Abstract
Purpose
Ethyl pyruvate has anti-inflammatory properties and protects organs from ischemia/reperfusion (I/R)-induced tissue injury. The aim of this study was to determine whether ethyl pyruvate decreases the inflammatory response after regional I/R injury and whether ethyl pyruvate protects against delayed regional I/R injury in an in vivo rat heart model after a 24 hours reperfusion.
Materials and Methods
Rats were randomized to receive lactated Ringer's solution or ethyl pyruvate dissolved in Ringer's solution, which was given by intraperitoneal injection 1 hour prior to ischemia. Rats were subjected to 30 min of ischemia followed by reperfusion of the left coronary artery territory. After a 2 hours reperfusion, nuclear factor κB, myocardial myeloperoxidase activity, and inflammatory cytokine levels were determined. After the 24 hours reperfusion, the hemodynamic function and myocardial infarct size were evaluated.
Results
At 2 hours after I/R injury, ethyl pyruvate attenuated I/R-induced nuclear factor κB translocation and reduced myeloperoxidase activity in myocardium. The plasma circulating levels of inflammatory cytokines decreased significantly in the ethyl pyruvate-treated group. At 24 hours after I/R injury, ethyl pyruvate significantly improved cardiac function and reduced infarct size after regional I/R injury.
Conclusion
Ethyl pyruvate has the ability to inhibit neutrophil activation, inflammatory cytokine release, and nuclear factor κB translocation. Ethyl pyruvate is associated with a delayed myocardial protective effect after regional I/R injury in an in vivo rat heart model.
Keywords: Ethyl pyruvate, myocardium, reperfusion injury, inflammation
INTRODUCTION
Pyruvate (CH3COCOO-), a key intermediate in the oxidative and anaerobic metabolism of glucose, has a potent reactive oxygen species scavenging effect. The protective effects of pyruvate against oxidative stress have been reported.1,2 Pyruvate also ameliorates organ injury or dysfunction in animal models of myocardial,3 cerebral,4 intestinal,5 and hepatic6 ischemia/reperfusion (I/R) injury. However, the use of pyruvate as a therapeutic agent is limited by its aqueous instability; thus, its ethyl ester form is used as a practical pyruvate precursor for administration.7 Ethyl pyruvate (EP) is a lipophilic ester derivative of pyruvate, which has a myocardial protective effect in an in vivo model by various mechanisms.8,9
EP is an effective anti-inflammatory agent in a variety of in vitro and in vivo model systems.10-12 In the present study, we used a regional myocardial I/R injury model to compare the effects of treatment with lactated Ringer's solution and EP dissolved in Ringer's solution on several physiological and biochemical variables.
Myocardial I/R injury is a major disease entity faced in many clinical situations such as coronary thrombolytic therapy, percutaneous transluminal coronary angioplasty, coronary artery bypass graft, and cardiac transplantation. In this situation, choosing the appropriate fluids to reduce myocardial I/R injury is critical.
The objectives of the study were to determine whether EP decreases inflammatory response in myocardium and plasma at 2 hours after I/R injury and whether EP has delayed myocardial protective effects after regional I/R injury at 24 hours post-I/R injury.
MATERIALS AND METHODS
Animal preparation
Male Sprague-Dawley rats (Sam Tako Inc., Osan, Korea) weighing between 220 and 250 g were used for this study. All animals were maintained in accordance with the Guidelines for the Care and Use of Laboratory Animals published by the US National Institutes of Health in 1996. Animal preparation and surgery were done according to previous report.13 Animals received general anesthesia with an intramuscular injection of 80 mg/kg ketamine (ketamine HCl®, Huons, Seoul, Korea) and 8 mg/kg zylazine (Rompun®, Bayer Korea, Seoul, Korea). Once the rat tail moved, we judged the rat as awakening from the anesthesia. We additionally injected 40 mg/kg ketamine and 4 mg/kg zylazine to maintain the level of anesthesia. The trachea was intubated, and the rats were mechanically ventilated (tidal volume, 8 mL/kg; respiratory rate, 80 bpm) with ambient air using a volume-controlled rodent ventilator (Type 7025; Ugo Basile, Comerio, Italy). Arterial blood pH and gases were maintained within normal physiological limits (pH 7.35-7.45, PaCO2 35-40 mmHg, PaO2 70-100 mmHg).
