Abstract
Photodynamic therapy (PDT) with low light fluence rate has rarely been studied in protocols that use short drug–light intervals and thus deliver illumination while plasma concentrations of photosensitizer are high, creating a prominent vascular response. In this study, the effects of light fluence rate on PDT response were investigated using motexafin lutetium (10 mg/kg) in combination with 730 nm light and a 180-min drug–light interval. At 180 min, the plasma level of photosensitizer was 5.7 ng/μl compared to 3.1 ng/mg in RIF tumor, and PDT-mediated vascular effects were confirmed by a spasmodic decrease in blood flow during illumination. Light delivery at 25 mW/cm2 significantly improved long-term tumor responses over that at 75 mW/cm2. This effect could not be attributed to oxygen conservation at low fluence rate, because 25 mW/cm2 PDT provided little benefit to tumor hemoglobin oxygen saturation. However, 25 mW/cm2 PDT did prolong the duration of ischemic insult during illumination and was correspondingly associated with greater decreases in perfusion immediately after PDT, followed by smaller increases in total hemoglobin concentration in the hours after PDT. Increases in blood volume suggest blood pooling from suboptimal vascular damage; thus the smaller increases after 25 mW/cm2 PDT provide evidence of more widespread vascular damage, which was accompanied by greater decreases in clonogenic survival. Further study of low fluence rate as a means to improve responses to PDT under conditions designed to predominantly damage vasculature is warranted.
INTRODUCTION
The benefits of low fluence rate in photodynamic therapy (PDT) have been found to follow the oxygen-conserving nature of this treatment. Namely, slower rates of photochemical oxygen consumption favor maintenance of tumor oxygenation during PDT, and this in turn has been associated with increases in direct tumor cell killing and vascular damage (1). However, the effects of lower fluence rate have been studied mainly with photosensitizers and drug–light intervals designed to favor drug clearance from the bloodstream prior to illumination. Studies using Photofrin (24-h drug–light interval), Photoclor [HPPH, 2-(1-hexyloxyethyl)-2-devinyl pyropheophorbide-a; 24-h drug–light interval], and Foscan (mTHPC, meta-tetrahydroxyphenylchlorin; 24–72-h drug–light interval) in a variety of tumor models and normal tissues have found a cytotoxic benefit to either low-fluence-rate illumination (2–5) or an alternative approach of hyperfractionating illumination, which allows for recovery of oxygenation during the interruptions in light delivery (6, 7). Similar advantages have been found in studies with the photosensitizer proto-porphyrin IX (PpIX), which is produced cellularly after administration of aminolevulinic acid (ALA) (8, 9). However, less is known about the effects of fluence rate under PDT conditions that target the tumor vasculature.
It has been hypothesized that low fluence rate would have little effect on response to vascular-targeted PDT since damage would be localized to the blood vessels, which are assumed to be well supplied with oxygen, even during PDT (8). Others have also predicted that PDT of the vasculature would be unaffected by fluence rate (10). However, the study of Iinuma et al. (8) suggested that low fluence rate increased response to vascular-targeted PDT using benzoporphyrin derivative (BPD). This drug was used at a 60-min drug–light interval to damage the vasculature, which was expected to be independent of fluence rate, but actually demonstrated a greater fluence-rate-dependent cytotoxicity than PDT with the more cellular ALA-induced PpIX. The authors concluded that the fluence-rate dependence of the BPD treatment stemmed primarily from drug that had localized to the tumor cells during the 60-min interval prior to illumination. More recently, other more specific studies of PDT-created vascular damage using the chick chorioallantoic membrane showed that in BPD-PDT up to 11% of damage to blood vessels could be accounted for by fluence-rate effects. In PDT with the photosensitizer motexafin lutetium, up to 74% of damage to the blood vessels under certain conditions could be accounted for by the effects of fluence rate (11).
One potential explanation for a benefit of low fluence rate in PDT of the vasculature could be that, contrary to early expectations, PDT can deplete tumor oxygenation even in tissue immediately adjacent to the vascular endothelium (12). Accordingly, low fluence rate could allow for better oxygenation of and thus greater damage to the vascular wall. Because the aforementioned study used Photofrin at a 24-h drug–light interval, which favors photosensitizer accumulation in the tumor cells compared to the vascular space, a more pronounced effect may be expected with a drug that is more concentrated in the bloodstream at the time of illumination.
Another potential explanation for a benefit of low fluence rate in vascular-damaging PDT can be gleaned from recent studies using the photosensitizer HPPH and a 24-h drug–light interval in which the longer treatment times associated with low-fluence rate provided an advantage to the vascular component of the PDT response (13). This suggests that although longer treatments may not be clinically desirable in conjunction with current light delivery technologies, they may improve PDT responses under conditions that cause predominantly vascular damage.
In the present study, we evaluated the fluence-rate dependence of response to PDT using the photosensitizer motexafin lutetium (14–16) under conditions that produce a predominantly vascular response. We examined the potential contribution of fluence-rate effects on vascular oxygenation and considered the effects of extended treatment time at low fluence rate. The lower fluence rate improved PDT response, and the extended treatment time at the lower rate provided a more thorough vascular shutdown measured as greater reductions in tumor perfusion.
MATERIALS AND METHODS
Tumor Model and PDT
RIF tumors were propagated on 9–11-week-old C3H mice (NCI-Frederick, Frederick, MD) by the intradermal injection of 3 × 105 cells on the shoulder of the animal, unless otherwise indicated. Approximately 7–9 days later, the tumor and the surrounding area were depilated (Nair), and the animals received 10 mg/kg motexafin lutetium (gift from Pharmacyclics, Inc, Sunnyvale CA) via tail vein injection; 180 min later a 1.0–1.1-cm-diameter field centered on the tumor was illuminated. Tumors ≤100 mm3 in volume were used in tumor response studies, while tumors up to 200 mm3 were used in studies requiring tumor excision or monitoring. Illumination to doses indicated in the text was performed using a 730-nm diode laser (provided by Pharmacyclics; manufactured by Diomed Inc., Andover, MA). Light was delivered through microlens-tipped fibers (Cardio-Focus, Norton, MA), and laser output was measured with a power meter (Coherent, Auburn, CA) and adjusted to deliver the prescribed irradiance at the tissue surface. During PDT, mice were anesthetized by inhalation of isoflurane in medical air delivered through a nosecone (VetEquip anesthesia machine, Pleasanton, CA). Animal studies were reviewed and approved by the University of Pennsylvania Institutional Animal Care and Use Committee, and animal facilities are accredited by the American Association for the Accreditation of Laboratory Animal Care (AAALAC).
Photosensitizer Concentration
Tumor-bearing animals were administered motexafin lutetium (10 mg/kg, i.v.), and 180 min later tumor or blood was collected. Animals from which tumors were collected were perfused with saline to remove blood prior to collection of the tissue. Blood was collected by cardiac puncture into tubes with sodium heparin, from which plasma was isolated. Extraction of motexafin lutetium involved homogenization of 10 mg of tumor (100 μl of blood) in 400 μl (1 ml) of 24 mM phosphate buffer, followed by addition of 400 μl (1 ml) each of chloroform and methanol. After centrifugation, the chloroform layer was collected and run on the spectrofluorometer as described previously (17).
