Abstract
Purpose
Marginal grafts should be used more actively in Asian countries where deceased donor transplantation is unpopular. We modified a quantitative donor scoring system proposed by Nyberg and his colleagues and developed a donor scoring system in order to assess the quality of deceased donor grafts and their prognostic value as an initial effort to promote usage of marginal donors.
Materials and Methods
We retrospectively evaluated 337 patients.
Results
A scoring system was derived from six donor variables [age, 0-25; renal function, 0-4; history of hypertension, 0-4; Human Leukocyte Antigen (HLA) mismatch, 0-3; body weight, 0-1; cause of death, 0-3 points]. Donor grafts were stratified by scores: grade A, 0-10; grade B, 11-20; grade C, 21-30; and grade D, 31-40 points. Donor grades significantly correlated with estimated glomerular filtration rate (eGFR) at 6 months (A, 64.0 mL/min/1.73 m2; B, 57.0 mL/min/1.73 m2; C, 46.8 mL/min/1.73 m2; p < 0.001). The five-year graft survival rate was also lower in grade C than grade A (74% vs. 93%, p = 0.002). Donors in grade C and D were regarded as marginal donors. The proportion of marginal donors was much lower in Korea, compared with data from the United Network for Organ Sharing (15.2% vs. 29%).
Conclusion
Considering the scarcity of deceased donor kidneys and the relatively better graft outcome with lower grade-donors in Korea, it is worth increasing the usage of marginal grafts.
Keywords: Kidney transplantation, cadaver, donor selection
INTRODUCTION
With the worsening shortage of donor kidneys, kidneys from marginal donors have become an important source for deceased donor kidney transplantation. The number of people waiting for deceased kidney transplantation increases by more than 10% every year in Korea, where deceased kidney transplantation is not popular for cultural reasons. The deceased kidney transplantation rate per million population in Korea was 5.47 in 2007, whereas those in Spain and in USA were 45.9 and 34.8, respectively.1 The inclusion of an older donor, since 1991, has led to a dramatic increase in deceased kidney donations and a decrease in the waiting time for transplantation in Spain.2,3 In the United States, a definition of expanded criteria donors (ECDs) and a targeted allocation policy for kidneys from these donors were approved by the United Network for Organ Sharing (UNOS) board in 2001.4,5
A quantitative donor scoring system proposed by Nyberg and his colleagues was another effort to assess the quality of donor kidneys and their prognostic value.6,7 It is important to inform patients of the prognosis of marginal kidney grafts when deciding whether to receive a marginal kidney. The prognosis of deceased renal grafts is affected by donor variables, some of which vary according to ethnic, social, and environmental backgrounds. Therefore, it is necessary to establish an objective grading system to assess the quality of deceased kidneys, which can predict graft outcomes in Korea. As an initial effort to facilitate the use of marginal grafts in Korea, we modified a quantitative donor scoring system proposed by Nyberg and his colleagues and suggested a Korean donor scoring system and analyzed graft outcomes according to the quality of donors.
MATERIALS AND METHODS
Patients
We reviewed the records of 441 patients who received deceased kidney transplants at two transplantation centres (Seoul National University Hospital and Samsung Medical Center) between June 1994 and April 2008. A non heartbeating donor was not used in our centers. We restricted our cohort to recipients with at least 1 year of documented follow-up, in order to use their renal function at 6 months and 1 year after transplantation as prognostic outcomes. Fifty recipients who received kidneys from donors younger than 15 years were excluded. Another 34 recipients were also excluded, because their follow-up periods were less than 1 year. We excluded 2 patients who died of surgical complications and 18 patients with insufficient information about the donor kidneys. Finally, 337 patients were included in the study. Five-year graft survival rates were calculated only for patients who had been followed for at least 5 years or lost their grafts within 5 years (n = 224). Graft failure was regarded as an estimated glomerular filtration rate (eGFR) less than 10 mL/min/1.73 m2 or conversion to dialysis. We also reviewed the records of 102 independent patients who received deceased kidney transplants at Asan Medical Centre between April 1995 and June 2003 in order to validate the new scoring system.
Determination of glomerular filtration rate (GFR)
GFR, estimated by the abbreviated the modification of diet in renal disease (MDRD) equation,8 was used as the measure of renal function. eGFR was also estimated by the Cockcroft-Gault equation, which uses body weight in its calculation, to find out if body weight was an independent predictor of recipient eGFR. We used the final serum creatinine level obtained before procurement to calculate the donor eGFR. Recipient eGFR was calculated with serum creatinine values obtained every 6 months for up to 5 years after transplantation.