A paramedian sternotomy was performed, and the pericardium was opened to expose the heart. For the I/R experiments, a 4-0 black silk, nonabsorbable suture (Mersilk; Ethicon Inc., Somerville, NJ, USA) was passed around the left coronary artery (LCA) territory to induce regional myocardial ischemia. Coronary artery occlusion was achieved by tightening the snare through a small vinyl chloride tube and clamping it with a mosquito hemostat. Reperfusion was achieved by releasing the clamp. Myocardial ischemia was verified by the appearance of regional epicardial cyanosis over the myocardial surface and arrhythmia. After 30 min of ischemia, the myocardium was reperfused by loosening the snare, which was maintained for 2 or 24 hours of reperfusion. Successful reperfusion was confirmed by the disappearance of epicardial cyanosis and production of epicardial hyperemia and arrhythmia after releasing the snare. The body temperature was monitored with a rectal thermometer (Sirecust 1260; Siemens Medical Electronics, MA, USA) and maintained at 36-38℃ with an electrical heating pad.
Experimental protocols
Each drug was administered intraperitoneally 1 hour before the ischemic insult. We grasped the abdominal wall with two fingers to protect the bowel organs from the drug injection. We injected the drugs into the intraperitoneal space with a 23 G needle and syringe. All animals underwent a 30 min LCA occlusion followed by 2 or 24 hours of reperfusion. The animals were randomly allocated into two sets of experiments, with each set consisting of four groups. The randomization was allocated by one author with a numbered container. The sample size calculation is based on our preliminary study. The appropriate sample size was 7 by using PASS 2008 (Statistical & Power Analysis Software; NCSS, Utah, USA).
The first set of experiment
In the first set of experiments, 48 rats were divided into four groups as follows. 1) Sham + 4 mL lactated Ringer's solution (LR) group, sham-operated rats in which the LCA was not tightened (n = 12); 2) sham + EP50 group, sham-operated rats in which 50 mg/kg EP dissolved in 4 mL Ringer's solution was administered intraperitoneally 1 hour before the sham operation (n = 12); 3) I/R + LR group, rats in which 4 mL LR was administered intraperitoneally 1 hour before ischemic insult (n = 12); and 4) I/R + EP50 group, rats in which 50 mg/kg EP dissolved in 4 mL Ringer's solution was administered intraperitoneally 1 hour before ischemic insult (n = 12). Vehicle (LR 4 mL) or EP (50 mg/kg) dissolved in 4 mL Ringer's solution was administered by intraperitoneal injection 1 hour before ischemia. The animals were exposed to 30 min of ischemia followed by 2 hours of reperfusion, and tissue samples (n = 6) were used to detect NF-κB translocation and myeloperoxidase (MPO) activity. Blood samples (1 mL in each group, n = 6) were collected at baseline and after 2 hours reperfusion in tubes containing heparin to measure the tumor necrosis factor (TNF)-α and interleukin (IL)-β concentrations. The entire sample was immediately centrifuged. Plasma was collected and frozen at -70℃ until analyzed.
The second set of experiment
In the second set of experiments, animals were divided into four groups as follows. 1) Sham + 4 mL lactated Ringer's solution (LR) group, sham-operated rats in which the LCA was not tightened (n = 15); 2) I/R + LR group, rats in which 4 mL LR was administered intraperitoneally 1 hour before ischemic insult (n = 15); 3) I/R + EP25 group dissolved in 4 mL Ringer's solution, rats in which 25 mg/kg EP was administrated intraperitoneally 1 hour before ischemic insult (n = 15); and 4) I/R + EP50 group, rats in which 50 mg/kg EP dissolved in 4 mL Ringer's solution was administered intraperitoneally 1 hour before ischemia insult (n = 15). The animals were exposed to 30 min of ischemia followed by a 24 hours reperfusion, and infarct size and heart function was estimated.
Preparation of nuclear and cyotplasmic proteins
The isolation of nuclear and cytoplasmic protein was done according to previous report.14 The heart sample area at risks (AARs) were suspended in a buffer [10 mM Tris (pH 7.5), 1.5 mM MgCl2, 10 mM KCl, and 0.1% Triton X-100], and lysed by homogenisation. The lysed sample was isolated by microcentrifugation at 7,500 rpm for 5 min. The supernatant, which contained cytoplasmic protein, was collected and stored at -80℃ prior to Western blot analysis. Nuclear proteins were extracted at 4℃ by resuspending the nuclear pellets in a buffer [20 mM Tris (pH 7.5), 20% glycerol, 1.5 mM MgCl2, 420 mM NaCl, 0.2 mM ethylenediaminetetraacetic acid (EDTA), and 0.1% Triton X-100]. The nuclear pellets were left at 4℃ with occasional vortex during 1 hour incubation. The pellets were centrifuged at 13,000 rpm for 15 min, the supernatant specimen that contained nuclear protein was collected. The protein concentrations of the samples were equal in all experiments.