Tumor Blood Flow
Tumor blood flow was measured using a previously described and validated diffuse correlation spectroscopy (DCS) instrument (18), which measures rapid temporal fluctuations of transmitted light (785 nm) through tissues and then uses the autocorrelation functions associated with these fluctuations to extract information about the motion of tissue scatterers, in this case red blood cells. The DCS probe consists of nine source and four detector fibers that are arranged in a circular pattern to cover the entire tumor surface, thereby allowing monitoring without the need to move the probe. The probe is focused onto the tumor surface through a camera lens from a distance of 15 cm; the use of optical notch filters to block the treatment light from reaching the detectors allowed monitoring to occur continuously throughout PDT. Data were collected as a function of the distance between the source and detector pairs to a depth of ~2.2 mm, which is within the ~3-mm depth of the tumors studied. Average blood flow response was calculated as the mean of data collected from different source-detector pairs and expressed as a relative change by normalizing to flow in the same tumor in the min before PDT began.
Tumor pO2
Tumor pO2 was measured using the Oxylab pO2 (Oxford Optronix, Oxford, UK) in anesthetized (isoflurane) animals after 5 min for probe stabilization. Values are the average of 15 measurements from a given location. A total of six locations were measured in each tumor.
Tumor Response Assay
After PDT or control treatment, mice were followed daily to determine the number of days until tumor volume equaled or exceeded 400 mm3 (time to 400 mm3). Tumors were measured in two orthogonal directions, and volumes were calculated using the formula volume = diameter × width2 × 3.14/6. A cure was defined as an absence of tumor regrowth at 90 days after PDT.
Tumor Vascular Physiological Properties
Quantities for tumor hemoglobin oxygen saturation (SO2), total hemoglobin concentration (THC), oxyhemoglobin concentration (cHbO2), and deoxyhemoglobin concentration (cHb) were measured by continuous-wave broadband diffuse reflectance spectroscopy (DRS) as described previously (19, 20). The system consists of a 250-W quartz tungsten halogen lamp (Cuda Fiberoptics, Jacksonville, FL), a hand-held surface contact fiber-optic probe, a monochromator (Acton Research, Acton, MA) to disperse light from the detection fibers, and a liquid nitrogen-cooled CCD camera (Roper Scientific, Trenton, NJ) to image the reflectance spectra from multiple detection fibers simultaneously. The fiber-optic probe consists of a 400-μm-diameter source fiber and ten co-linear 400-μm-diameter detection fibers; source-detector separations between 1.2 mm and 4 mm were used, depending on the diameter and curvature of the tumor at the position where the probe contacted its surface. Measurements were made at 10–20 locations (acquisition time of 100 ms/measurement) on the tumor. The depth of measurement extended from ~0.6 mm to ~2 mm into the tumor. Spectra were collected in the 400–900-nm wavelength range and calibrated based on measurements in a 6-inch-diameter integrating sphere (LabSphere Inc., North Sutton, NH). Measurements were made immediately before and after PDT but not during light delivery. Data were fitted as described previously to determine cHbO2 and cHb, from which THC (THC = cHbO2 + cHb) and SO2 (cHbO2/THC) were calculated (19, 20). Relative changes in these quantities were calculated as the ratios after PDT to before PDT in the same tumor.
Histological Analyses
For assessment of PDT-related toxicity, tumor and normal tissue were collected from the euthanized animal at 24 h after PDT, fixed in formalin, and embedded in paraffin. Sections cut from these blocks were stained with hematoxylin and eosin, then read by a veterinary pathologist.
For assessment of vascular perfusion at the conclusion of PDT, Hoechst 33342 (Sigma, St. Louis, MO; 30 mg/kg) was administered via the orbital plexus 90 s prior to the completion of illumination. Immediately after PDT, the tumor was excised, coated in Tissue-Tek Oct compound, and frozen on a dry-ice-cooled aluminum plate. Cryosections were cut, fixed in 4% paraformaldehyde, and photographed for vascular-associated Hoechst fluorescence (LabPhot microscope with a 100-W high-pressure mercury arc lamp and Photometrics Quantix CCD digital camera). Sections were then blocked and stained with a primary (CD31; 1:100 for 1 h; BD Pharmingen, San Diego, CA) and secondary antibody (Cy5-conjugated mouse anti-rat; 1:50 for 45 min; Jackson Immunoresearch, West Grove, PA) to label blood vessel structure, as described previously (12). Photography of CD31-labeled blood vessels was performed at the same spatial coordinates that had been used to collect images of Hoechst-labeled perfusion in the same section. Tissue-containing areas on the section were additionally identified by flooding each section with 20 μM of Hoechst 33342 and photographing again at the same coordinates. Images of CD31 staining, perfused Hoechst, and tissue-labeling Hoechst were masked to identify stained areas, overlaid and then analyzed to determine the percentage of CD31-labeled blood vessels with Hoechst perfusion within the tumor cross section. Vessels were identified as contiguous units of CD31 staining and were counted as perfused if overlaid in whole or in part by the perfused Hoechst label. All analyses were performed using custom routines in MATLAB (MathWorks, Natick MA), with the exception of masking of the Hoechst images, which were analyzed in Adobe Photoshop (Adobe Systems Inc., San Jose, CA). Controls included slides stained with secondary antibody, but no primary antibody; these controls demonstrated no staining.
In Vivo/In Vitro Clonogenic Assay
Tumor-bearing animals were treated with PDT or as controls and then killed humanely by CO2 inhalation. Tumors were excised, weighed, minced and enzymatically digested using a technique described previously (19) in a trypsinizing flask containing 3000 units deoxyribonuclease (Sigma-Aldrich, St. Louis, MO), 2000 units collagenase (Sigma-Aldrich), and 3 mg protease (Sigma-Aldrich) dissolved in 12 ml of Hanks’ balanced salt solution. Cells were plated on 100 mm tissue culture dishes in triplicate, and after a ~10-day incubation (37°C in 95% air/5% CO2) colonies were fixed, stained (2.5 mg/ml methylene blue in 30% alcohol), and counted. The number of clonogenic cells per gram was calculated as the number of cells per gram of tumor multiplied by the ratio of the number of colonies to the number of cells plated.
Statistics
We used Cox regression analysis to determine the effect of fluence rate on time to a tumor volume of 400 mm3. The analysis was stratified to allow for a common estimate of the hazard ratio for fluence rate across fluence strata while providing an independent baseline within each stratum. The Wald χ2 test was used to test the assumption that the hazard ratio was similar across strata. Tumor response data were plotted as Kaplan-Meier product-moment estimates of survival (21), with “surviving” animals defined as those alive with a tumor volume of less than 400 mm3. Animals that survived to 90 days after PDT without tumors were incorporated as censored data points. Wilcoxon rank sum tests were used for all other comparisons among treatment groups, including those that used measurements of tumor pO2, THC, cHbO2, cHb and SO2, as well as histological and clonogenic analyses. For all tests, P < 0.05 was considered significant.