A system for assessing deceased donor kidneys
A donor assessment system for deceased donor kidneys was developed using donor variables that showed statistically significant impact on renal function 6 months after transplantation. The system was then applied to renal function 1 year after transplantation and 5-year graft survival rates. Donor variables included age, cause of death, anti-cytomegalovirus antibody status, history of hypertension (HTN), human leukocyte antigen (HLA) typing, serum creatinine, sex, weight, and donor-recipient weight ratio. The cause of death in the donor was stratified into two categories: cerebrovascular accident or non-cerebrovascular accident. The HLA types of the donor and recipient were compared on the basis of six antigens at A, B, and DR loci to identify donor-recipient mismatches.
Validation of the new donor scoring system
After application of the new donor scoring system to our population, we also applied the system to an independent group of patients in a different hospital to validate its prognostic ability.
Statistical analysis
Statistical analysis was performed using the SPSS version 13.0 (SPSS Inc, Chicago, IL, USA) for Microsoft Windows. We used a Student t-test, analysis of variances and linear regression analysis in order to assess the significance of donor variables. Graft survival rates were determined by the Kaplan-Meier method and were compared by the log rank test. p values of < 0.05 were considered statistically significant for all comparisons.
RESULTS
Significant donor-derived prognostic factors for short-term graft function
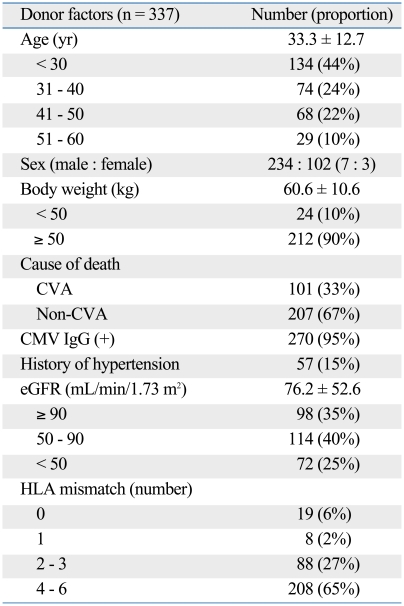
Clinical characteristics of donors are summarized in Table 1. Six donor variables (age, history of HTN, renal function, cause of death, HLA mismatch, and body weight) were found to be significantly associated with recipient GFR 6 months after transplantation (Table 2).
Table 1.
Clinical Characteristics of Donors
CVA, cerebrovascular accident; CMV, cytomegalovirus; eGFR, estimated glomerular filtration rate by the modification of diet in renal disease (MDRD) equation; HLA, human leukocyte antigen.
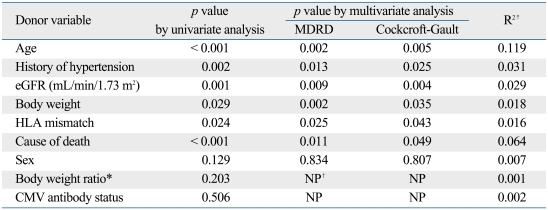
Table 2.
Association between Donor Variables and Recipient eGFR at 6 Months
MDRD, the modification of diet in renal disease; eGFR, estimated glomerular filtration rate by either MDRD equation or Cockcroft-Gault equation; HLA, human leukocyte antigen; CMV, cytomegalovirus; NP, not performed.
*Donor-recipient body weight ratio.
†Analysis not performed.
‡Backward linear regression analysis was used.
