Abstract
Aims
Endothelial progenitor cells (EPC) may participate in the repair of injured coronary endothelium. We have recently identified EPC co-expressing the osteoblastic marker osteocalcin [OCN (+) EPC] and found that their numbers are increased in patients with early and late coronary atherosclerosis. The current study was designed to test the hypothesis that early coronary atherosclerosis is associated with the retention of osteogenic EPC within the coronary circulation.
Methods and results
Blood samples were taken simultaneously from the proximal aorta and the coronary sinus from 31 patients undergoing invasive coronary endothelial function testing. Using flow cytometry, peripheral blood mononuclear cells were analysed for EPC markers (CD133, CD34, KDR) and OCN. The net gradient of EPC was calculated by multiplying the coronary blood flow by the arteriovenous EPC gradient (a negative net gradient indicating retention of EPC). Similarly, serum samples were analysed for stromal cell-derived factor-1 alpha (SDF-1 alpha) and interleukin-8 (IL-8) and their net production calculated. Compared with controls (n = 17) patients with endothelial dysfunction (ED, n = 14) had a significant net retention of CD34+/CD133−/KDR+/OCN+ EPC [118.38 (0.00, 267.04) vs. −112.03 (838.36, 0.00), P = 0.004]. The retention of OCN (+) EPC correlated with the degree of ED. Patients with ED also showed a net retention of CD34+/CD133−/KDR+ EPC (P = 0.010). Net production of IL-8 was positive in ED [1540.80 (−300.40, 21744.10)pg/mL] but negative in controls [−3428.50 (−11225.00, 647.48), P = 0.025].
Conclusion
Our study demonstrates that patients with early coronary atherosclerosis are characterized by retention of OCN (+) EPC within the coronary circulation, potentially leading to progressive coronary calcification rather than normal repair.
Keywords: Endothelial progenitor cells, Osteocalcin, Endothelial dysfunction, Early atherosclerosis, Flow cytometry
Introduction
Accumulating evidence suggests that circulating bone marrow-derived cells may play a role not only in the repair and regeneration of injured endothelium1,2 but also in the pathogenesis of atherosclerotic lesion formation.3 After Asahara et al.1 showed that peripherally administered human umbilical cord CD34+ endothelial progenitor cells (EPC) contributed to angiogenesis in mouse and rabbit models of hind limb ischaemia, Sata et al.3 demonstrated in mouse models of hyperlipidaemia and iatrogenic endothelial injury that bone marrow derived cells contributed to atherosclerotic lesion formation.
Cytokines like the stromal cell-derived factor-1 alpha (SDF-1) and interleukin-8 (IL-8) likely facilitate the homing of circulating EPC into the injured endothelium. Indeed, SDF-1 has been shown to induce neovascularization by recruiting EPC into ischaemic tissues through increased SDF1-alpha/CXCR4 coupling.4,5 In addition, the inflammatory cytokine IL-8 has been found to be a chemoattractant for EPC in ischaemic tissue.6
Endothelial dysfunction (ED) is regarded as an early stage of atherosclerosis and has been found to be a strong, independent predictor of cardiovascular events.7 The endothelium, especially in early atherosclerosis, undergoes a constant process of injury and repair in order to maintain normal vascular function and structure.8 We have recently identified a subgroup of bone marrow derived, circulating EPC with an osteogenic phenotype (co-staining in flow cytometry for the osteoblast marker osteocalcin [OCN, OCN (+) EPC]).9 We found that patients with coronary ED have significantly higher numbers of circulating OCN (+) EPC compared with patients with normal endothelial function.9 In additional cell culture experiments we showed that circulating progenitor cells from patients with high OCN co-staining of EPC were capable of forming mineralized deposits.9
Moreover, using spectral analysis of intravascular ultrasound (IVUS) radiofrequency data, we have recently demonstrated that coronary artery segments with ED are not only characterized by features of plaque vulnerability but also more extensive coronary calcification.10
Thus, the current study was designed to test the hypothesis that—in contrast to controls—patients with coronary ED retain osteogenic, OCN (+) EPC within the coronary circulation facilitated by the release of SDF-1 and IL-8. The retention of osteogenic EPC for endothelial repair may lead to the induction and progression of coronary calcification rather than normal repair. In order to address this hypothesis we obtained simultaneous blood samples from the ostium of the left coronary artery and the coronary sinus, determined trans-cardiac EPC, SDF-1, and IL-8 gradients and calculated net gradients by multiplication with invasively measured coronary blood flow (CBF).