Western blotting
Proteins were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and incubated with monoclonal antibodies to nuclear factor (NF)-κB p65 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 4℃ overnight. Proteins were detected with horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) [1 : 5,000 in Tris-buffered saline and Tween 20 (TBS-T) containing 5% skimmed milk powder for 1 hour at room temperature] and were visualised by enhanced chemiluminescence.
Plasma cytokine measurement
ELISA kits (R&D Systems, Minneapolis, MN, USA) were used for detection of TNF-α and IL-1β according to the manufacturer's instructions as earlier described.14 The plasma samples reacted with the assay reagents in the TNF-α and IL-1β kits, respectively, and analyzed spectrophotometrically (Infinite® F200; Tecan Group, Mannedorf, Switzerland) at an absorbance of 450 nm.
Evaluation of MPO activity
MPO activity, a marker for neutrophil accumulation, was evaluated according to the method of Bradley, et al. Myocardial tissue (ca. 500 mg) was excised from the AAR after 2 hours reperfusion and homogenized in 50 mM/L potassium phosphate buffer at pH 6.0. The samples were centrifuged for 30 min at 20,000 g and 4℃ before extraction. The resulting pellets were used to measure MPO activity after suspension in 50 mM/L potassium phosphate buffer containing 0.5% hexadecyltrimethyl ammonium bromide. The pellets were centrifuged at 20,000 g for 15 min; a 0.2 mL supernatant specimen was added to 0.8 mL of 50 mM/L potassium phosphate buffer (pH 6.0) containing 0.167 mg/mL 3,3'-dimethoxybenzidine dihydrochloride and 0.0005% hydrogen peroxide. Absorbance was measured spectrophotometrically at 460 nm using a spectrophotometer (Lambda EZ 210; PerkinElmer, Waltham, MA, USA) for 2 min. One unit of MPO was defined as the activity that degraded 1 µM/min peroxide at 25℃.
Measurement of hemodynamic variables and myocardial contractility
After the 24 hours reperfusion, rats were anesthetised with 30 mg/kg ketamine and 3 mg/kg xylazine. The right common carotid artery was cannulated with a 2-Fr Millar catheter (Model SPR-407; Millar Instruments, Houston, TX, USA) and the catheter was advanced into the left ventricle (LV) to measure heart rate (HR), left ventricular end diastolic pressure (LVEDP), and maximum rate of LV pressure increase (+dP/dtmax) and decrease (-dP/dtmax).
Determination of the area at risk and infarct size
After the cardiac function analysis, the LCA was occluded again and 2 mL of 1% Evans blue dye was injected into the pulmonary artery to facilitate AAR determination. The Evans blue dye stained the perfused myocardium, whereas the occluded vascular bed remained unstained. The atria, right ventricles, and major vessels were removed from the heart, and the AAR was separated from the non-ischemic area. The AAR was cut into small pieces and incubated in 2% 2,3,5-triphenyltetrazolium chloride (TTC) at 37℃ for 20 min. The TTC stained the non-infarcted myocardium a deep red color, and the infarcted area (IA) remained pale yellow. The sections were fixed in 2% formalin solution for 24 hours. The LV, AAR, and IA weights were calculated. The AAR was expressed as a percentage of the LV weight, and the IA as a percentage of the AAR weight.
Statistical analysis
All values are expressed as the mean ± SD. All statistical analyses were performed using statistics software (ver. 10.0 for Windows; SPSS Inc., Chicago, IL, USA). Differences in the relationship amongst groups were evaluated by the Friedman one-way ANOVA and post hoc Duncan's multiple comparison test. Statistical significance was determined as p < 0.05.
RESULTS
EP modulates inflammatory signalling pathways and modifies NF-κB activation
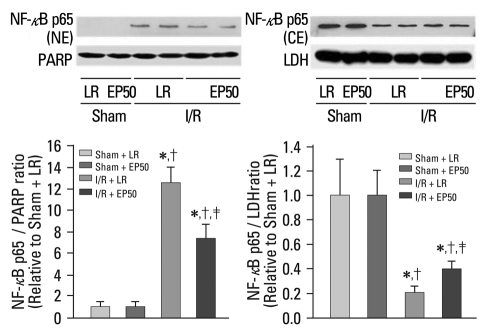
The changes in the cytosolic and nuclear extractions are shown in Fig. 1. NF-κB expression in the cytosolic fraction was attenuated significantly in the I/R + LR group compared to the sham + LR group. In contrast, expression in the nuclear fraction was enhanced in the I/R + LR group; however, this change was inhibited by EP pretreatment, which suggests that I/R-induced NF-κB translocation into the nucleus was inhibited by EP.