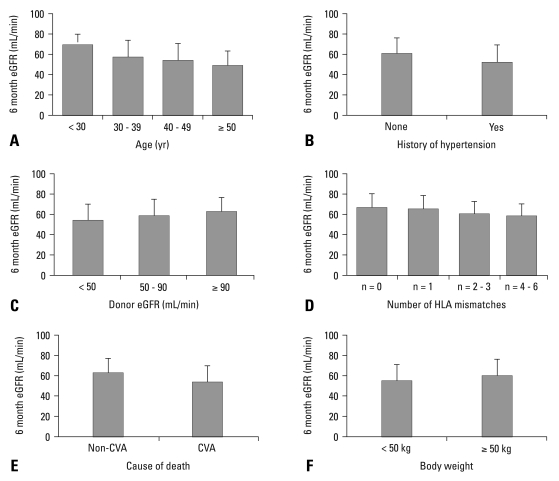
The donor age correlated with recipient renal function. The recipient renal function decreased as the donor's age increased above 30 years. Statistically significant decrements in eGFR were noted between each of age groups (< 30 vs. 30-39, 30-39 vs. ≥ 50, Fig. 1A; p < 0.001, Table 2). The presence of hypertension was an important donor factor that correlated with graft function (p = 0.002, Table 2). Approximately 15% of the donors had a history of hypertension, which negatively correlated with renal function 6 months after transplantation (p = 0.002, Fig. 1B). The effect of the duration of hypertension on graft function could not be evaluated, because there was little information about this. The eGFR of the donor before procurement positively correlated with the eGFR of the recipient 6 months after transplantation (p = 0.001, Table 2, Fig. 1C). When GFR was estimated by the Cockcroft-Gault equation, a similar correlation was found (p = 0.004, data not shown). The eGFR of the recipient was highest when there were no HLA mismatches with the deceased kidney. HLA match was divided into four groups based on recipient renal func-tion 6 months after transplantation (Fig. 1D). A negative correlation was observed between the number of HLA mismatches and recipient renal function (p = 0.024, Table 2). In 33% of the donors, the cause of death was cerebrovascular accident. Renal function 6 months after transplantation was significantly lower in recipients of kidneys from donors with a cerebrovascular cause of death than other causes of death (53.1 mL/min/1.73 m2 vs. 62.1 mL/min/ 1.73 m2, p < 0.001, Fig. 1E; Table 2). Interestingly, low donor body weight (i.e., less than 50 kg) was a significant risk factor for a low eGFR after transplantation (p = 0.029, Table 2). Body weight was less than 50 kg in more than 10% of donors. The mean eGFR 6 months after transplantation was 54.4 mL/min/1.73 m2 when donor body weight was less than 50 kg and 59.8 mL/min/1.73 m2 when it was 50 kg or more (p < 0.026, Fig. 1F). When donor eGFR was estimated by Cockcroft-Gault equation, body weight remained a significant predictor of recipient eGFR (Table 2). However, disparities in recipient and donor body weight measured by their ratio [WtDONOR (kg)/WtRECIPIENT (kg)] did not show a statistically significant impact on recipient renal function 6 months after transplantation (p = 0.203).
Fig. 1.
Impacts of donor age (A), history of donor hypertension (B), donor renal function (C), number of (HLA) mismatch (D), cerebrovascular cause of death (E), and donor body weight (F) on the estimated glomerular filtration rate (eGFR) of recipients 6 months after deceased kidney transplantation. HLA, human leukocyte antigen; CVA, cerebrovascular accident.
Among 283 donors tested for cytomegalovirus (CMV) IgG antibody, 95% were positive. The presence of CMV antibody did not influence graft function (55.3 mL/min/1.73 m2 CMV negative vs. 58.3 mL/min/1.73 m2 CMV positive, p = 0.476). The influence of donor sex on recipient renal function also was not significant (60.5 mL/min/1.73 m2 with male donors vs. 57.4 mL/min/1.73 m2 with female donors, p = 0.079). Diabetes had been diagnosed in 3 donors and statistical analysis was not done. The mean cold ischemic time was 6.4 hours and the impact of this time on graft survival was negligible.
Development of a modified donor scoring system for deceased donor
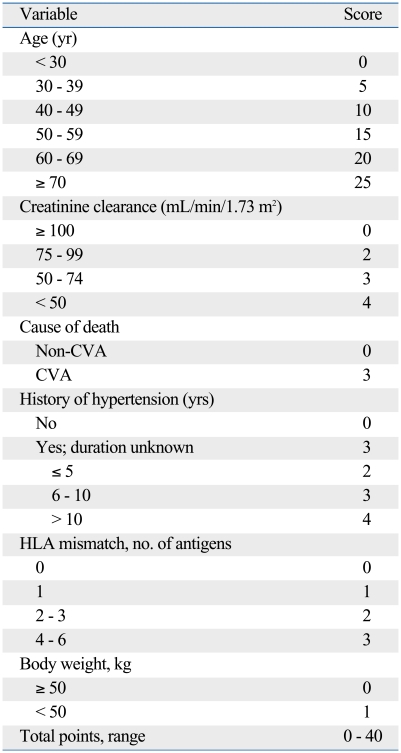
The quantitative scoring system by Nyberg and his colleagues was convenient and useful in assessing deceased donor kidneys in Korea.6,7 We modified the system based on our results of significant donor variables. The modified donor scoring system was developed from six donor variables (age, 0-25 points; history of hypertension, 0-4; estimated glomerular filtration rate before procurement, 0-4; cause of death, 0-3; HLA mismatch, 0-3; body weight, 0-1) that showed significant associations with recipient renal function. The details of the scoring system were provided in Table 3. In each category, a higher score was assigned to factors associated with worse renal function in recipients. A total of 40 points were distributed among the six variables. One point was given to body weight, because it was a new factor not included in the scoring system devised by Nyberg and his colleagues, and allocation of higher points decreased the prognostic value of the total system. The medical records did not include information on the duration of hypertension in most donors and therefore we increased the score to 3 points to donors with unknown duration of hypertension to reflect the relative importance of this factor. Deceased donor kidneys were assigned a letter grade based on the total score (A, 0-10 points; B, 11-20 points; C, 21-30 points; D, 31-40 points).
