Abstract
In this paper we posit a domain-general principle that may account for the improvement that is observed in several aspects of perceptual development over the first years of life. Development during this time frame is characterized by a process of perceptual narrowing, whereby the discrimination of perceptual information is broadly tuned at first, and then narrows to more selective levels with experience. This process appears to cut across both the visual and auditory modalities, and may reflect the development of a common neural architecture.
Keywords: Perceptual Narrowing, Face Perception, Speech Perception
Introduction
A question that permeates the study of developmental psychology is whether there are domain-general principles that generalize across perceptual domains. For example, previous theories of the development of speech and face perception posit similar developmental mechanisms that account for changes in the discriminatory abilities of infants. Indeed, there is a recent accumulation of evidence from research on the development of phonemic perception (e.g. Kuhl et al., 2006), face perception (e.g. Pascalis, de Haan, & Nelson, 2002; Pascalis et al., 2005), intersensory perception (Lewkowicz & Ghazanfar, 2006), visual language discrimination (Weikum et al., in press) and the discrimination of culturally specific musical rhythms (Hannon & Trehub, 2005a, 2005b) that collectively suggest the emergence of a domain-general principle of perceptual development. Across the aforementioned areas of development, perceptual discrimination is tuned to environmentally relevant distinctions by 9-12 months of age, whereas the discrimination of environmentally irrelevant, or less frequently encountered, distinctions declines. Importantly, this does not suggest a developmental regression, but a progression towards becoming more efficient at perceiving and processing salient compared to less salient environmental input.
An integration of the behavioral evidence with recent studies examining the neural correlates of perceptual narrowing suggest that in the absence of behavioral discrimination there is evidence of neural discrimination (Rivera-Gaxiola, Silva-Pereyra, & Kuhl, 2005; Scott, Shannon, & Nelson, 2006). Thus the brain may maintain the ability to perceptually discriminate but this discrimination is not manifest in behavior unless recruited though experience. We will start by reviewing the evidence for this account. We then review the research examining the neural correlates of perceptual narrowing. Finally, we discuss a connection between auditory and visual environmental input and posit neural mechanisms that may mediate perceptual narrowing.
Evidence of Perceptual Narrowing Across Domains
Development of phonemic perception
The ontogeny of phonemic perception is characterized by a decline in the discrimination of speech sounds not present in one's native language. Thus, native language experience functions to tune, maintain, and facilitate the perception of phonemes. Currently, there is substantial evidence suggesting that this specialization occurs between 6- and 12-months of age and is dependent on perceptual exposure to native, relative to non-native phonemic contrasts (Werker & Tees, 2005). For example, there is a decline in the ability of English speaking infants to distinguish Hindi the phonemic contrasts /Ta/ and /ta/ from 6 to 12 months of age (Werker & Tees, 1984). In addition to experience-driven maintenance of native contrasts, there is also evidence of facilitation of these native contrasts (Kuhl, et al., 2006). Thus, evidence suggests phonemic contrast detection in the native language is not simply maintained over development, but that there is environmental facilitation of the contrasts infants have experienced.