Methods
Study subjects
The study was approved by the Institutional Review Board of Mayo Foundation and all study subjects provided written, informed consent. A total of 31 patients undergoing coronary angiography and coronary endothelial function testing for clinical purposes (referred to the catheterization laboratory for recurrent angina with or without a positive stress test) who met the criteria outlined below for inclusion into the two study groups were recruited. Patients with acute coronary syndromes (unstable angina or acute myocardial infarction), heart failure (ejection fraction <50%), or severe renal or liver disease were excluded. None of the subjects had a clinical diagnosis of Paget's disease or a fracture within the past 5 years. The Framingham risk score was calculated as previously described.11 The two groups were defined as follows: controls, patients without significant structural coronary lesions on angiography (<30% stenosis) and normal endothelial function, as assessed by intra-coronary acetylcholine challenge (see below), n = 17; and patients with ED, defined by the absence of significant structural coronary lesions on angiography but with abnormal endothelial function, n = 14. We assessed both epicardial and microvascular endothelial function, however, for the clinical diagnosis of ED and inclusion into the study, patients had to have epicardial ED. All but 3 of the 14 patients in the ED group had both epicardial as well as microvascular ED.
Coronary angiography and invasive endothelial function testing
Patients underwent a diagnostic coronary angiography using standard clinical protocols. Patients who met the inclusion criteria were studies and a 5 F multipurpose Amplatz left catheter was placed into the coronary sinus under radiographic guidance via a 7 F femoral vein access. The correct position of the catheter within the coronary sinus was verified by pressure transduction, contrast injection, and oxygen saturation.12,13
Simultaneous arterial blood (20 mL) was drawn from the left coronary and the coronary sinus catheter for flow cytometry and biochemical analyses.12,13 All subjects underwent assessment of endothelium-dependent coronary vasoreactivity, as previously described.14,15 In brief, 5000 units of heparin were given intravenously and a Doppler guidewire (Flowire, Volcano Inc.) within a coronary-infusion catheter (Ultrafuse, SciMed Life System) was positioned into the mid-portion of the left anterior descending coronary artery. Acetylcholine at increasing concentrations (10−6 to 10−4) was infused into the left anterior descending coronary artery to assess endothelium-dependent vasoreactivity. Haemodynamic data, Doppler measurements, and a coronary angiogram were obtained after each infusion. Coronary artery diameter was measured by an independent investigator in the segment 5 mm distal to the tip of the Doppler wire using a computer-based image analysis system. Average peak velocity (APV) was derived from the Doppler flow velocity spectra and CBF was determined as π(coronary artery diameter/2)2 × (APV/2). As previously described, microvascular ED was defined as an increase in CBF of <50% and epicardial ED was defined as a decrease in epicardial coronary artery diameter of more than 20% in response to the maximal dose of acetylcholine (10−4 M).16 Endothelium-independent microvascular function was determined by the coronary flow reserve (CFR), which is the ratio of the APV at maximal hyperaemia [induced by intracoronary adenosine (24–60 μg)] to the APV at baseline.9
Flow cytometry
Peripheral blood mononuclear cells were isolated from fresh blood samples using a Ficoll density gradient and immunofluorescent cell staining was performed using the following fluorescent conjugated antibodies: CD34-PerCP Cy 5.5 (Beckton-Dickinson), CD133-phycoerythrin (PE) (Miltenyi Biotec GmbH) and kinase insert domain receptor KDR-APC (R&D Systems) and the appropriate isotype controls. In addition, OCN+ cells were identified using an anti-human OCN antibody (Santa Cruz Biotechnology) and a fluorescein isothiocyanate secondary antibody (Jackson ImmunoResearch), as previously described.17,18 Cell fluorescence was measured immediately after staining (Becton Dickinson, FACS Calibur) and data were analysed using CellQuest software (Becton Dickinson). A total of 150 000 events were counted and final data were obtained within the lymphocyte gate. The person doing the cell analysis was not aware of the results of the patient classification.
We determined the intra-assay coefficient of variation (CV, i.e. a patient's blood sample was analysed twice on the same day using the methods described above) for different subsets of EPC in 12 patients. The CVs for CD34+, CD34+/KDR+, CD34+/CD133−/KDR+, and CD34+/CD133−/KDR+/OC+ cells were 13, 19, 30, and 30%, respectively.