Table 3.
A Donor Scoring System for Korean Deceased Kidney Transplantation
CVA, cerebrovascular accident; HLA, human leukocyte antigen.
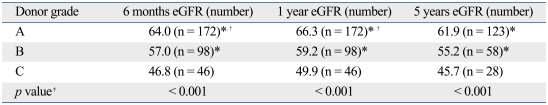
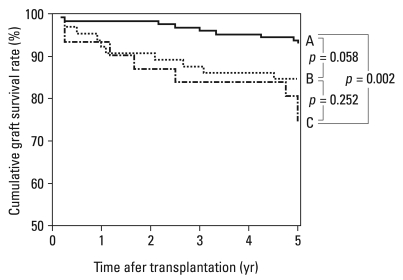
When we applied this scoring system to our study population, the proportion of each grade was 53.4% in grade A, 31.4% in grade B, 14.9% in grade C, and 0.32% in grade D. Only one deceased donor kidney was classified as a grade D, and it was excluded for further statistical analysis. The 6-month renal function was inversely correlated with the level of scores (R2 = 0.153, p < 0.001). The 6-month renal function of recipients decreased with lower grades of donor kidney (grade A, 64.0 mL/min/1.73 m2; grade B, 57.0 mL/min/1.73 m2; grade C, 46.8 mL/min/1.73 m2, p < 0.001, Table 4). When this scoring system was tested for its ability to predict renal function 12 months after transplantation, the results showed the same tendency as those at 6 months (grade A, 66.3 mL/min/1.73 m2; grade B, 59.2 mL/min/1.73 m2; grade C, 49.9 mL/min/1.73 m2, p < 0.001, Table 4). We also determined 5-year graft survival rates for recipients of grade A to C kidneys. 5-year graft survival rates among recipients of grade A, B, and C grafts were approximately 93%, 85%, and 74%, respectively, with a statistically significant difference between A and C (p = 0.002; Fig. 2). Taken together, our system had good predictive power for graft prognosis. Compatible with the system devised by Nyberg and his colleagues, donors of grade C and D were regarded as marginal donors.6
Table 4.
Recipient Renal Function 6 Months, 1 and 5 Years after Transplantation According to the Donor Grade
eGFR, estimated glomerular filtration rate (mL/min/1.73 m2).
*p < 0.05 vs. grade C.
†p < 0.05 vs. grade B.
‡ANOVA test.
Fig. 2.
Impacts of the deceased donor grade on the graft survival. The grade of donor kidneys had a consistent influence on graft survival after transplantation. The 5-year graft survival of grades A, B, and C kidneys were, 93%, 85%, and 74%, respectively. The difference of 5-year graft survival rates was statistically significant between grade A and grade C (p = 0.002).
Validation of the new donor scoring system
Our donor scoring system was assessed for its prognostic power using a new, independent cohort. There was a steady decline in 5-year renal function with advanced grades. Five-year eGFRs for grade A, B, and C in a new cohort were 67.7 mL/min/1.73 m2 (n = 42), 57.3 mL/min/1.73 m2 (n = 38), and 52.0 mL/min/1.73 m2 (n = 11), respectively (p = 0.047). However, impacts of the donor grade on the recipient renal function 6-months after transplantation were not as evident as on the 5-year renal function, maybe because the number of patients was relatively small, especially in grade C (grade A, 70.4 mL/min/1.73 m2, n = 47; grade B, 63.1 mL/min/1.73 m2, n = 44; grade C, 61.8 mL/min/1.73 m2, n = 11, p = 0.165).