Development of face perception
It has been hypothesized that the ability to discriminate between faces not consistently present in the infant's early environment declines between 6- and 9-months of age (Nelson, 2001). Support for this hypothesis comes from recent reports looking at the development of discriminatory abilities when infants view monkey faces, and faces of an unfamiliar race (Kelly et al., in press; Pascalis, de Haan, & Nelson, 2002; Pascalis, et al., 2005). In these experiments, a visual-paired comparison (VPC) task, which capitalizes on infant's preference for novelty, was used to infer perceptual discrimination. In the VPC task, participants are first familiarized to a single stimulus. Then the familiarized stimulus is paired with an unfamiliar or novel stimulus. Using this task, 6-month-old infants exhibit novelty preferences for monkey faces, however neither 9-month olds nor adults show evidence of such discrimination (Pascalis, et al., 2002). These data suggest that younger infants exhibit a more broadly tuned face processing system, which can discriminate amongst exemplars within multiple categories of faces (e.g. two human or two monkey faces) and that this system becomes more specific (e.g. discrimination only amongst human faces) and less flexible with age. A very similar pattern of results is observed for ‘other-race’ faces, suggesting that 3- and 6-month-old infants exhibit discrimination of faces from other ethnic groups, whereas 9-month-olds only show discrimination for faces within their own ethnic group (Kelly et al., in press)
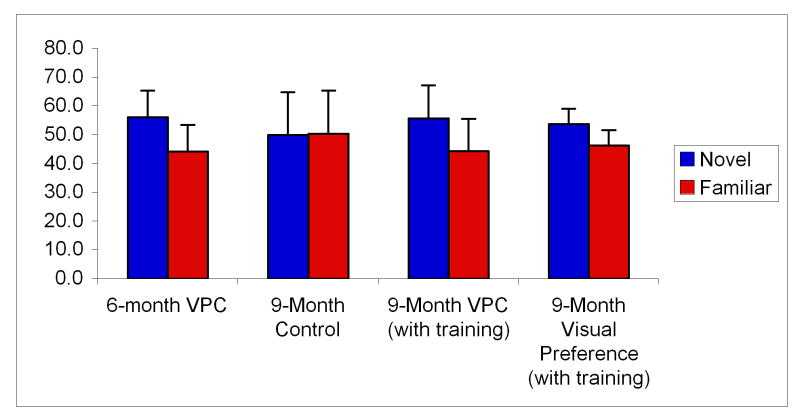
To investigate further the role of experience in the tuning of face processing, Pascalis et al. (2005) gave 6-month-old infants 3 months of perceptual experience with monkey faces by sending them each home with monkey face picture book (See Figure 1). After this experience, 9-month olds were found to have maintained the ability to discriminate monkey faces (see Figure 2). Importantly, discrimination was not only found for faces the infants learned in the picture book, but also discrimination generalized to previously unseen monkey faces.
Figure 1.
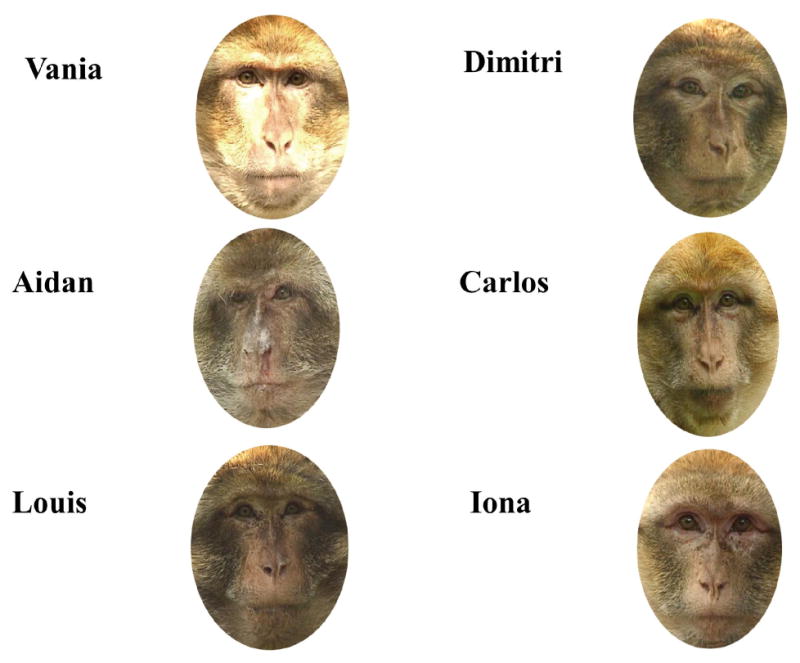
Examples of trained monkey faces and their corresponding names.
Figure 2.
Infant pre- and post-training preferential looking times.
