. Reinstatement of alcohol-seeking behavior by drug-associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology. 1999;20:471–9. doi: 10.1016/S0893-133X(98)00084-0. [DOI] [PubMed] [Google Scholar]
- 43.Liu X, Weiss F. Reversal of ethanol-seeking behavior by D1 and D2 antagonists in an animal model of relapse: differences in antagonist potency in previously ethanol-dependent vs nondependent rats. J Pharmacol Exp Ther. 2002;300:882–9. doi: 10.1124/jpet.300.3.882. [DOI] [PubMed] [Google Scholar]
- 44.Dayas CV, Liu X, Simms JA, Weiss F. Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biol Psychiatry. 2007;61:979–89. doi: 10.1016/j.biopsych.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schroeder JP, Spanos M, Stevenson JR, Besheer J, Salling M, Hodge CW. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP. Neuropharmacology. 2008;55:546–54. doi: 10.1016/j.neuropharm.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maccioni P, Bienkowski P, Carai MA, Gessa GL, Colombo G. Baclofen attenuates cue-induced reinstatement of alcohol-seeking behavior in Sardinian alcohol-preferring (sP) rats. Drug Alcohol Depend. 2008;95:284–7. doi: 10.1016/j.drugalcdep.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci. 2006;26:9967–74. doi: 10.1523/JNEUROSCI.2384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bachteler D, Economidou D, Danysz W, Ciccocioppo R, Spanagel R. The effects of acamprosate and neramexane on cue-induced reinstatement of ethanol-seeking behavior in rat. Neuropsychopharmacology. 2005;30:1104–10. doi: 10.1038/sj.npp.1300657. [DOI] [PubMed] [Google Scholar]
- 49.Vengeliene V, Leonardi-Essmann F, Perreau-Lenz S, et al. The dopamine D3 receptor plays an essential role in alcohol-seeking and relapse. FASEB J. 2006;20:2223–33. doi: 10.1096/fj.06-6110com. [DOI] [PubMed] [Google Scholar]
- 50.Sanchis-Segura C, Borchardt T, Vengeliene V, et al. Involvement of the AMPA receptor GluR-C subunit in alcohol-seeking behavior and relapse. J Neurosci. 2006;26:1231–8. doi: 10.1523/JNEUROSCI.4237-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bienkowski P, Koros E, Kostowski W, Bogucka-Bonikowska A. Reinstatement of ethanol seeking in rats: behavioral analysis. Pharmacol Biochem Behav. 2000;66:123–8. doi: 10.1016/s0091-3057(00)00194-5. [DOI] [PubMed] [Google Scholar]
- 52.Bienkowski P, Kostowski W, Koros E. The role of drug-paired stimuli in extinction and reinstatement of ethanol-seeking behaviour in the rat. Eur J Pharmacol. 1999;374:315–9. doi: 10.1016/s0014-2999(99)00244-7. [DOI] [PubMed] [Google Scholar]
- 53.Nie H, Janak PH. Comparison of reinstatement of ethanol- and sucrose-seeking by conditioned stimuli and priming injections of allopregnanolone after extinction in rats. Psychopharmacology (Berl) 2003;168:222–8. doi: 10.1007/s00213-003-1468-0. [DOI] [PubMed] [Google Scholar]
- 54.Ciccocioppo R, Martin-Fardon R, Weiss F. Effect of selective blockade of mu1 or delta opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology. 2002;27:391–9. doi: 10.1016/S0893-133X(02)00302-0. [DOI] [PubMed] [Google Scholar]
- 55.Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F. Reinstatement of ethanol-seeking behavior by drug cues following single vs multiple ethanol intoxication in the rat: effects of naltrexone. Psychopharmacology (Berl) 2003;168:208–15. doi: 10.1007/s00213-002-1380-z. [DOI] [PubMed] [Google Scholar]
- 56.Ciccocioppo R, Economidou D, Fedeli A, et al. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology (Berl) 2004;172:170–8. doi: 10.1007/s00213-003-1645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ciccocioppo R, Angeletti S, Weiss F. Long-lasting resistance to extinction of response reinstatement induced by ethanol-related stimuli: role of genetic ethanol preference. Alcohol Clin Exp Res. 2001;25:1414–9. doi: 10.1097/00000374-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 58.Backstrom P, Hyytia P. Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. Eur J Pharmacol. 2005;528:110–8. doi: 10.1016/j.ejphar.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 59.Backstrom P, Hyytia P. Ionotropic glutamate receptor antagonists modulate cue-induced reinstatement of ethanol-seeking behavior. Alcohol Clin Exp Res. 2004;28:558–65. doi: 10.1097/01.alc.0000122101.13164.21. [DOI] [PubMed] [Google Scholar]