Marginal donor usage and its prognosis
Next, we roughly compared our population with the UNOS database for marginal donor usage and its prognosis. The graft scoring system devised by Nyberg and his colleagues was used instead of our new scoring system due to inaccessibility of the raw UNOS data.6 The proportion of grade C-D donors in our population was much lower than that in the UNOS database (15.2% vs. 29%). One year estimated GFRs for grade A, B, and C in our population were 66.7 mL/min/1.73 m2, 59.5 mL/min/1.73 m2, and 50.0 mL/min, respectively, and these values were about 9.3%, 11.5%, and 11.7% higher than those of the corresponding grades in UNOS data (for grade A, for grade B, and for grade C).6 We also found that 5-year graft survival rates of all grades in our study population were about 10% higher than those of the corresponding grades in UNOS data (Fig. 2). Overall, these results showed that marginal donors are used less in Korea and the graft outcomes from marginal donors in Korea seemed to be at least as good as those in the States, irrespective of their grades.
DISCUSSION
The donor scoring system proposed by Nyberg and his colleagues is a good tools for evaluating marginal donors.6,7 However, donor ethnicity could influence recipient renal function at 6 months, as they noted. In particular, renal function was significantly lower in Asian donors, compared to those of other ethnicities in their study. Other important factors such as cold ischemic time and CMV serostatus can be different among different countries. Therefore, it seemed necessary to optimize the donor scoring system according to the local situation, such as ethnic backgrounds and socio-environmental factors. Here, we proposed a donor scoring system derived from Korean data as a baseline data to promote usage of marginal donors.
We found that lower body weight in deceased donors was associated with worse renal function (i.e., lower eGFR) after transplantation. There have been debates on the significance of body weight in kidney transplantation.9,10 Body size, measured as body surface area or body weight, correlates with glomerular volume,11,12 kidney weight,11,12 and GFR.13,14 Even though Nyberg and his colleagues excluded donors weighing less than 40 kg, small donors were not excluded in our study, because Korean people tend to weigh less than those in the US. We analyzed data using donor GFRs estimated by both the MDRD equation and the Cockcroft-Gault equation. The Cockcroft-Gault equation is designed to compensate for the effect of weight on estimated GFR, whereas the MDRD equation does not include body weight in the estimation of GFR. When donor GFR was estimated by the Cockcroft-Gault equation involving weight, a body weight below 50 kg was still a significant prognostic factor in graft outcomes (data not shown). The mean ratio of donor-to-recipient body weight was 0.83 for donors weighing less than 50 kg and 1.09 for donors weighing 50 kg or more (p < 0.05). When the donor-recipient body weight ratio was low, the renal function in recipients 6 months after transplantation was lower (< 0.8 ratio, mean eGFR = 55.5 mL/min/1.73 m2; 0.8-1.2 of ratio, mean eGFR = 58.1 mL/min/1.73 m2; ≥ 1.2 of ratio, mean eGFR = 60.3 mL/min/1.73 m2). However, these differences in eGFR were not statistically significant (p = 0.452). Therefore, other body weight-related factors such as malnutrition might explain the impact of donor body weight on the graft outcome. Cold ischemic time was very short in most of Korean deceased transplantations (mean 6.4 hours) and the impact of this variable on graft survival was negligible. Due to high positivity of 95% among Korean donors, the impact of CMV serostatus was also negligible in our study. Overall, our new scoring system was not only convenient, but also predictive of graft function.
When we roughly compared our Korean data and UNOS data using the Nyberg's scoring system, the proportion of grade C-D donors (marginal donors) was much lower in Korea. We found that graft survival rate of grade C kidneys was higher in our Korean group than that of USA by about 10%. Even though comparison of graft survival between the two groups in this way have limitations, good graft outcome might result from better quality of deceased donor kidneys in Korea, which could be attributed to uniformly shorter cold ischemic time in Korean donors as well as relatively homogenous ethnicity.
Thirty-four recipients were excluded, because their follow-up periods were less than 1 year in the initial period of our study. We evaluated their results after a year and found that only one among them experienced graft failure. Four recipients received steroid pulse therapy due to acute cellular rejection but recovered renal function perfectly. Nineteen recipients received a kidney of A grade, 9 recipients that of a B grade, 6 recipients that of a C grade. The proportion of each grade was 55.9% in grade A, 26.5% in grade B, 17.6% in grade C, and 0.0% in grade D, and not different with the study group (p = 0.342).