Overall, similar to the development of phonemic perception, face processing abilities develop based on the types of faces present in the visual environment. To date, there have not been any studies examining whether the discrimination of commonly experienced types of faces is better characterized as being maintained or facilitated. For example, does the decline of discrimination in one category (i.e. monkey faces) occur in the service of better discrimination in another category (i.e. human faces) or is the ability to discriminate commonly experienced faces simply maintained over development? If perceptual narrowing cuts across perceptual domains, one would expect to obtain similar facilitation effects for face processing, as found for speech processing.
Development of intersensory perception
To test whether perceptual narrowing operates in the development of intersensory perception, Lewkowicz and Ghazanfar (2006) investigated infants' ability to match face and voice pairings across development. Infants aged 4-, 6-, 8-, and 10-months viewed two side-by-side images of vocalizing monkey faces while listening to one of the two vocalizations. Results reveal that only the younger infants were able to correctly match the vocalization they heard with the monkey face making that vocalization, as indicated by a looking preference for the sound-face match. However, by 8-10 months of age, no sound-face match was made.
Evidence in support of the multi-modal nature of perceptual narrowing also comes from research investigating the development of language discrimination using silently presented articulations (Weikum, et al., in press). Weikum and colleagues report that English and French monolingual 4- and 6-month-old infants are able to discriminate both French and English silent articulations. However, after 8-months of age, only French-English bilingual infants discriminate these same silent articulations. Combined, these investigations provide direct evidence that perceptual narrowing operates in the development of intersensory perception in addition to previous unimodal visual and auditory perception.
Development of the perception of musical rhythm
The development of the perception and discrimination of musical rhythms has also been studied in infants and adults (Hannon & Trehub, 2005a; 2005b). North American music often differs in metrical structure from other musical cultures (e.g. metrical structure in North American music contains simple duration ratios whereas metrical structure in Bulgarian music contains complex duration ratios). Whereas, North American adults can identify violations in music containing simple, but not complex metrical structure, Bulgarian adults can identify violations in music containing complex structure (Hannon & Trehub, 2005a). Furthermore, 6-month-old North American infants can also discriminate violations in both complex- and simple-meter contrasts (Hannon & Trehub, 2005a; 2005b). It is not until 12-months of age that the detection of violations in musical rhythm becomes adult-like (Hannon & Trehub, 2005b). However, if 12-month-olds are briefly exposed to music containing complex metrical structure, they are able to later perceive these violations. This same exposure in adults does not influence the ability to detect complex metrical violations (Hannon & Trehub, 2005b).
Neural Correlates of Perceptual Narrowing
The studies discussed above have recently been complemented by investigations into the neural correlates of perceptual narrowing. However, this research is limited to the development of speech and face perception, and to the recording of event-related potentials (ERPs). ERPs reflect the activity of simultaneously active populations of neurons that can be recorded from the scalps surface. This activity results in electrical signals that can be recorded from the scalp in response to the presentation of images or sounds. Therefore, ERPs can be used to index neural activity correlated with specific cognitive processes.
Recently, ERPs to native and non-native speech contrasts were examined by following 7-month old infants until they were 11-months of age (Rivera-Gaxiola, Silva-Pereyra & Kuhl, 2005). Overall, this investigation suggests that the electrophysiological response discriminates both the native and non-native speech contrasts at 7-months, but at 11-months, two groups of 11-month-old infants emerged. Both of these 11-month -groups exhibited ERP discrimination of non-native contrasts, but one group discriminated early in processing (150-250 ms after stimulus onset) and the other late in processing (250-550 ms). These data suggest that although previous behavioral work suggest that infants show behavioral evidence for a decline in the ability to discriminate non-native contrasts, neural differentiation of this ability is maintained at 11-months of age.