Determination of endothelial progenitor cells net gradient
Endothelial progenitor cells absolute counts (per 100 000 cell counts) were determined in the aortic and coronary sinus sample of each study patient. The trans-cardiac gradient was determined by subtracting the aortic EPC number from that of the coronary sinus.12,13 The net gradient of EPC was calculated by multiplying the trans-cardiac gradient with the CBF as determined by intracoronary Doppler.19 A negative net gradient indicated retention, a positive net gradient release of EPC from the coronary circulation.
Net production of stromal cell-derived factor-1 alpha and interleukin-8
A custom cytokine Quantibody Array (RayBiotech, Inc., Norcross, GA, USA) consisted of a glass slide that has been spotted with 16 arrays of specific antibodies. A standard curve and samples were assayed in each well simultaneously through a method similar to a sandwich ELISA. After antibody incubations and washes the fluorescent signals were detected using the Axon GenePix laser scanner (Toronto, Canada). The generated signals of the samples were compared with the signals of the standard curves for each of the cytokines and the data were then analysed using the RayBio Q Analyser software. Net production of SDF-1 alpha and IL-8 was calculated by multiplying their trans-cardiac gradients with CBF.
Biochemical assays
Serum lipids were measured using enzymatic colorimetry and LDL cholesterol calculated from these parameters. Haemoglobin A1c was measured using ion-exchange high performance chromatography (BIO-RAD Variant II Turbo Hemoglobin A1c program, Hercules, CA, USA). Free insulin levels were measured by an automated chemiluminescent immunoenzymatic assay (ACCESS, Beckman-Coulter Inc., Fullerton, CA, USA). High sensitivity C-reactive protein (hs-CRP) levels were measured using a latex particle-enhanced immunoturbidemetric assay on a Hitachi 912 automated analyser. Serum creatinine was measured and glomerular filtration rate (GFR) was estimated as described before.9
Statistical analyses
Pre-specified comparisons between the ED and control subjects were made using the Mann–Whitney U test for continuous variables (SPSS, 18.0). Dichotomous variables were compared using Fisher's exact test.
Because some of the data are not normally distributed, they are presented as median [interquartile (25th to 75th percentile) range]. Spearman correlations were used to describe relationships between the OCN (+) EPC net gradients with % changes in coronary diameter to 10−4 mol/L acetylcholine, % changes in CBF to 10−4 mol/L acetylcholine, and length of the dysfunctional coronary artery segment. Any P-values of <0.05 were considered statistically significant.
Results
Patient characteristics
The relevant clinical and biochemical data as well as the detailed cardiac catheterization data in the study subjects are shown in Tables 1 and 2. There was no statistically significant difference in the prevalence of coronary disease risk factors or Framingham score between the groups. According to the definition of the groups, ED patients did not have significant structural coronary artery disease and as expected, parameters of epicardial and microvascular endothelial function were significantly different in ED when compared with the patients with normal endothelial function. The median length of coronary artery segment with significant ED was 39.88 (26.82, 87.84) mm.
Table 1.
Patient demographics, clinical data
| Control (n = 17) | ED (n = 14) | P-value | |
|---|---|---|---|
| Age (years) | 45 (42.50, 51.50) | 49 (38.25, 55.50) | 0.564 |
| Gender (% male) | 29 | 36 | 0.720 |
| Cholesterol (mmol/L) | 4.81 (4.02, 5.33) | 4.91 (3.53, 5.95) | 0.721 |
| LDL (mmol/L) | 2.69 (2.31, 3.06) | 3.10 (1.82, 3.30) | 0.634 |
| HDL (mmol/L) | 1.34 (1.15, 1.47) | 1.23 (1.11, 1.61) | 0.619 |
| hs-CRPa (mg/L) | 1.8 (0.45, 4.65) | 1.5 (0.70, 2.93) | 0.756 |
| Fasting glucose (mmol/L)b | 5.22 (4.92, 5.72) | 5.44 (5.25, 5.89) | 0.201 |
| Haemoglobin A1c (%) | 5.3 (5.05, 5.65) | 5.45 (5.3, 5.73) | 0.240 |
| Diabetes mellitus (n) | 0 | 0 | |
| Insulin (pmol/L) | 5.9 (4.45, 8.05) | 9.35 (4.45, 14.48) | 0.177 |
| Framingham risk score | 4 (−1, 7) | 3.5 (1, 6.5) | 0.604 |
| BPsyst (mmHg) | 129 (106.50, 141) | 124 (105, 136.75) | 0.487 |
| BPdiast | 72 (65, 82.50) | 73.5 (64, 77.75) | 0.766 |
| GFR (mL/min/1.73m2) | 79 (71.50, 90) | 78.5 (70.50, 96.75) | 0.781 |
| BMI (kg/m2) | 29.1 (23.35, 36) | 29.4 (26.18, 36.95) | 0.606 |
| Statin therapy (%) | 41 | 50 | 0.508 |
aAvailable in 12 of the 14 ED patients.