Fig. 1.
Effect of EP on I/R-induced translocation of NF-κB. Representative illustration of NF-NF-κB expression in the cytosolic and nuclear fractions of rat ischemic myocardium by Western blotting. Sham + LR, sham-operated rats in which no tightening of the left coronary artery was performed; sham + EP50, sham-operated rats in which 50 mg/kg EP was administered intraperitoneally 1 hour before ischemic insult; I/R + LR, rats in which 4 mL LR was administered intraperitoneally 1 hour before ischemic insult; I/R + EP50, rats in which 50 mg/kg EP was administered intraperitoneally 1 hour before ischemic insult. NF-NF-κB, nucl-ear factor-NF-κB; NE, nuclear extraction; CE, cytosolic extraction; I/R, ischemia/reperfusion; EP, ethyl pyruvate; LR, lactated Ringer's solution. *p < 0.05 vs. sham + LR. †p < 0.05 vs. sham + EP50. ‡p < 0.05 vs. I/R + LR.
EP decreases production of inflammatory mediators and MPO activity
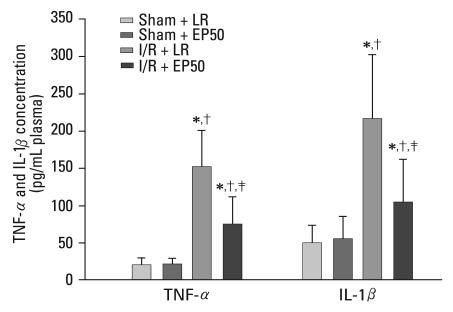
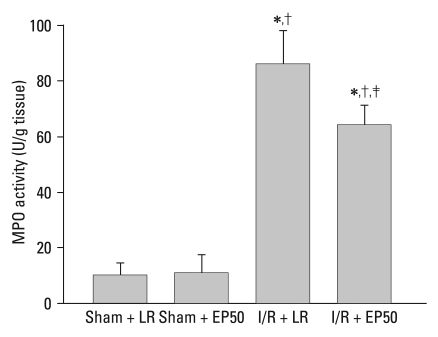
I/R induced a significant increase in the plasma levels of inflammatory cytokines after the 2 hours reperfusion (Fig. 2). However, in the I/R + EP50 group, the elevation in plasma TNF-α concentration was inhibited significantly compared to that in the I/R + LR group (75 ± 36.7 vs. 152 ± 48.9 pg/mL plasma, p = 0.010). The IL-1β level was also reduced in the I/R + EP50 group compared to the I/R + LR group (105 ± 56 vs. 216 ± 85 pg/mL plasma, p = 0.040). The TNF-α and IL-1β levels were not significantly different between the Sham + LR and Sham + EP50. MPO activity in the ischemic area of the myocardium was reduced significantly in the I/R + EP50 group compared to the I/R + LR group (64 ± 7.4 vs. 86 ± 12.1 U/g tissue, p = 0.030) after regional I/R injury (Fig. 3).
Fig. 2.
Cardiac production of pro-inflammatory cytokines. Plasma levels of TNF-α and IL-1β at 2 hours post-reperfusion in each group. Values are the mean ± SD (n = 6 in each group). Sham + LR, sham-operated rats in which no tightening of the left coronary artery was performed; sham + EP50, sham-operated rats in which 50 mg/kg EP was administered intraperitoneally 1 hour before ischemic insult; I/R + LR, rats in which 4 mL LR was administered intraperitoneally 1 hour before ischemic insult; I/R + EP50, rats in which 50 mg/kg EP was administered intraperitoneally 1 hour before ischemic insult. TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; I/R, ischemia/reperfusion; EP, ethyl pyruvate; LR, lactated Ringer's solution. *p < 0.05 vs. sham + LR. †p < 0.05 vs. sham + EP50. ‡p < 0.05 vs. I/R + LR.
Fig. 3.
MPO activity was expressed as U/g area at risk (AAR) obtained from the sham + LR, sham + EP50, I/R + LR, and I/R + EP50 groups. Values are the mean ± SD (n = 6 in each group). Sham + LR, sham-operated rats in which no tightening of the left coronary artery was performed; sham + EP50, sham-operated rats in which 50 mg/kg EP was administered intraperitoneally 1 hour before ischemic insult; I/R + LR, rats in which 4 mL LR was administered intraperitoneally 1 hour before ischemic insult; I/R + EP50, rats in which 50 mg/kg EP was administered intraperitoneally 1 hour before ischemic insult. MPO activity, myeloperoxidase activity; I/R, ischemia/reperfusion.; EP, ethyl pyruvate; LR, lactated Ringer's solution. *p < 0.05 vs. sham + LR. †p < 0.05 vs. sham + EP50. ‡p < 0.05 vs. I/R + LR.