RESULTS
Low Fluence Rate Improves Response of Tumor Vasculature to PDT
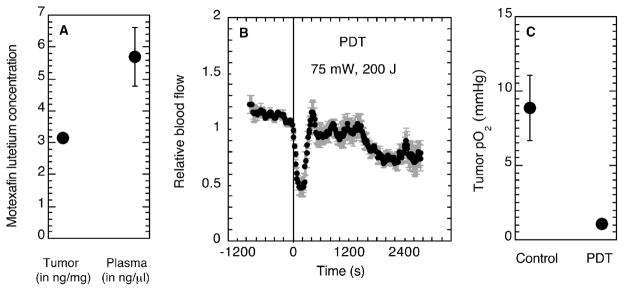
PDT was performed with the photosensitizer motexafin lutetium (10 mg/kg i.v.) at a drug–light interval of 180 min (730 nm light). This drug–light interval was chosen for its relevance to the short drug–light interval in clinical trials (17) and because of reports that the selectivity of motexafin lutetium for tumor blood vessels was lost at drug–light intervals of less than 180 min (22). For these photosensitization conditions, localization of the photosensitizer in the blood was confirmed: plasma concentrations of motexafin lutetium were 5.7 ng/μl compared to 3.1 ng/mg in the tumor itself (Fig. 1A). Consistent with the high plasma concentrations of motexafin lutetium at the time of illumination, a strong vascular response to PDT was seen as an acute decrease in blood flow within seconds of initiating treatment (Fig. 1B). A significant decrease (P < 0.001) in tumor pO2 (average ± SE) to 1.0 ± 0.04 mmHg at 180 min after PDT compared to 8.9 ± 2.2 mmHg in light-treated control tumors (Fig. 1C) suggests propagation of the vascular damage over the several hours after treatment.
FIG. 1.
Motexafin lutetium concentration (average ± SE) extracted from tumor (N = 5) or plasma (N = 3) (panel A). Effects of motexafin lutetium PDT on relative tumor blood flow (panel B) and oxygenation (panel C). Tumor blood flow during PDT (75 mW/cm2, 200 J/cm2) is expressed relative to blood flow in the minute prior to initiating illumination (indicated by vertical line); plots show the average (± SD) relative blood flow from a representative animal among six measured. Tumor oxygenation (average ± SE) was measured at 180 min after PDT (75 mW/cm2, 200 J/cm2) or control light treatment (N = 3 per group). Motexafin lutetium was injected at 10 mg/kg at 180 min prior to tissue collection or light delivery.
The effect of fluence rate on the long-term response of tumors to illumination at 25 and 75 mW/cm2 was examined (Fig. 2). At a fluence of 135 J/cm2, PDT at 25 mW/cm2 improved tumor cure rates to 17% from 0% at 75 mW/cm2; at a fluence of 200 J/cm2, PDT at 25 mW/cm2 improved tumor cure rates to 45% from 8% at 75% mW/cm2. Using Cox regression (stratified by fluence), we found a significant advantage to lower fluence rate [hazard ratio = 2.11 (CI 95% 1.08–4.13), P = 0.03]. The benefit of low fluence rate was not dominated by response at 200 compared to 135 J/cm2 because the hazard ratios (1.95 and 2.31, respectively) were similar between these two groups. However, the delivery of equivalent fluences was essential to demonstrate this benefit of low fluence rate. For example, if PDT was performed to an equivalent exposure time of 45 min at 75 and 25 mW/cm2, responses were similarly poor, with an 8% and 0% cure rate, respectively. Attempts to compare fluence-rate effects at longer treatment times of 90 min (i.e., time required for 135 J/cm2 at 25 mW/cm2) were unsuccessful due to the high total fluence (405 J/cm2) at 75 mW/cm2 and the resulting associated morbidity. Therefore, in an alternative approach, fluence rate was lowered further to 8.4 mW/cm2 to allow longer treatment times (Table 1). No additional benefit to tumor response was gained by reduction from 25 to 8.4 mW/cm2. This suggests that in PDT-induced vascular damage gains in tumor response were obtained through only moderate reductions in fluence rate and that further decreases below a threshold fluence rate provided no additional benefit.
FIG. 2.
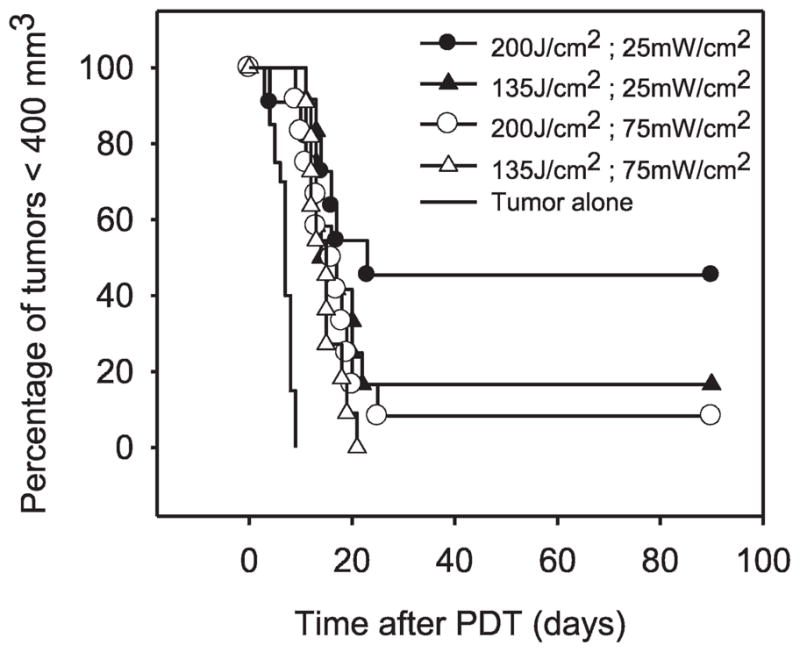
Fluence-rate effects on tumor response to motexafin lutetium (10 mg/kg, 180-min drug–light interval) PDT. Light controls (no photosensitizer) and photosensitizer controls (no light) were indistinguishable from the untreated controls (N = 11–12 per PDT group).
TABLE 1.
Median Time in Days of Tumor Regrowth to a Volume of 400 mm3 (percentage cures) after Motexafin Lutetium PDTa
| 75 mW/cm2b | 25 mW/cm2c | 8.4 mW/cm2d | |
|---|---|---|---|
| 67 J/cm2 | NDe | 10 (0%) | 11 (0%) |
| 135 J/cm2 | 15 (0%) | 16 (17%) | 13 (17%) |
| 200 J/cm2 | 17 (8%)f | 23 (45%) | ND |
PDT at the indicated fluences and fluence rates was performed at 180 min after i.v. injection of 10 mg/kg motexafin lutetium (N = 6–12 animals per group).
Delivery of 135 and 200 J/cm2 at 75 mW/cm2 required 30 and 45 min, respectively.
Delivery of 67,135 and 200 J/cm2 at 25 mW/cm2 required 45, 90, and 135 min, respectively.
Delivery of 67 and 135 J/cm2 at 8.4 mW/cm2 required 135 and 268 min, respectively.
Not done.
One death occurred after PDT with these conditions.