bAvailable in 13 of the 14 ED patients.
Table 2.
Coronary catheterization data
| Control (n = 17) | ED (n = 14) | P-value | |
|---|---|---|---|
| CFR | 3.2 (2.5, 3.45) | 3.25 (2.65, 4.05) | 0.512 |
| % Changes in CBF (%) | 194.33 (64.03, 268.54) | 23.02 (−36.93, 55.32) | 0.000 |
| % Changes in epicardial diameter (%)a | −2.52 (−7.27, 3.96) | −24.83 (−37.88, −19.97) | 0.000 |
CFR, coronary flow reserve, endothelium-independent microvascular function; CBF, coronary blood flow to 10−4 mol/L acetylcholine, microvascular endothelial function.
aCoronary diameter to 10−4 mol/L acetylcholine.
Flow cytometry
In preliminary experiments, we determined that the vast majority of the CD34+/KDR+ cells were in the small lymphocyte gate, as has also been noted by other investigators.20,21
Total numbers of circulating osteocalcin (−) endothelial progenitor cells and osteocalcin (+) endothelial progenitor cells (aortic sample)
The total numbers of circulating EPC were higher in patients with ED compared with controls [CD34−/CD133+/KDR+/OCN−: 42.75 (26.5, 74.75) vs. 12 (2.25, 18.64), P = 0.001; CD34+/CD133+/KDR+/OCN−: 18.5 (12.45, 37.5) vs. 5.5 (1.49, 12.5), P = 0.002; CD34+/CD133−/KDR+/OCN−: 69.5 (16.09, 126.75) vs. 27 (11.76, 45.75), P = 0.049].
Similarly, patients with ED had higher numbers of circulating OCN (+) EPC [CD34−/CD133+/KDR+/OCN+: 19.5 (8.25, 95.65) vs. 4 (0.5, 30.5), P = 0.067; CD34+/CD133+/KDR+/OCN+: 20 (11.18, 30.05) vs. 6 (2, 16), P = 0.016; CD34+/CD133−/KDR+/OCN+: 15 (4.3, 24.88) vs. 1.5 (0, 9), P = 0.022].
Net gradients of endothelial progenitor cells and osteocalcin (+) endothelial progenitor cells
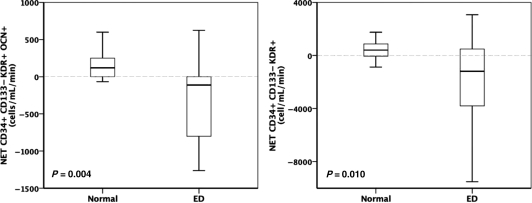
Figure 1 (left panel) shows that patients with ED show a statistically significant net retention of osteogenic, OCN (+) EPC within the coronary circulation (P = 0.004), in contrast to controls, who show an equivocal net gradient. Patients with ED also showed a negative net gradient of EPC in general, which was significantly different to the again equivocal net gradient of controls (Figure 1, right panel, P = 0.010).
Figure 1.
Net gradients of CD34+/CD133−/KDR+/OCN+ and CD34+/CD133−/KDR+ in cells/mL/min. Patients with endothelial dysfunction showed retention of both cell populations in contrast to controls with positive/equivocal net gradients.
There was a significant correlation between the degree of microvascular ED (% changes in CBF to 10−4 mol/L acetylcholine <50%) and the coronary retention of CD34+/KDR+/OCN+ EPC (r = 0.763, P = 0.002). Moreover, we observed a significant correlation between % changes in coronary diameter to 10−4 mol/L acetylcholine and the net retention of CD34+/KDR+/OCN+ EPC (r = 0.609, P = 0.021).