- 60.Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63:152–7. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 61.Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–9. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crombag HS, Grimm JW, Shaham Y. Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology. 2002;27:1006–15. doi: 10.1016/S0893-133X(02)00356-1. [DOI] [PubMed] [Google Scholar]
- 63.Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31:2197–209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–30. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ludwig AM, Wikler A, Stark LH. The first drink: psychobiological aspects of craving. Arch Gen Psychiatry. 1974;30:539–47. doi: 10.1001/archpsyc.1974.01760100093015. [DOI] [PubMed] [Google Scholar]
- 66.O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry. 1992;49:881–7. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- 67.Monti PM, Rohsenow DJ, Hutchison KE, et al. Naltrexone’s effect on cue-elicited craving among alcoholics in treatment. Alcohol Clin Exp Res. 1999;23:1386–94. [PubMed] [Google Scholar]
- 68.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–63. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 70.Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- 71.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 72.Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–8. [PubMed] [Google Scholar]
- 73.Howard EC, Schier CJ, Wetzel JS, Gonzales RA. The dopamine response in the nucleus accumbens core-shell border differs from that in the core and shell during operant ethanol self-administration. Alcohol Clin Exp Res. 2009;33:1355–65. doi: 10.1111/j.1530-0277.2009.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–63. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mogenson GJ, Yang CR. The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. Adv Exp Med Biol. 1991;295:267–90. doi: 10.1007/978-1-4757-0145-6_14. [DOI] [PubMed] [Google Scholar]
- 76.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–52. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 77.Blaiss CA, Janak PH. The nucleus accumbens core and shell are critical for the expression, but not the consolidation, of Pavlovian conditioned approach. Behav Brain Res. 2009;200:22–32. doi: 10.1016/j.bbr.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–76. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- 79.Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2002;78:625–36. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- 80.Weiss F, Porrino LJ. Behavioral neurobiology of alcohol addiction: recent advances and challenges. J Neurosci. 2002;22:3332–7. doi: 10.1523/JNEUROSCI.22-09-03332.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101:129–52. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- 82.Rewal M, Jurd R, Gill TM, He DY, Ron D, Janak PH. Alpha4-containing GABAA receptors in the nucleus accumbens mediate moderate intake of alcohol. J Neurosci. 2009;29:543–9. doi: 10.1523/JNEUROSCI.3199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- 84.Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 85.Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154:261–74. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bowers MS, Hopf FW, Chou JK, et al. Nucleus accumbens AGS3 expression drives ethanol seeking through Gbg. Proc Natl Acad Sci U S A. 2008;105:12533–8. doi: 10.1073/pnas.0706999105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2008;200:545–56. doi: 10.1007/s00213-008-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.O’Keefe J. A review of the hippocampal place cells. Prog Neurobiol. 1979;13:419–39. doi: 10.1016/0301-0082(79)90005-4. [DOI] [PubMed] [Google Scholar]
- 89.Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- 90.Phillips RG, Eichenbaum H. Comparison of ventral subicular and hippocampal neuron spatial firing patterns in complex and simplified environments. Behav Neurosci. 1998;112:707–13. doi: 10.1037//0735-7044.112.3.707. [DOI] [PubMed] [Google Scholar]
- 91.Sharp PE, Green C. Spatial correlates of firing patterns of single cells in the subiculum of the freely moving rat. J Neurosci. 1994;14:2339–56. doi: 10.1523/JNEUROSCI.14-04-02339.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martin PD, Ono T. Effects of reward anticipation, reward presentation, and spatial parameters on the firing of single neurons recorded in the subiculum and nucleus accumbens of freely moving rats. Behav Brain Res. 2000;116:23–38. doi: 10.1016/s0166-4328(00)00249-7. [DOI] [PubMed] [Google Scholar]