To date, no longitudinal investigations of the neural correlates of visual perceptual narrowing have been conducted. However, cross-sectional investigations provide evidence of a gradual experience-dependent cortical specialization of face processing (e.g. Halit, de Haan, & Johnson, 2003). Comparisons of ERPs in response to human and monkey faces at different ages reveal adult-like patterns of neural activity are not present until 12-months (Halit, de Haan & Johnson, 2003).
ERPs were also recorded from typically developing 9-month-olds while they were presented with pictures of newly familiarized and unfamiliar monkey or human faces in both a frontal and profile orientation (Scott, Shannon, & Nelson, 2006). Although differential ERP responses were found between human and monkey faces, there was also evidence of neural differentiation of familiar and unfamiliar monkey faces. More specifically, as illustrated in Figure 3, the ERP amplitude of the infant P400 component differentiated amongst familiar and unfamiliar frontal and profile human faces, whereas the response to monkey faces only differentiated familiar and unfamiliar faces (not frontal and profile faces). Thus, response to human faces was more specific than the response to monkey faces, but unlike behavioral responses, ERPs did discriminate familiar and unfamiliar monkey faces. This suggests that while human faces are discriminated at a more specific level than monkey faces, there is still residual neural evidence of the ability to discriminate monkey faces.
Figure 3.
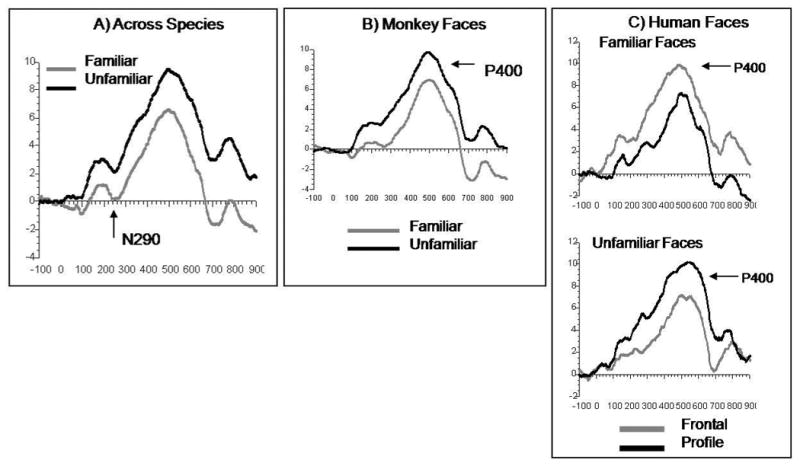
ERP results for the 9-month old P400 component. The P400 component is a postive component occurring approximately 400 milliseconds after the presentation of the stimulus. An average of electrodes A1, A2, T5, T6, O1, O2 is pictured. A) ERP response to monkey faces. The familiar monkey face (collapsed across orientations) elicited greater amplitude P400 than the unfamiliar monkey face. Frontal and profile monkey faces were not electrophysiologically distinguished. B) ERPs in response to human faces indicating more specific perceptual processing relative to monkey faces. For familiar faces, the frontal face elicits a greater P400, however for the unfamiliar face, the profile face elicits a greater P400.
Proposed Mechanisms of Perceptual Narrowing
Why do discriminatory abilities in the visual and auditory systems appear follow a common developmental trajectory? A recent study suggests that, in an immediate paired comparison task, newborns discriminate static images of their mother's face from a stranger's face if postnatal exposure to the mother's voice-face combination was available. If the infant is tested prior to seeing their mother's voice-face paired, discrimination of the mother's face was not demonstrated (Sai, 2005). This, combined with research finding perceptual narrowing when infants view silent articulations (Weikum, et al., in press), suggest that face and speech perception are linked from an early age. Neural evidence of this link comes from a Positron Emission Tomography (PET) study with infants (Tzourio-Mazoyer et al., 2002). The PET technique uses radioactively labelled substances, which are introduced into the bloodstream, to visualize neural activity. Tzouriro-Mazoyer and colleagues report adult-like brain activation, when presented with faces at 2-months of age. However, faces also activated areas that are typically devoted to language in adults, supporting the existence of an early link between the visual and auditory systems.