There was no statistically significant correlation between the net gradient of OCN (+) EPC across the coronary circulation and the total length of the epicardial coronary artery segment showing ED.
Net production of stromal cell-derived factor-1 alpha and interleukin-8
Interleukin-8 net production was significantly higher in patients with ED (n = 9) compared with controls [n = 13, controls: −3428.50 (−11225.00, 647.48) vs. ED: 1540.80 (−300.40, 21744.10) pg/mL, P = 0.025]. Net production of SDF-1 alpha was higher in patients with ED compared with controls without reaching statistical significance [controls: 0.00 (−207 980.00, 281 530.00) vs. ED: 12195.60 (0.00, 1 339 100.00) pg/mL, P = 0.281].
Discussion
The current study demonstrates for the first time that patients with early coronary artery disease (angiographically normal coronary arteries but ED) retain circulating EPC with an osteoblastic phenotype [OCN (+) EPC] within the coronary circulation.
In contrast, subjects with normal endothelial function have lower numbers of circulating OCN (+) EPC and do not retain them within the coronary circulation.
Thus, we expand the findings of our recent studies in which we identified OCN (+) EPC and demonstrated that patients with severe obstructive or early coronary atherosclerosis show significantly higher percentages of EPC co-expressing OCN compared with patients with normal coronaries and normal endothelial function.9 Speculatively, patients with ED not only have higher numbers of circulating OCN (+) EPC but may also use these cells for endothelial regeneration, which in turn may actually induce coronary artery calcification rather than normal repair. The role of osteogenic EPC in the process of vascular injury and repair is underscored by the shown correlation between OCN (+) EPC retention and the degree of ED at the epicardial and microcirculatory levels and emphasizes the diffuse nature of ED and the extension of the process into the microcirculatory level.
One can speculate that patients with early coronary artery disease have an intrinsic pro-inflammatory and oxidative stress state, which leads to recurrent endothelial injury and an increased osteogenic phenotyping of circulating EPC. This is supported by recent studies demonstrating a net production of inflammatory and oxidative factors across the coronary circulation in patients with ED.9,10 The number of EPC and EPC–OCN released from the bone marrow is likely higher than the number of cells retained within the coronary circulation, leading to an overall higher number of circulating EPC and EPC–OCN in patients with ED compared with controls.
Using spectral analysis of IVUS radiofrequency data our group recently demonstrated that patients with coronary ED show significantly more calcification in coronary artery segments with endothelial dysfunctional compared with adjacent segments with normal endothelial function.10 Thus, the retention of osteogenic EPC in these patients may lead to initiation as well as progression of coronary artery calcification rather than normal repair. This proposed mechanism may also account for the conflicting results showing EPC contribute to endothelial regeneration and neo-angiogenesis1,22,23 on the one hand but also to atherosclerotic lesion progression with calcification on the other hand.24,25
Similar to the findings in OCN (+) EPC, patients with early coronary atherosclerosis showed higher numbers as well as retention of circulating (CD34+/CD133−/KDR+) EPC in general. These data suggest that, in contrast to patients with normal endothelial function, patients with ED retain EPC within their coronary circulation likely intended for the repair of the injured coronary endothelium. We speculated that an increased release of homing factors (like IL-8 and SDF-1 alpha) that preferentially facilitate homing of osteogenic, OCN (+) EPC may, however, lead to coronary calcification rather than normal endothelial repair.
Stromal cell-derived factor-1 alpha has been shown to induce neovascularization through recruitment of EPC into ischaemic tissue by virtue of SDF-1 alpha/CXCR4 coupling.4,5 We have recently demonstrated that CD34+/KDR+/OCN+ EPC more frequently co-express the homing factor receptor CXCR4 than OCN-EPC.26 Owing to this strong association between OCN and CXCR4 expression, increased SDF-1 alpha release, therefore, likely facilitates homing of OCN (+) EPC rather than non-OCN EPC. In the current study, the net production of SDF-1 alpha was indeed higher in patients with ED than in controls. In addition, the net production of IL-8, an inflammatory cytokine that has been found to be a chemoattractant for EPC in ischaemic tissue,6 was also higher in patients with ED than in controls. Thus, the present findings corroborate and expand previous observations from patients with myocardial ischaemia showing that also in patients with ED, cardiac SDF-1 alpha and IL-8 release likely facilitate abnormal repair of injured endothelium.