In summary, deceased kidney transplantation in a Korean population involved less marginal grafts, but these grafts had relatively good outcomes. We developed a donor scoring system with good correlation with graft outcomes. In order to overcome the severe donor shortage, it is worth increasing usage of deceased organs with lower grades after careful evaluation with the donor scoring system optimized for the Korean population. We will try to answer the question 'who should receive them?' and the 'old for old' policy may be considered as an option. We need to obtain enough data to persuade them to receive a marginal kidney. The prospective study already provides a step in achieving this.
ACKNOWLEDGEMENTS
This work was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (Project No.: A040004).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.International Registry Organ Donation and Transplantation. http://www.tpm.org.
- 2.Miranda B, Gonzalez Alvarez I, Cuende N, Naya MT, De Felipe C. Update on organ donation and retrieval in Spain. Nephrol Dial Transplant. 1999;14:842–845. doi: 10.1093/ndt/14.4.842. [DOI] [PubMed] [Google Scholar]
- 3.Port FK, Bragg-Gresham JL, Metzger RA, Dykstra DM, Gillespie BW, Young EW, et al. Donor characteristics associated with reduced graft survival: an approach to expanding the pool of kidney donors. Transplantation. 2002;74:1281–1286. doi: 10.1097/00007890-200211150-00014. [DOI] [PubMed] [Google Scholar]
- 4.Metzger RA, Delmonico FL, Feng S, Port FK, Wynn JJ, Merion RM. Expanded criteria donors for kidney transplantation. Am J Transplant. 2003;3(Suppl 4):114–125. doi: 10.1034/j.1600-6143.3.s4.11.x. [DOI] [PubMed] [Google Scholar]
- 5.Nyberg SL, Baskin-Bey ES, Kremers W, Prieto M, Henry ML, Stegall MD. Improving the prediction of donor kidney quality: deceased donor score and resistive indices. Transplantation. 2005;80:925–929. doi: 10.1097/01.tp.0000173798.04043.af. [DOI] [PubMed] [Google Scholar]
- 6.Nyberg SL, Matas AJ, Kremers WK, Thostenson JD, Larson TS, Prieto M, et al. Improved scoring system to assess adult donors for cadaver renal transplantation. Am J Transplant. 2003;3:715–721. doi: 10.1034/j.1600-6143.2003.00111.x. [DOI] [PubMed] [Google Scholar]
- 7.Nyberg SL, Matas AJ, Rogers M, Harmsen WS, Velosa JA, Larson TS, et al. Donor scoring system for cadaveric renal transplantation. Am J Transplant. 2001;1:162–170. [PubMed] [Google Scholar]
- 8.Levey AS, Greene T, Kusek J, Beck GJ, Group MS. A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract] J Am Soc Nephrol. 2000;11:A0828. [Google Scholar]
- 9.Cho YW, Terasaki PI, Cecka JM, Gjertson DW, Takemoto S. Should excessive height and weight differences between the kidney donor and recipient be avoided? Transplant Proc. 1997;29:104–105. doi: 10.1016/s0041-1345(96)00023-1. [DOI] [PubMed] [Google Scholar]
- 10.Kasiske BL, Snyder JJ, Gilbertson D. Inadequate donor size in cadaver kidney transplantation. J Am Soc Nephrol. 2002;13:2152–2159. doi: 10.1097/01.asn.0000024564.22119.3d. [DOI] [PubMed] [Google Scholar]
- 11.Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec. 1992;232:194–201. doi: 10.1002/ar.1092320205. [DOI] [PubMed] [Google Scholar]
- 12.Kasiske BL, Umen AJ. The influence of age, sex, race, and body habitus on kidney weight in humans. Arch Pathol Lab Med. 1986;110:55–60. [PubMed] [Google Scholar]
- 13.White AJ, Strydom WJ. Normalisation of glomerular filtration rate measurements. Eur J Nucl Med. 1991;18:385–390. doi: 10.1007/BF02258428. [DOI] [PubMed] [Google Scholar]
- 14.Newman EV, Bordley J, Winternitz J. The interrelationships of glomerular filtration rate (mannitol clearance), extracellular fluid volume, surface area of the body, and plasma concentration of mannitol. Johns Hopkins Med J. 1944;75:253–268. [Google Scholar]