Another mechanism, which may mediate perceptual narrowing, is discriminatory learning occurring at the subordinate or individual level. As described above, there is behavioral evidence showing that 9-month-olds can discriminate monkey faces when infants were given experience with individually named monkey faces (Pascalis et al., 2005). Infants thus learned the faces using both the picture of the face and the corresponding name of each face. This experience not only increased their ability to later discriminate these learned faces but it also led to better discrimination of new monkey faces. One question that arises is whether experience naming these faces individually leads to a maintenance of perceptual discrimination than if, for example, each monkey was labeled at the category level (i.e. all monkey's were named “Monkey”) or if infants were trained without any labels.
This review presents an accumulation of evidence of developmental specialization in perceptual discriminatory abilities during the first year of life. This specialization corresponds to improved discriminatory efficiency for stimuli predominant in the child's environment relative to a decline in discriminating stimuli not present in the surrounding environment. What is currently not well understood is what mechanisms are responsible for this narrowing and/or maintenance/facilitation with experience. We also do not know how a decline in discriminatory abilities is represented in the brain. However, coincident with this decline, the brain is experiencing an exuberance of synaptic connections, followed by the pruning of these connections to adult levels. We hypothesize that this pruning is the neural mechanism mediating perceptual narrowing across domains, leading to entrenched discriminatory abilities. Related, Hebbian Learning, or the strengthening of neural circuits and synaptic connections with repeated use, may also be operating to increase synaptic efficacy of environmentally relevant information. This use/disuse principle of cellular learning may contribute to the observed behavioral phenomenon of perceptual narrowing. For example, as perceptual experience to particular types of human faces increases, the strength of the neural circuit responding to these types of human faces is strengthened. On the other hand, the lack of experience to monkey faces leads to neural disuse and thus inefficient processing. This may be why we do not observe behavioral, but do observe neural, discrimination of monkey faces at 9-months of age. Moreover, recent ERP evidence suggests that perceptual narrowing may not reflect the complete erasure of neural connections, making these systems flexible and ready to be reactivated at a latter period in time. The flexibility of perceptual systems to learn to discriminate new visual and auditory distinctions after the formation of neural circuitity is a likely hypothesis. However, the effects of continued disuse across the lifespan and its influence on both the behavioral and neural correlates of perceptual abilities has yet to be fully investigated.
The findings described above have implications spanning multiple fields within the area of developmental psychology, however more research is needed in order to answer some very important questions about the nature of perceptual narrowing. First, it is currently unclear whether learning to discriminate commonly experienced perceptual stimuli at 9-12 months represents maintenance or facilitation of an ability present at 6 months. Furthermore, if facilitation is occurring across multiple domains, is there competition for neural resources within each domain? In other words, can the developmental progression be characterized as the dividing up of limited resources for efficient processing? As alluded to above, what is narrowing? Is learning to individuate amongst exemplars (whether it be faces, phonemes, musical rhythms, or cross-modal matching) an important factor, or does mere exposure influence the specificity of this system? Finally, can research on perceptual narrowing lead to specific hypotheses about how neural development is responsive to environmental input? Answer these questions and others will lead to a clearer understanding of the development of perceptual abilities within and beyond the first year of life.
Acknowledgments
This work was supported in part by funds to the Lisa Scott from the James S. McDonnell foundation and Perceptual Expertise Network, by funds from both the College of Social and Behavioral Sciences and the Department of Psychology at the University of Massachusetts- Amherst, to Olivier Pascalis from the National Institutes of Health (HD46526-01), and to Charles Nelson from the National Institutes of Health (NS032976-07; MH078829).
Contributor Information
Lisa S. Scott, University of Massachusetts at Amherst.
Olivier Pascalis, University of Sheffield.