Whereas a negative net gradient indicates retention of EPC within the coronary circulation, interpretation of a positive net gradient may be more complex. One potential reason for a positive net gradient of EPC may be a pool or reservoir of resident EPC within the vascular wall. Indeed, Bearzi et al.27 demonstrated recently that EPC are nested in vascular niches throughout the human coronary circulation, corroborating similar studies by Hu et al.28 and Rodriguez-Menocal et al.29 in rodent endothelial injury models. They showed furthermore that these resident EPC differentiate predominantly into endothelial and smooth muscle cells. Likely, EPC from these vascular niches as well as bone marrow-derived EPC participate in endothelial regeneration and atherosclerotic lesion progression.3,28–30 In addition, a potential delay/slow-down of the migration of circulating, bone marrow derived EPC through the dense network of vasa vasorum within the vascular vessel wall may also contribute to a positive net gradient:31 after entry, the time to release of EPC from the vascular wall into either the main coronary lumen or into draining venous vasa vasorum31 may be conceivably delayed and result in a positive net gradient simply by virtue of an additive effect.
Limitations
Our study design does not allow demonstration of the actual implantation of EPC into the coronary circulation or the mechanism by which patients with ED retain OCN (−/+) EPC. We did not perform IVUS or coronary calcium scoring and, hence, are unable to comment on coronary artery calcification in the present study population. In addition, we were, thus, not able to classify the patients according to IVUS assessment of the degree of coronary atherosclerosis.
Conclusion
The current study is the first to evaluate dynamics of EPC across the coronary circulation and demonstrates that patients with early atherosclerosis show retention of EPC with osteogenic properties. Increased numbers of circulating OCN (+) EPC, their retention and preferential homing within the coronary circulation of patients with ED may lead to abnormal vascular repair, initiation and progression of coronary artery disease and calcification. Future studies examining whether EPC phenotyping and/or EPC homing can be altered by medical interventions and, thereby, the atherosclerotic process be prevented or delayed need to be undertaken.
Funding
The study was supported by the National Institute of Health (NIH, AG004875, AG031750, HL64924, HL085307, DK77013, DK73608, and HL77131) and the Mayo Foundation.
Conflict of interest: none declared.
Acknowledgements
We thank Rebecca E. Nelson for helping with patient recruitment, Kelley A. Hoey for helping with the experiments and Ryan J. Lennon for statistical support.
References
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation. 2009;119:547–557. doi: 10.1161/CIRCULATIONAHA.108.788653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 4.Hiasa K, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S, Sata M, Ichiki T, Takeshita A, Egashira K. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004;109:2454–2461. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 6.Kocher AA, Schuster MD, Bonaros N, Lietz K, Xiang G, Martens TP, Kurlansky PA, Sondermeijer H, Witkowski P, Boyle A, Homma S, Wang SF, Itescu S. Myocardial homing and neovascularization by human bone marrow angioblasts is regulated by IL-8/Gro CXC chemokines. J Mol Cell Cardiol. 2006;40:455–464. doi: 10.1016/j.yjmcc.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 8.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 9.Gossl M, Modder UI, Atkinson EJ, Lerman A, Khosla S. Osteocalcin expression by circulating endothelial progenitor cells in patients with coronary atherosclerosis. J Am Coll Cardiol. 2008;52:1314–1325. doi: 10.1016/j.jacc.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavi S, Bae JH, Rihal CS, Prasad A, Barsness GW, Lennon RJ, Holmes DR, Jr, Lerman A. Segmental coronary endothelial dysfunction in patients with minimal atherosclerosis is associated with necrotic core plaques. Heart. 2009;95:1525–1530. doi: 10.1136/hrt.2009.166017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 12.Lavi S, McConnell JP, Rihal CS, Prasad A, Mathew V, Lerman LO, Lerman A. Local production of lipoprotein-associated phospholipase A2 and lysophosphatidylcholine in the coronary circulation: association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation. 2007;115:2715–2721. doi: 10.1161/CIRCULATIONAHA.106.671420. [DOI] [PubMed] [Google Scholar]
