Abstract
An improved and very fast algorithm dealing with the extraction of vessels in three-dimensional imaging is described. The approach is based on geometrical moments and a local cylindrical approximation. A robust estimation of vessel and background intensity levels, position, orientation and diameter of the vessels with adaptive control of key parameters, is provided during vessel tracking. Experimental results are presented for lower limb arteries in multidetector CT scanner.
Keywords: Algorithms; Artifacts; Data Display; Femoral Artery; radiography; Humans; Ischemia; Leg; blood supply; Models, Cardiovascular; Popliteal Artery; radiography; Radiographic Image Enhancement; methods; Thrombosis; radiography; Tomography, X-Ray Computed; methods
INTRODUCTION
The ability to depict the vascular network is of critical importance for the diagnosis of vascular abnormalities, pre-operative planning as well as per-operative actions. The recent advances provided by 3-D Magnetic Resonance Angiography (MRA) and multidetector CT scanner ( CTA) leads to new perspectives for objective and quantitative analysis of vascular networks as far as efficient algorithms are designed. These imaging modalities lead to very large data sets (several hundreds of megabytes) from which the vessels must be extracted under very strict time constraints to face the clinical requirements. Most of the current segmentation methods bring already relevant delineations of vessels but with too high computation time. They rely on intensity-based methods [1], generalized cylinder approximations [2], multiscale [3,4] and skeletonization [5] schemes, and deformable model approaches [6,8] applied on successive 2-D slices or on volume data.
The challenge is therefore to reach similar performance in few seconds on a standard PC platform. The present work is a step towards this objective. It relies on a moment-based algorithm [9] allowing, after interactive selection of 3-D seed points, to track the vessels and to locally estimate both the diameter and the orientation. Several improvements are brought including an efficient initialisation, iterative positioning of the computation window, adaptive control of its size, and multi-background handling. These new features will be described after a short summary of the initial algorithm. Some preliminary results are then displayed on CTA data.
METHOD
Using the assumption that a vessel can be locally approximated by a cylinder, it has been shown in [9] that the three-dimensional geometrical moments of up to order 2 lead to analytical expressions of the orientation of the cylinder axis, the diameter when two homogeneous regions are considered, and the estimated mean gray levels inside and around the vessel. The discrete computation of these moments is performed on the set of elementary volumes (e.g. voxels) arranged on an isotropic grid using a spherical window, associated to a subvoxel decomposition. This way, the computation is reduced to mask convolutions. The tracking is performed by shifting the computation window according to the estimated orientation, and the stopping rule is stated as a decision between two hypotheses, presence or absence of the vessel. This solution has been validated on simulated data, for different signal to noise ratio and window sizes. As far as the window is larger than the vessel, diameter precision in the parameters estimation and robustness to noise have been demonstrated. The method was successfully applied to low field MRA data [9]. The core of the method remains unchanged and only the improvements will be reported here which allow to deal with some specific features of CT images, for instance the presence of more than two tissues.
Algorithm initialisation
In order to avoid the precise pointing of successive seed points located inside the several vascular branches of interest, a region is now defined by the user, in any of the three orthogonal cross-sections. Maxima of intensities, corresponding to potential vessels, are then looked for in this region.
Window centering
Either centered or non-centered moments can be used for the parameter computation but the estimations are better when using the former expressions. The orientation estimated at a given position of the window does not guarantee a precise positioning at the next point along the vessel in particular when highly curved vessels are tracked. An iterative centering is therefore performed by computing the first order moments and then, the other cylinder parameters are estimated.
Window size adjustment
The size of the vessels can widely vary (from tens to a very few voxels) when large segments of the body are explored or when diseases are concerned. In addition, the analytical diameter formulation as reported in [9] depends directly on the window size as well as the precision of the estimations mentioned above. The window size is then adjusted during the vessel tracking according to the previously computed width.
Multi-region handling
In contrast with MRA, CT based imaging modalities display other organs and tissues, and the enhanced vessels in CTA can be over or close to them, in other words the computation window can include more than two regions. This problem is solved by exploring the vessel surroundings at each step along equally spaced directions in a plane perpendicular to the currently estimated vessel axis. The profiles along these directions are determined and the highest level, outside the vessel, is used to set the background value to almost zero.
Re-initialisation procedure
The tracking can be stopped when the model does not fit with the local shape ( bifurcation, high curvature of small vessels), in presence of artefacts or very severe stenosis. A cubic box, twice the size of the computation window, centred on the endpoint, is then open and an intersection of vessels with the faces of the box is searched. The previously visited voxels are marked in order to avoid a potential backtrack.
RESULTS AND DISCUSSION
This new version of the approach has been evaluated on three CTA data sets of the lower limbs acquired on a multidetector spiral CT ( Siemens Somatom 4 plus Volume Zoom, Erlangen). A partial view of a volume image (see Figure 1) representing the lower limbs is depicted using the Maximum Intensity Projection (MIP) technique which emphasizes the difficulties for the physicians to discriminate between the enhanced vascular network and the bone structures. Three orthogonal views of the extracted vessels are shown where the rendering is performed by using the local cylinder surface approximations. The computation time is less than one second on a PC Pentium III 450Mhertz.
Figure 1.