Charles A. Nelson, Harvard Medical School and Children's Hospital Boston
References
- Halit H, de Haan M, Johnson MH. Cortical specialisation for face processing: face-sensitive event-related potential components in 3-and 12-month-old infants. Neuroimage. 2003;19:1180–1193. doi: 10.1016/s1053-8119(03)00076-4. [DOI] [PubMed] [Google Scholar]
- *Hannon EE, Trehub SE. Metrical categories in infancy and adulthood. Psychological Science. 2005a;16:48–55. doi: 10.1111/j.0956-7976.2005.00779.x. [DOI] [PubMed] [Google Scholar]
- Hannon EE, Trehub SE. Tuning in to musical rhythms: Infants learn more readily than adults. Proceedings of the National Academy of Sciences. 2005b;102:12639–12643. doi: 10.1073/pnas.0504254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Ge L, Pascalis O. The other-race effect develops during infancy: Evidence of perceptual narrowing. Psychological Science. doi: 10.1111/j.1467-9280.2007.02029.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK, Stevens E, Hayashi A, Deguchi T, Kiritani S, et al. Infants show a facilitation effect for native language phonetic perception between 6 and 12 months. Developmental Science. 2006;9:F13–F21. doi: 10.1111/j.1467-7687.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- *Lewkowicz DJ, Ghazanfar AA. The decline of cross-species intersensoty perception in human infants. Proceedings of the National Academy of Sciences. 2006;103:6771–6774. doi: 10.1073/pnas.0602027103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Nelson CA. The development and neural bases of face recognition. Infant and Child Development. 2001;10:3–18. [Google Scholar]
- Pascalis O, de Haan M, Nelson CA. Is face processing species-specific during the first year of life? Science. 2002;296:1321–1323. doi: 10.1126/science.1070223. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Scott LS, Kelly DJ, Shannon RW, Nicholson E, Coleman M, et al. Plasticity of face processing in infancy. Proceedings of the National Academy of Sciences. 2005;102:5297–5300. doi: 10.1073/pnas.0406627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Gaxiola M, Silva-Pereyra J, Kuhl PK. Brain potentials to native and non-native speech contrasts in 7- and 11-month-old American infants. Developmental Science. 2005;8:162–172. doi: 10.1111/j.1467-7687.2005.00403.x. [DOI] [PubMed] [Google Scholar]
- Sai FZ. The role of the mother's voice in developing mother's face preference: Evidence for intermodal perception at birth. Infant and Child Development. 2005;14:29–50. [Google Scholar]
- Scott LS, Shannon RW, Nelson CA. Neural correlates of human and monkey face processing by 9-month-old infants. Infancy. 2006;10:171–186. doi: 10.1207/s15327078in1002_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, De Schonen S, Crivello F, Reutter B, Aujard Y, et al. Neural correlates of woman face processing by 2-month-old infants. NeuroImage. 2002;15:454–461. doi: 10.1006/nimg.2001.0979. [DOI] [PubMed] [Google Scholar]
- Weikum WM, Vouloumanos A, Navarra J, Soto-Faraco S, Sebastian-Galles N, Werker J. Visual Language Discrimination in Infancy. Science. doi: 10.1126/science.1137686. in press. [DOI] [PubMed] [Google Scholar]
- Werker JF, Tees RC. Cross-language speech perception: Evidence for perceptual reorganization during the first year of life. Infant Behavioral and Development. 1984;7:49–63. [Google Scholar]
- Werker JF, Tees RT. Speech perception as a window for understanding plasticity and commitment of language systems of the brain. Developmental. 2005 doi: 10.1002/dev.20060. [DOI] [PubMed] [Google Scholar]
Recommended Readings
- Hannon EE, Trehub SE. 2005b See reference list. [Google Scholar]
- Lewkowicz DJ, Ghazanfar AA. 2006 doi: 10.1073/pnas.0602027103. See reference list. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA. 2001 See reference list. [Google Scholar]
- Werker JF, Tees RT. 2005 See reference list. [Google Scholar]