- 13.Lavi S, Yang EH, Prasad A, Mathew V, Barsness GW, Rihal CS, Lerman LO, Lerman A. The interaction between coronary endothelial dysfunction, local oxidative stress, and endogenous nitric oxide in humans. Hypertension. 2008;51:127–133. doi: 10.1161/HYPERTENSIONAHA.107.099986. [DOI] [PubMed] [Google Scholar]
- 14.Hasdai D, Gibbons RJ, Holmes DR, Jr, Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96:3390–3395. doi: 10.1161/01.cir.96.10.3390. [DOI] [PubMed] [Google Scholar]
- 15.Han SH, Bae JH, Holmes DR, Jr, Lennon RJ, Eeckhout E, Barsness GW, Rihal CS, Lerman A. Sex differences in atheroma burden and endothelial function in patients with early coronary atherosclerosis. Eur Heart J. 2008;29:1359–1369. doi: 10.1093/eurheartj/ehn142. [DOI] [PubMed] [Google Scholar]
- 16.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 17.Eghbali-Fatourechi GZ, Lamsam J, Fraser D, Nagel D, Riggs BL, Khosla S. Circulating osteoblast-lineage cells in humans. N Engl J Med. 2005;352:1959–1966. doi: 10.1056/NEJMoa044264. [DOI] [PubMed] [Google Scholar]
- 18.Eghbali-Fatourechi GZ, Modder UI, Charatcharoenwitthaya N, Sanyal A, Undale AH, Clowes JA, Tarara JE, Khosla S. Characterization of circulating osteoblast lineage cells in humans. Bone. 2007;40:1370–1377. doi: 10.1016/j.bone.2006.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Traverse JH, Nesmelov YE, Crampton M, Lindstrom P, Thomas DD, Bache RJ. Measurement of myocardial free radical production during exercise using EPR spectroscopy. Am J Physiol Heart Circ Physiol. 2006;290:H2453–H2458. doi: 10.1152/ajpheart.00412.2005. [DOI] [PubMed] [Google Scholar]
- 20.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 21.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 22.Cogle CR, Wainman DA, Jorgensen ML, Guthrie SM, Mames RN, Scott EW. Adult human hematopoietic cells provide functional hemangioblast activity. Blood. 2004;103:133–135. doi: 10.1182/blood-2003-06-2101. [DOI] [PubMed] [Google Scholar]
- 23.Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- 24.Shimizu K, Sugiyama S, Aikawa M, Fukumoto Y, Rabkin E, Libby P, Mitchell RN. Host bone-marrow cells are a source of donor intimal smooth- muscle-like cells in murine aortic transplant arteriopathy. Nat Med. 2001;7:738–741. doi: 10.1038/89121. [DOI] [PubMed] [Google Scholar]
- 25.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 26.Gossl M, Modder UI, Sarano M, Lerman LO, Khosla S, Lerman A. Circulating EPC co-expressing an osteoblastic phenotype are increased in severe calcific aortic valve stenosis. Circulation. 2009;120:S1117. (abstr) [Google Scholar]
- 27.Bearzi C, Leri A, Lo Monaco F, Rota M, Gonzalez A, Hosoda T, Pepe M, Qanud K, Ojaimi C, Bardelli S, D'Amario D, D'Alessandro DA, Michler RE, Dimmeler S, Zeiher AM, Urbanek K, Hintze TH, Kajstura J, Anversa P. Identification of a coronary vascular progenitor cell in the human heart. Proc Natl Acad Sci USA. 2009;106:15885–15890. doi: 10.1073/pnas.0907622106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez-Menocal L, St-Pierre M, Wei Y, Khan S, Mateu D, Calfa M, Rahnemai-Azar AA, Striker G, Pham SM, Vazquez-Padron RI. The origin of post-injury neointimal cells in the rat balloon injury model. Cardiovasc Res. 2009;81:46–53. doi: 10.1093/cvr/cvn265. [DOI] [PubMed] [Google Scholar]
- 30.Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 31.Gossl M, Rosol M, Malyar NM, Fitzpatrick LA, Beighley PE, Zamir M, Ritman EL. Functional anatomy and hemodynamic characteristics of vasa vasorum in the walls of porcine coronary arteries. Anat Rec A Discov Mol Cell Evol Biol. 2003;272:526–537. doi: 10.1002/ar.a.10060. [DOI] [PubMed] [Google Scholar]