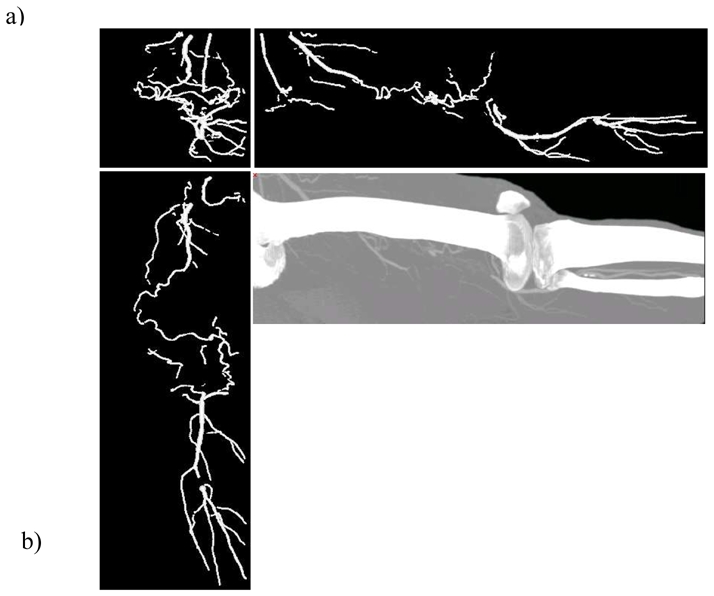
CTA images of left (a) and right (b) limbs (raw data : 200x200 pixels, 12 bits resolution, 400 slices, 600 after a linear interpolation). In (a) the vessels have been extracted from the interactive selection of 3 ROI and 3 points, in (b) from 2 ROI and 2 points.
It can be seen that the most significant vessels are extracted. This patient suffering from lower limb ischemia has a bilateral thrombosis of the femoral superficial artery. This can be assessed by the study of the axial slices and of the MIP. There is an excellent correlation with the results obtained with our method: the thrombosed vessel is not visible and the collateral vessels coming from the deep femoral artery are nicely depicted. Furthermore, the popliteal artery and the run off are clearly visible. Some discontinuities are recovered and low contrasted and stenosed segments as well as curved vessel segments are preserved.
These results point out the capability of the algorithm to detect and track in CTA the vascular network. It provides a structured description of the vessels (e.g. the axes) and an estimation of the main local features. The extracted data can be displayed using surface or volume rendering techniques and easily analyzed in clinical setting.
However, additional issues have to be considered. The model is no more valid in forking cases. The conducted experiments have shown that, if the tracking is not affected, the parameters are biased ( the diameter for instance is overestimated). A solution may rely on a two steps analysis with a detection of the deviation with regard to the model and, then, through the use of the search box, an approximation by means of two cylinders.
Another concern is related to the presence of calcifications. The high contrast they bring in the immediate surrounding of the vessel exemplifies the need, already mentioned, to take into account multiple regions. But in this case, if the vessel tracking can be ensured, a reliable estimation of the cylinder parameters cannot be directly expected. The approach under study consists to segment the calcifications detected during the tracking through region growing, to subtract them from the volume image and to repeat the parameter estimation of the model.
CONCLUSION
An algorithm has been proposed to extract the vessels from volume image and the experiments conducted on CTA data have been reported and discussed. The emphasis has been put on a critical feature in image analysis, the time computation. This issue will be more and more important in the future with the avaibility of very large data sets in order to answer to the clinical constraints in daily practice.
Acknowledgments
The authors are grateful to Brigitte Le Bruno and Jean Claude Galisson, from Siemens France, for supporting this work and for providing the image data, and to Johanne Bezy who helped in managing the data sets.
References
- 1.Shiffman S, Rubin GD, Napel S. Medical Image segmentation using analysis of isolable-contour maps. IEEE Trans Medical Imaging. 2000;19:11. doi: 10.1109/42.896782. to appear. [DOI] [PubMed] [Google Scholar]
- 2.Verdonck B, Bloch I, Maitre H. Accurate segmentation of blood vessels from 3-D medical images. Proc Intern Conf Image Processing. 1996:311–314. [Google Scholar]
- 3.Sato Y, Nakajima S, Shiraga N, Atsumi H, Yoshida S, Koller T, Gerig G, Kikinis R. 3-D multi-scale line filter for segmentation and visualization of curvilinear structures in medical images. Medical Image Analysis. 1998;2(2):143–168. doi: 10.1016/s1361-8415(98)80009-1. [DOI] [PubMed] [Google Scholar]
- 4.Summers P, Bhalero AH, Hawkes DJ. Multiresolution, model-based segmentation of MR angiograms. J Magnetic Resonance Imaging. 1997;7(6):950–957. doi: 10.1002/jmri.1880070603. [DOI] [PubMed] [Google Scholar]
- 5.Wilson DL, Noble JA. Segmentation of cerebral vessels and aneurysms from MR angiography data. Proc Intern Conf Image Processing. 1997:423–428. [Google Scholar]
- 6.Hernandez-Hoyos M, Orkisz M, Roux JP, Douek P. Inertia-based vessel axis extraction and stenosis quantification in 3-D MRA images. Proc Computer assisted Radiology and surgery. 1999:189–193. [Google Scholar]
- 7.McInerney T, Terzopoulos D. Topology adaptive snakes. Medical Image Analysis. 2000;4(2):73–92. doi: 10.1016/s1361-8415(00)00008-6. [DOI] [PubMed] [Google Scholar]
- 8.Lorigo LM, Faugeras OD, Grimson WEL, Keriven R, Kikinis R, Nabavi A, Westin C-F. CURVES: Curve evolution for vessel segmentation. Medical Image Analysis. 2000 doi: 10.1016/s1361-8415(01)00040-8. to appear. [DOI] [PubMed] [Google Scholar]
- 9.Reuzé P, Coatrieux JL, Luo LM, Dillenseger JL. A 3-D moment based approach for blood vessel detection and quantification in MRA. Technology and Health Care. 1993;1:181–188. doi: 10.3233/THC-1993-1209. [DOI] [PubMed] [Google Scholar]