Because of the morbidity associated with 90 min of PDT at 75 mW/cm2, we performed complete necropsies and histopathological analyses. We found that the treated tumors demonstrated vascular congestion and hemorrhage, further confirming a vascular mechanism of tumor damage. However, the animals’ lungs also contained platelet thrombi, and lymphoid tissues of the intestine, thymus, and spleen all contained extensive individual cell necrosis, which we interpreted as apoptosis. When tumors were propagated on the flanks of mice, instead of on their shoulders, no histopathological abnormalities were found in the lungs, intestines, spleen or thymus after PDT. It is therefore likely that delivery of high-fluence/fluence-rate 730-nm light to the shoulder exposed normal tissue in the vicinity of shoulder tumors (e.g. lung) to doses sufficient to trigger a stress response. Thus subsequent experiments focused on maximum light fluences of 200 J/cm2, which produced little morbidity while demonstrating a strong fluence-rate effect.
Low Fluence Rate Minimally Benefits Vascular Oxygenation
The above tumor response studies established that lowering the PDT fluence rate from a moderately high fluence rate of 75 mW/cm2 to a lower fluence rate of 25 mW/cm2 provided significant advantages to long-term tumor response. One mechanism by which low fluence rate can increase tumor response is by slowing oxygen depletion, allowing for better maintenance of tumor oxygenation during PDT. Because this is a vascular-damaging regimen, we examined fluence-rate effects on oxygenation at the level of the blood vessel. Figure 3 shows the effect of PDT on tumor hemoglobin oxygen saturation (SO2). PDT at both fluence rates significantly depleted SO2. Thus lowering the fluence rate from 75 mW/cm2 to 25 mW/cm2 does not provide a significant advantage to SO2 (P > 0.05). This suggests that the benefit to tumor response at low fluence rate was mediated primarily by a component of treatment at a low fluence rate other than its potential to affect oxygen depletion.
FIG. 3.
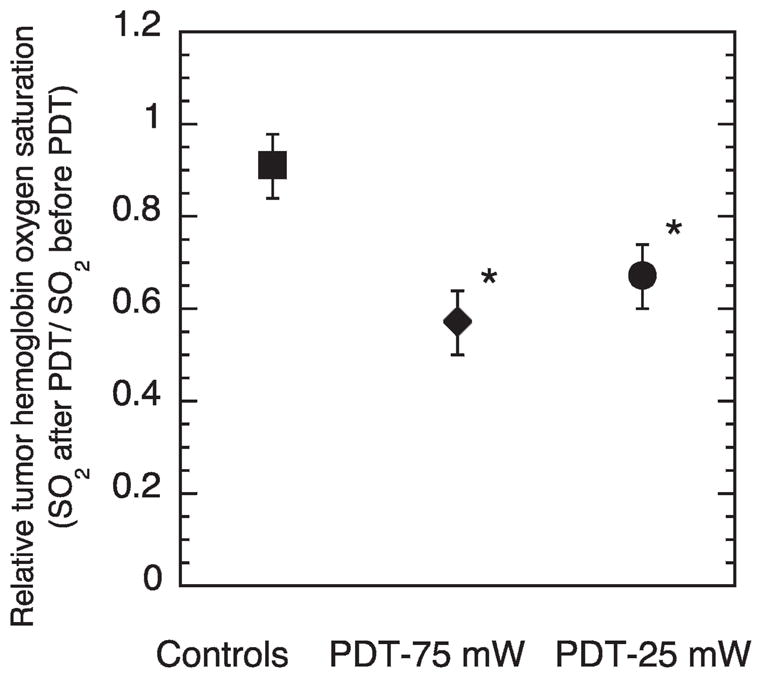
Tumor hemoglobin oxygen saturation (SO2) expressed relative to the pre-illumination value in the same tumor average ± SE; motexafin lutetium (10 mg/kg, 180-min drug–light interval) PDT performed to 200 J/cm2 at either 25 mW/cm2 or 75 mW/cm2, as indicated. Controls received light but no photosensitizer. Asterisk indicates P < 0.05 relative to controls; N = 11–14 per group.
Treatment Length Benefits Vascular Response to Low Fluence Rate
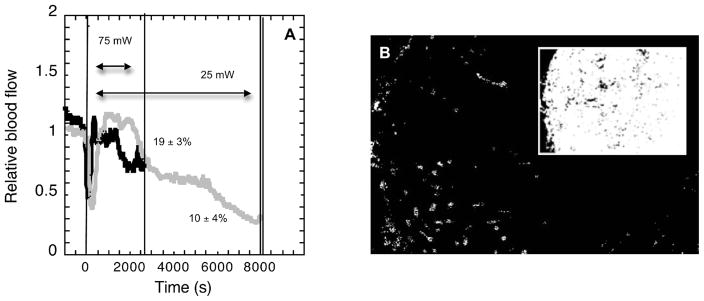
Another characteristic of low-fluence-rate PDT is that a longer treatment time is required to deliver a given fluence than if a higher fluence rate is used. Thus the extension of the treatment time could increase tumor response. As shown in Fig. 1B, motexafin lutetium-PDT at 75 mW/cm2 triggers a rapid decrease in blood flow, followed by a recovery and then a more gradual reduction. Figure 4A shows that the same pattern of vascular response is found during low-fluence-rate PDT, but due to the longer treatment time the duration of the second reduction in blood flow is longer at the lower fluence rate. Thus, although the fluence rate does not appear to affect the general pattern of blood flow response during PDT, a more prolonged period of low blood flow does occur during the more lengthy illumination at low fluence rate. Consequences to tumor perfusion were quantified in fluorescence micrographs of tumor sections from animals exposed to an in vivo label of vascular function. In these sections an average (SE) of 19 ± 3% of the immunohistochemically identified blood vessels were perfused at the conclusion of 75 mW/cm2 PDT compared to 10 ± 4% at the conclusion of 25 mW/cm2 PDT. This difference was statistically significant (P = 0.03), suggesting that the prolonged reduction in blood flow led to a significant reduction in vascular function at the conclusion of 25 mW/cm2 PDT. Among tumors that still exhibited blood flow at the conclusion of PDT, many of the functional vessels were clustered along the edge of the tumor, and more prominent vascular shutdown was noted in the tumor center (Fig. 4B).
FIG. 4.
Relative tumor blood flow (panel A) during motexafin lutetium (10 mg/kg, 180-min drug–light interval) PDT to 200 J/cm2 at 25 mW/cm2 (gray) and 75 mW/cm2 (black). Plots indicate representative average relative blood flow (error bars not shown for clarity) normalized to blood flow in the minute prior to beginning illumination. Single vertical lines indicate the start of illumination and the end of illumination at 75 mW/cm2. Double vertical line indicates the end of illumination at 25 mW/cm2. The percentage of blood vessels perfused at treatment completion, as measured by histological analysis, is indicated next to each blood flow plot; these values represent the average ± SE from five animals per group and are significantly different by Wilcoxon Rank Sums analysis. Representative histological image (panel B) exemplifies the pattern of incomplete vascular shutdown at PDT conclusion, with greater perfusion visible at the tumor periphery; inset depicts tumor area within the section and shows the location of the tumor periphery along the left side of the image. No perfused blood vessels are present in the area obstructed by the inset.