- 93.Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, Silva AJ. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav Neurosci. 1998;112:863–74. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
- 94.Selden NR, Everitt BJ, Jarrard LE, Robbins TW. Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience. 1991;42:335–50. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- 95.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 96.Sanders MJ, Wiltgen BJ, Fanselow MS. The place of the hippocampus in fear conditioning. Eur J Pharmacol. 2003;463:217–23. doi: 10.1016/s0014-2999(03)01283-4. [DOI] [PubMed] [Google Scholar]
- 97.Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci. 2001;21:1720–6. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16:174–82. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- 99.Riedel G, Micheau J, Lam AG, et al. Reversible neural inactivation reveals hippocampal participation in several memory processes. Nat Neurosci. 1999;2:898–905. doi: 10.1038/13202. [DOI] [PubMed] [Google Scholar]
- 100.Topple AN, Hunt GE, McGregor IS. Possible neural substrates of beer-craving in rats. Neurosci Lett. 1998;252:99–102. doi: 10.1016/s0304-3940(98)00574-6. [DOI] [PubMed] [Google Scholar]
- 101.Fuchs RA, Evans KA, Ledford CC, et al. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- 102.McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 103.Furtak SC, Wei SM, Agster KL, Burwell RD. Functional neuroanatomy of the parahippocampal region in the rat: the perirhinal and postrhinal cortices. Hippocampus. 2007;17:709–22. doi: 10.1002/hipo.20314. [DOI] [PubMed] [Google Scholar]
- 104.Groenewegen HJ, Wright CI, Beijer AV. The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog Brain Res. 1996;107:485–511. doi: 10.1016/s0079-6123(08)61883-x. [DOI] [PubMed] [Google Scholar]
- 105.Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–8. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- 106.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–52. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 107.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–38. doi: 10.1023/a:1025048802629. [DOI] [PubMed] [Google Scholar]
- 108.Hatfield T, Han JS, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. J Neurosci. 1996;16:5256–65. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tye KM, Stuber GD, de Ridder B, Bonci A, Janak PH. Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning. Nature. 2008;453:1253–7. doi: 10.1038/nature06963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–61. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ishikawa A, Ambroggi F, Nicola SM, Fields HL. Contributions of the amygdala and medial prefrontal cortex to incentive cue responding. Neuroscience. 2008;155:573–84. doi: 10.1016/j.neuroscience.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kruzich PJ, See RE. Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. J Neurosci. 2001;21:RC155. doi: 10.1523/JNEUROSCI.21-14-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tye KM, Janak PH. Amygdala neurons differentially encode motivation and reinforcement. J Neurosci. 2007;27:3937–45. doi: 10.1523/JNEUROSCI.5281-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fuchs RA, See RE. Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology (Berl) 2002;160:425–33. doi: 10.1007/s00213-001-0997-7. [DOI] [PubMed] [Google Scholar]
- 115.Yun IA, Fields HL. Basolateral amygdala lesions impair both cue- and cocaine-induced reinstatement in animals trained on a discriminative stimulus task. Neuroscience. 2003;121:747–57. doi: 10.1016/s0306-4522(03)00531-1. [DOI] [PubMed] [Google Scholar]
- 116.Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–6. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- 117.Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–98. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- 118.Dhillo WS. Appetite regulation: an overview. Thyroid. 2007;17:433–45. doi: 10.1089/thy.2007.0018. [DOI] [PubMed] [Google Scholar]
- 119.Leibowitz SF. Overconsumption of dietary fat and alcohol: mechanisms involving lipids and hypothalamic peptides. Physiol Behav. 2007;91:513–21. doi: 10.1016/j.physbeh.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Rev. 2002;39:107–40. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 121.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–82. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]