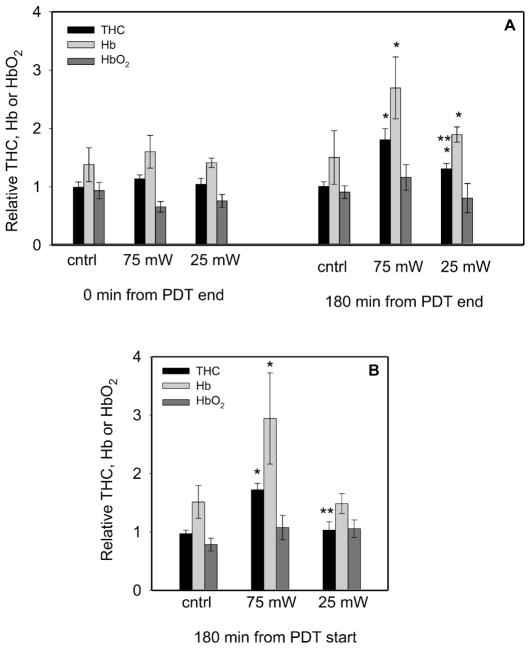
The time course of the PDT-induced vascular effect was assessed through the relative tumor total hemoglobin concentration (THC), a measure of blood volume. Vascular effects at 75 mW/cm2 manifested themselves through rises in THC in the 180 min after PDT, which were a result of increases in the concentration of deoxyhemoglobin (cHb). Simultaneously, small changes in the concentration of oxyhemoglobin (cHbO2) were found (Fig. 5A). An increase in cHb in the absence of corresponding increases in cHbO2 is consistent with disruption of the vascular network so as to allow blood delivery and leakage but limited egress from the tumor; i.e., it is in agreement with the above-noted pattern of vascular disruption that frequently involved greater vascular shutdown in the tumor center than at its edges. Over time after PDT, a semi-functional and permeabilized vascular system could lead to pooling of blood within the tumor and thus to the accumulation of deoxyhemoglobin and increases in THC.
FIG. 5.
PDT effect on the concentration of total hemoglobin (THC), deoxyhemoglobin (Hb), and oxyhemoglobin (HbO2) immediately and 180 min after the end of PDT (panel A) and 180 min after the start of PDT (panel B). Motexafin lutetium (10 mg/kg, 180-min drug–light interval) PDT was performed to 200 J/cm2 at either 25 mW/cm2 or 75 mW/cm2, as indicated. Controls (cntrl) received light (both fluence rates included) but no photosensitizer. Values were calculated as the ratios of the indicated parameter measured in the same tumor after PDT to those before PDT and are averages ± SE. *P < 0.05 compared to controls at the same time; **P < 0.05 for comparison between the fluence rates. N = 11–14 at 0 min and 4–7 (PDT) or 10 or 11 (cntrl) at other times.
Significant increases in relative THC and cHb were also noted at 180 min after PDT at 25 mW/cm2 (Fig. 5A), suggesting that incomplete vascular shutdown also occurs at the lower fluence rate; however, these increases were smaller than those found with 75 mW/cm2 PDT. Thus lower fluence rate leads to more complete vascular shutdown, as measured by smaller increases in THC after PDT. However, vascular shutdown was not complete because relative THC (on average) did not decrease below its pre-PDT value.
To assess the contribution of the extended treatment time at low fluence rate, we also collected data at 180 min after the beginning of PDT (Fig. 5B). At this time only the 75 mW/cm2-treated tumors demonstrated increases in THC and Hb concentration that are characteristic of (incomplete) vascular shutdown. The fact that PDT at 25 mW/cm2 was not associated with increases in THC at 180 min after the beginning of PDT suggests that the additional time of treatment at the lower fluence rate was necessary for the vascular effects detected 180 min after the end of PDT to develop.
Fluence-Rate Effects on Clonogenic Survival Follow Vascular Response
The above immunohistochemical and THC data suggest more complete vascular shutdown after 25 mW/cm2 PDT compared to 75 mW/cm2 PDT. In this same time frame, the in vivo/in vitro clonogenic assay was used to study fluence-rate effects on tumor cell survival, which could be affected by the differences in vascular response. Compared to controls, PDT at 75 mW/cm2 produced a modest but significant decrease in tumor clonogenicity, roughly 1 log of cell killing, at 180 min after the end of PDT (Fig. 6). In contrast, PDT at 25 mW/cm2 led to ~1 log of cell killing immediately after PDT, with an increase to ~3 logs of killing by 180 min after PDT; this decrease in clonogenicity was significantly greater than that found with 75 mW/cm2 PDT. At an equivalent time from the beginning of PDT (225 min) clonogenic survival was similar for the two fluence rates, which again suggests that the additional time incorporated at the lower fluence rate is of cytotoxic significance.
FIG. 6.
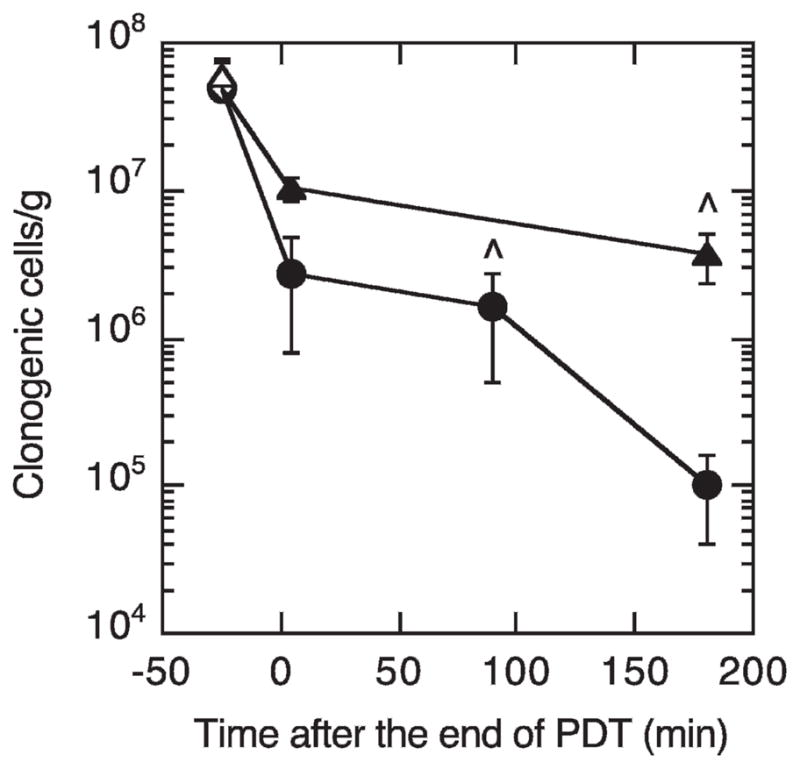
In vivo/in vitro clonogenic survival of cells from tumors treated with motexafin lutetium (10 mg/kg, 180–min drug–light interval) PDT to 200 J/cm2 at either 25 mW/cm2 (●) or 75 mW/cm2 (▲) and excised at the indicated times. Control tumors that were untreated, treated only with light (no photosensitizer), or treated only with photosensitizer (no light) are plotted at a negative time with open symbols. Points are averages ± SE (n = 3 or 4 animals/group). ● Indicates equivalent time from the beginning of PDT (225 min).
DISCUSSION
In the present study, we used motexafin lutetium as photosensitizer under conditions (10 mg/kg, 180-min drug–light interval) previously shown to lead to an acute, tumor-specific vascular response in treated tumors (22). Other studies found that motexafin lutetium uptake in mouse tissue peaked after 180 min [measured in mouse foot (23)], with its localization determined to be roughly equivalent in the tissue (SMT-F tumor) and plasma (24). In the RIF tumor model, we found that an interval of 180 min led to higher motexafin lutetium concentrations in the plasma than in the tumor. Illumination at this time led to a spasmotic decrease in blood flow, confirming the presence of an acute vascular response with this drug–light interval. This prominent vascular response is not unlike that found in PDT with other photosensitizers, such as BPD, Tookad, hypericin and chlorin e6, if light is delivered when plasma concentrations of the drugs are high (25–28). In the present study, the continuing progressive development of vascular damage over illumination and the time thereafter is seen in a decreased access of a perfusion marker to the tumor vessels and a significant increase in tumor hypoxia (pO2 of 1 mmHg at 180 min after PDT compared to 9 mmHg in controls). However, vascular damage was incomplete, because perfused vessels, although reduced in number compared to controls, were still detectable at the conclusion of PDT.
As found by others, we found that a lower fluence rate benefited PDT response to protocols that favor vascular damage (8). We sought to understand the mechanisms responsible for the low-fluence-rate enhancement in this study, which was assessed through improvements in long-term tumor response to PDT at 25 compared to 75 mW/cm2. A lower fluence rate can benefit PDT response by slowing the rate of photochemical oxygen consumption, thereby allowing for better maintenance of tumor oxygenation and increasing direct tumor cell killing (29, 30). Since the vasculature was the primary target under the conditions used, we evaluated fluence-rate effects on blood hemoglobin oxygen saturation (SO2) in microvasculature. PDT created significant decreases in SO2 at both fluence rates used. The fact that decreases in SO2 were detectable in the minutes after PDT (when it was technically feasible to make the measurements) suggests that this oxygen depletion was secondary to vascular effects that had developed during treatment. Similarly, the lack of a fluence-rate effect on oxygenation suggests that oxygen depletion did not result from photochemical consumption but rather was a downstream consequence of vascular effects. Under conditions of substantial decreases in tumor perfusion such as those found during PDT at both fluence rates, resulting decreases in tissue oxygen tensions could lead to increased oxygen extraction from hemoglobin and therefore decreases in oxyhemoglobin. However, SO2 cannot be expected to track precisely with vascular shutdown because it measures the oxygenation of only perfused vessels.
Further evidence that the fluence rate did not affect oxygen tensions or the direct PDT effect in tumor cells is apparent in the results of the clonogenicity studies. At the lower fluence rate there was no significant advantage to cell killing measured immediately after PDT. Clonogenicity immediately after PDT at both fluence rates was lower than that found in light controls, which suggests the presence of a fluence-rate-independent component to direct cell killing for these treatment conditions. However, in this study, assessment of direct cell death immediately after the completion of PDT could be confounded by cytotoxicity secondary to vascular effects that developed early in PDT, especially during PDT at the lower 25 mW/cm2 fluence rate, which required over 120 min of illumination and thus provided ample time for any effects of early vascular damage to propagate. As a result, it is not possible to attribute cell death measured at the conclusion of PDT solely to direct cell killing; nevertheless, any contribution of direct killing to response was small and independent of fluence rate.
In general, fluence rate has been found to affect many aspects of the PDT response, including not only direct cytotoxicity to tumor cells and vascular damage but also contributions from an immune response (5, 8, 31, 32), which may be important when suboptimal damage to the tumor vasculature permits an influx of immune cells after PDT. The presence of an immune system component to tumor response of the present study was not explicitly examined and thus cannot be ruled out. Neither of the fluence rates studied in the present investigation produced complete shutdown of blood vessels immediately after PDT, which would allow for accessibility to host immune cells. Conditions that increased vascular damage (lower fluence rate) were associated with better long-term efficacy; thus, as found by others, the presence of a strong vascular response may supersede contributions from an immune response (5).
Another characteristic of low-fluence-rate illumination is its association with longer illumination times for treatment to the same total dose (fluence) as that used at a higher fluence rate. In this study, PDT was significantly more effective when delivered to equivalent fluences at 25 mW/cm2 than at 75 mW/cm2. However, delivery of 200 J/cm2 required 45 min at 75 mW/cm2 and 135 min at 25 mW/cm2. Seshadri et al. (13) showed that the additional treatment time at lower fluence rate could be an independent factor favoring the vascular component of tumor response to HPPH-PDT. That study was performed under conditions that favored plasma clearance of the drug prior to illumination (33), as opposed to our present study, in which illumination was intentionally performed when plasma levels are high (24). Nevertheless, the HPPH PDT data suggest that treatment duration may also play an important role in fluence-rate effects for PDT of tumor blood vessels. In agreement, our data show that longer treatment times are needed for the lower fluence rate to demonstrate a benefit.
Although greater vascular damage was found in this study at a lower fluence rate of 25 mW/cm2 compared to 75 mW/cm2, further lowering the fluence rate to 8.4 mW/cm2 provided no additional tumor response. This suggests the presence of a threshold beyond which increasing the length of PDT and concurrently the length of time over which low tumor blood flow is maintained will provide no cytotoxic advantage. This finding is consistent with a hypothesis that an increase in vascular damage from low-fluence-rate PDT was a consequence of prolonged ischemia that led to vessel fragility; it is supported by the work of others documenting a relationship between the duration of ischemia and the development of detrimental biological effects (34). These results are also consistent with other PDT studies in which lowering the fluence rate below 7 mW/cm2 [HPPH PDT (13)] or 18 mW/cm2 [Photofrin-PDT (35)] reduced biological responses. At the very low fluence rates used in these and our studies there is the opportunity for greater repair of oxidative damage during the slow rate of light delivery (36), which could contribute to a threshold effect, as could the limitations imposed by penetration of low-fluence-rate light in solid tissues.
Evidence of PDT-induced vascular effects can be detected in this study as increases in THC, which are a consequence of increases in the concentration of deoxyhemoglobin, but not oxyhemoglobin. These findings are suggestive of incomplete vascular shutdown and vascular leakage after PDT, which could lead to pooling of deoxygenated blood. Similar findings have been reported by others after both vascular- and cell-targeting PDT regimens. For example, a threefold increase in blood volume within the treated field on mouse skin was reported at 60 min after PDT with BPD with either a 15-min (vascular-targeting) or a 180-min (cell-targeting) drug–light interval (37). Chen and colleagues found that BPD-PDT with a 15-min drug–light interval increased tumor vascular permeability over the hour after PDT (38); moreover, they noted that greater vascular extravasation occurs in the tumor periphery compared to its center (39). This latter observation is particularly relevant to the results of our study. We note that incomplete vascular shutdown after PDT is characterized by maintenance of blood flow within a tumor’s periphery and more widespread vascular showdown in the tumor center. Others have also documented that PDT can lead to sparing of vessels in the tumor periphery for a variety of different tumor models and photosensitizing conditions (26, 27, 40, 41).
In conclusion, this study establishes that a lower fluence rate can improve PDT response under conditions in which treatment is designed to have predominantly vascular effects. The improvement in response cannot be associated with improvements in vascular oxygenation at the lower fluence rate but instead appears to be related to the prolongation of ischemia during treatment with the lower fluence rate. More widespread vascular damage occurs at the lower fluence rate, which is detectable as smaller increases in blood volume and less accumulation of deoxygenated hemoglobin within the treated tumor. Increases in cytotoxicity at the lower fluence rate follow the vascular effects. These data indicate that further studies of low fluence rate in PDT regimens that predominantly damage the tumor vasculature are needed.
Acknowledgments
We gratefully acknowledge Elizabeth Rickter, Amanda Maas, Min Yuan and Shirron Carter, Department of Radiation Oncology, for technical support. We also thank Prof. Arjun Yodh for advice on the DCS and DRS studies. This work was supported by NIH grants CA085831 and CA87971.
References
- 1.Henderson BW, Busch TM, Snyder JW. Fluence rate as a modulator of PDT mechanisms. Lasers Surg Med. 2006;38:489–493. doi: 10.1002/lsm.20327. [DOI] [PubMed] [Google Scholar]
- 2.Coutier S, Bezdetnaya LN, Foster TH, Parache RM, Guillemin F. Effect of irradiation fluence rate on the efficacy of photodynamic therapy and tumor oxygenation in meta-tetra (hydroxyphenyl) chlorin (mTHPC)-sensitized HT29 xenografts in nude mice. Radiat Res. 2002;158:339–345. doi: 10.1667/0033-7587(2002)158[0339:eoifro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Sitnik TM, Hampton JA, Henderson BW. Reduction of tumour oxygenation during and after photodynamic therapy in vivo: effects of fluence rate. Br J Cancer. 1998;77:1386–1394. doi: 10.1038/bjc.1998.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsutsui H, MacRobert AJ, Curnow A, Rogowska A, Buonaccorsi G, Kato H, Bown SG. Optimisation of illumination for photodynamic therapy with mTHPC on normal colon and a transplantable tumour in rats. Lasers Med Sci. 2002;17:101–109. doi: 10.1007/s101030200017. [DOI] [PubMed] [Google Scholar]
- 5.Henderson BW, Gollnick SO, Snyder JW, Busch TM, Kousis PC, Cheney RT, Morgan J. Choice of oxygen-conserving treatment regimen determines the inflammatory response and outcome of photodynamic therapy of tumors. Cancer Res. 2004;64:2120–2126. doi: 10.1158/0008-5472.can-03-3513. [DOI] [PubMed] [Google Scholar]
- 6.Foster TH, Murant RS, Bryant RG, Knox RS, Gibson SL, Hilf R. Oxygen consumption and diffusion effects in photodynamic therapy. Radiat Res. 1991;126:296–303. doi: 10.2307/3577919. [DOI] [PubMed] [Google Scholar]
- 7.van Geel IP, Oppelaar H, Marijnissen JP, Stewart FA. Influence of fractionation and fluence rate in photodynamic therapy with Photofrin or mTHPC. Radiat Res. 1996;145:602–609. doi: 10.2307/3579279. [DOI] [PubMed] [Google Scholar]
- 8.Iinuma S, Schomacker KT, Wagnieres G, Rajadhyaksha M, Bamberg M, Momma T, Hasan T. In vivo fluence rate and fractionation effects on tumor response and photobleaching: photodynamic therapy with two photosensitizers in an orthotopic rat tumor model. Cancer Res. 1999;59:6164–6170. [PubMed] [Google Scholar]
- 9.Angell-Petersen E, Spetalen S, Madsen SJ, Sun CH, Peng Q, Carper SW, Sioud M, Hirschberg H. Influence of light fluence rate on the effects of photodynamic therapy in an orthotopic rat glioma model. J Neurosurg. 2006;104:109–117. doi: 10.3171/jns.2006.104.1.109. [DOI] [PubMed] [Google Scholar]
- 10.Maugain E, Sasnouski S, Zorin V, Merlin JL, Guillemin F, Bezdetnaya L. Foscan-based photodynamic treatment in vivo: correlation between efficacy and Foscan accumulation in tumor, plasma and leukocytes. Oncol Rep. 2004;12:639–645. [PubMed] [Google Scholar]
- 11.Hammer-Wilson MJ, Cao D, Kimel S, Berns MW. Photodynamic parameters in the chick chorioallantoic membrane (CAM) bioassay for photosensitizers administered intra-peritoneally (IP) into the chick embryo. Photochem Photobiol Sci. 2002;1:721–728. doi: 10.1039/b205471j. [DOI] [PubMed] [Google Scholar]
- 12.Busch TM, Wileyto EP, Emanuele MJ, Del Piero F, Marconato L, Glatstein E, Koch CJ. Photodynamic therapy creates fluence rate-dependent gradients in the intratumoral spatial distribution of oxygen. Cancer Res. 2002;62:7273–7279. [PubMed] [Google Scholar]
- 13.Seshadri M, Bellnier DA, Vaughan LA, Spernyak JA, Mazurchuk R, Foster TH, Henderson BW. Light delivery over extended time periods enhances the effectiveness of photodynamic therapy. Clin Cancer Res. 2008;14:2796–2805. doi: 10.1158/1078-0432.CCR-07-4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guldi DM, Mody TD, Gerasimchuk NN, Magda D, Sessler JL. The influence of large metal cations on the photophysical properties of texaphyrin, a rigid aromatic chromophore. J Am Chem Soc. 2000;122:8289–8298. [Google Scholar]
- 15.Grossweiner LI, Bilgin MD, Berdusis P, Mody TD. Singlet oxygen generation by metallotexaphyrins. Photochem Photobiol. 1999;70:138–145. [Google Scholar]
- 16.Mody TD, Fu L, Sessler JL. Texaphyrins: Synthesis and Development of a Novel Class of Therapeutic Agents. Wiley; Chichester: 2001. [Google Scholar]
- 17.Patel H, Mick R, Finlay J, Zhu TC, Rickter E, Cengel KA, Malkowicz SB, Hahn SM, Busch TM. Motexafin lutetium-photodynamic therapy of prostate cancer: short- and long-term effects on prostate-specific antigen. Clin Cancer Res. 2008;14:4869–4876. doi: 10.1158/1078-0432.CCR-08-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu G, Durduran T, Zhou C, Wang HW, Putt ME, Saunders HM, Sehgal CM, Glatstein E, Yodh AG, Busch TM. Noninvasive monitoring of murine tumor blood flow during and after photodynamic therapy provides early assessment of therapeutic efficacy. Clin Cancer Res. 2005;11:3543–3552. doi: 10.1158/1078-0432.CCR-04-2582. [DOI] [PubMed] [Google Scholar]
- 19.Wang HW, Rickter E, Yuan M, Wileyto EP, Glatstein E, Yodh A, Busch TM. Effect of photosensitizer dose on fluence rate responses to photodynamic therapy. Photochem Photobiol. 2007;83:1040–1048. doi: 10.1111/j.1751-1097.2007.00139.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang HW, Putt ME, Emanuele MJ, Shin DB, Glatstein E, Yodh AG, Busch TM. Treatment-induced changes in tumor oxygenation predict photodynamic therapy outcome. Cancer Res. 2004;64:7553–7561. doi: 10.1158/0008-5472.CAN-03-3632. [DOI] [PubMed] [Google Scholar]
- 21.Hosmer DW, Lemeshow S. Applied Survival Analysis. Wiley; New York: 1999. [Google Scholar]
- 22.Fingar VH, Taber SW, Haydon PS, Harrison LT, Kempf SJ, Wieman TJ. Vascular damage after photodynamic therapy of solid tumors: a view and comparison of effect in pre-clinical and clinical models at the University of Louisville. In Vivo. 2000;14:93–100. [PubMed] [Google Scholar]
- 23.Kostenich G, Orenstein A, Roitman L, Malik Z, Ehrenberg B. In vivo photodynamic therapy with the new near-IR absorbing water soluble photosensitizer lutetium texaphyrin and a high intensity pulsed light delivery system. J Photochem Photobiol B. 1997;39:36–42. doi: 10.1016/s1011-1344(96)00005-x. [DOI] [PubMed] [Google Scholar]
- 24.Young SW, Woodburn KW, Wright M, Mody TD, Fan Q, Sessler JL, Dow WC, Miller RA. Lutetium texaphyrin (PCI-0123): a near-infrared, water-soluble photosensitizer. Photochem Photobiol. 1996;63:892–897. doi: 10.1111/j.1751-1097.1996.tb09647.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen B, Roskams T, de Witte PA. Antivascular tumor eradication by hypericin-mediated photodynamic therapy. Photochem Photobiol. 2002;76:509–513. doi: 10.1562/0031-8655(2002)076<0509:atebhm>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 26.Chen B, Pogue BW, Goodwin IA, O’Hara JA, Wilmot CJ, Hutchins JE, Hoopes PJ, Hasan T. Blood flow dynamics after photodynamic therapy with verteporfin in the RIF-1 tumor. Radiat Res. 2003;160:452–459. doi: 10.1667/RR3059. [DOI] [PubMed] [Google Scholar]
- 27.Koudinova NV, Pinthus JH, Brandis A, Brenner O, Bendel P, Ramon J, Eshhar Z, Scherz A, Salomon Y. Photodynamic therapy with Pd-Bacteriopheophorbide (TOOKAD): successful in vivo treatment of human prostatic small cell carcinoma xenografts. Int J Cancer. 2003;104:782–789. doi: 10.1002/ijc.11002. [DOI] [PubMed] [Google Scholar]
- 28.Chin WW, Heng PW, Bhuvaneswari R, Lau WK, Olivo M. The potential application of chlorin e6-polyvinylpyr-rolidone formulation in photodynamic therapy. Photochem Photobiol Sci. 2006;5:1031–1037. doi: 10.1039/b605772a. [DOI] [PubMed] [Google Scholar]
- 29.Foster TH, Hartley DF, Nichols MG, Hilf R. Fluence rate effects in photodynamic therapy of multicell tumor spheroids. Cancer Res. 1993;53:1249–1254. [PubMed] [Google Scholar]
- 30.Sitnik TM, Henderson BW. Effects of fluence rate on cytotoxicity during photodynamic therapy. Proc SPIE. 1997;2972:95–102. [Google Scholar]
- 31.Thong PS, Olivo M, Kho KW, Bhuvaneswari R, Chin WW, Ong KW, Soo KC. Immune response against angiosarcoma following lower fluence rate clinical photodynamic therapy. J Environ Pathol Toxicol Oncol. 2008;27:35–42. doi: 10.1615/jenvironpatholtoxicoloncol.v27.i1.40. [DOI] [PubMed] [Google Scholar]
- 32.Busch TM, Xing X, Yu G, Yodh A, Wileyto EP, Wang HW, Durduran T, Zhu TC, Wang KK. Fluence rate-dependent intratumor heterogeneity in physiologic and cytotoxic responses to Photofrin photodynamic therapy. Photochem Photobiol Sci. 2009;8:1683–1693. doi: 10.1039/b9pp00004f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellnier DA, Henderson BW, Pandey RK, Potter WR, Dougherty TJ. Murine pharmacokinetics and antitumor efficacy of the photodynamic sensitizer 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a. J Photochem Photobiol B. 1993;20:55–61. doi: 10.1016/1011-1344(93)80131-r. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Nemoto M, Xu Z, Yu SW, Shimoji M, Andrabi SA, Haince JF, Poirier GG, Dawson TM, Koehler RC. Influence of duration of focal cerebral ischemia and neuronal nitric oxide synthase on translocation of apoptosis-inducing factor to the nucleus. Neuroscience. 2007;144:56–65. doi: 10.1016/j.neuroscience.2006.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Standish BA, Lee KK, Jin X, Mariampillai A, Munce NR, Wood MF, Wilson BC, Vitkin IA, Yang VX. Interstitial Doppler optical coherence tomography as a local tumor necrosis predictor in photodynamic therapy of prostatic carcinoma: an in vivo study. Cancer Res. 2008;68:9987–9995. doi: 10.1158/0008-5472.CAN-08-1128. [DOI] [PubMed] [Google Scholar]
- 36.Veenhuizen RB, Stewart FA. The importance of fluence rate in photodynamic therapy: is there a parallel with ionizing radiation dose-rate effects? Radiother Oncol. 1995;37:131–135. doi: 10.1016/0167-8140(95)01626-r. [DOI] [PubMed] [Google Scholar]
- 37.Kurohane K, Tominaga A, Sato K, North JR, Namba Y, Oku N. Photodynamic therapy targeted to tumor-induced angiogenic vessels. Cancer Lett. 2001;167:49–56. doi: 10.1016/s0304-3835(01)00475-x. [DOI] [PubMed] [Google Scholar]
- 38.He C, Agharkar P, Chen B. Intravital microscopic analysis of vascular perfusion and macromolecule extravasation after photodynamic vascular targeting therapy. Pharm Res. 2008;25:1873–1880. doi: 10.1007/s11095-008-9604-5. [DOI] [PubMed] [Google Scholar]
- 39.Chen B, Crane C, He C, Gondek D, Agharkar P, Savellano MD, Hoopes PJ, Pogue BW. Disparity between prostate tumor interior versus peripheral vasculature in response to verteporfin-mediated vascular-targeting therapy. Int J Cancer. 2008;123:695–701. doi: 10.1002/ijc.23538. [DOI] [PubMed] [Google Scholar]
- 40.Norum OJ, Bruland OS, Gorunova L, Berg K. Photochemical internalization of bleomycin before external-beam radiotherapy improves locoregional control in a human sarcoma model. Int J Radiat Oncol Biol Phys. 2009;75:878–885. doi: 10.1016/j.ijrobp.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 41.Schacht V, Szeimies RM, Abels C. Photodynamic therapy with 5-aminolevulinic acid induces distinct microcirculatory effects following systemic or topical application. Photochem Photobiol Sci. 2006;5:452–458. doi: 10.1039/b514128a. [DOI] [PubMed] [Google Scholar]