EP improves cardiac function after I/R injury
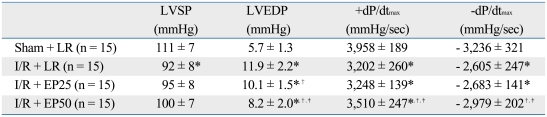
Hemodynamic measurements recorded at 24 hours after reperfusion revealed substantial LV dysfunction as demonstrated by an increased LVEDP (11.9 ± 2.2 vs. 5.7 ± 1.3 mmHg, p < 0.001) and decreased contractility. The +dP/dtmax shows the decreased contractility in the I/R + LR group compared to the sham + LR group (+3,202 ± 260 vs. +3,958 ± 189 mmHg/sec, p < 0.001). The -dP/dtmax shows the decreased contractivity in the I/R + LR group compared to the sham + LR group (- 2,605 ± 247 vs. - 3,236 ± 321 mmHg/sec, p < 0.001) (Table 1). The +dP/dtmax shows an inhibited decrease of contractility in the I/R + EP50 compared to the I/R + LR group (+3,510 ± 247 vs. +3,202 ± 260 mmHg/sec, p = 0.002). Also, the -dP/dtmax shows an inhibited decrease of contractility in the I/R + EP50 compared to the I/R + LR group (- 2,979 ± 202 vs. - 2,605 ± 247 mmHg/sec, p < 0.001). Moreover, the elevated LVEDP was significantly attenuated in the I/R + EP50 group (8.2 ± 2.0 vs. 11.9 ± 2.2 mmHg, p < 0.001). Consequently, EP significantly recovered heart function after myocardial I/R injury (Table 1).
Table 1.
Hemodynamic Data under Ischemia/Reperfusion Conditions
Values are the mean ± SD. Sham + LR, sham-operated rats in which no tightening of the left coronary artery was performed; I/R + LR, rats in which 4 mL LR was administered intraperitoneally 1 hour before ischemic insult; I/R + EP25, rats in which 25 mg/kg EP was administrated intraperitoneally 1 hour before ischemic insult; I/R + EP50, rats in which 50 mg/kg EP was administered intraperitoneally 1 hour before ischemic insult.
LVSP, left ventricular systolic pressure; LVEDP, left ventricular end diastolic pressure; I/R, ischemia/reperfusion; EP, ethyl pyruvate; LR, lactated Ringer's solution.
*p < 0.05 vs. sham + LR.
†p < 0.05 vs. I/R + LR.
‡p < 0.05 vs. I/R + EP25.
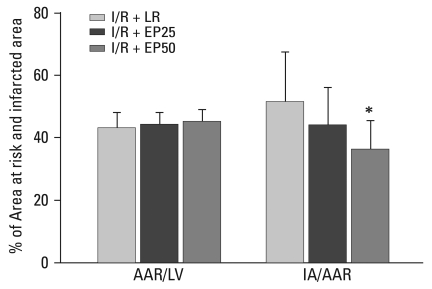
EP reduces infarct size
No significant differences were found in the AAR/LV induced by LCA occlusion among the groups. The IA/AAR was 51 ± 4% in the I/R + LR group and 44 ± 3% in the I/R + EP25 group (p = 0.040 vs. I/R + LR group). However, in the I/R + EP50 group, IA/AAR was reduced significantly to 36 ± 2% (p = 0.014 vs. I/R + LR group) (Fig. 4).
Fig. 4.
Comparison of myocardial infarct size in the I/R + LR, I/R + EP25, and I/R + EP50 groups. Values are means ± SD (n = 15 in each group). I/R + LR, rats in which 4 mL LR was administered intraperitoneally 1 hour before ischemic insult; I/R + EP25, rats in which 25 mg/kg EP was administrated intraperitoneally 1 hour before ischemic insult; I/R + EP50, rats in which 50 mg/kg EP was administered intraperitoneally 1 hour before ischemic insult. I/R, ischemia/reperfusion; LR, lactated Ringer's solution; EP, ethyl pyruvate; AAR, area at risk; LV, left ventricle; IA, infracted area. *p < 0.05 compared to the I/R + LR group.
DISCUSSION
We found that myocardial I/R injury activated the NF-κB-induced inflammatory response, whereas EP pre-treatment resulted in an attenuation of NF-κB translocation, inflammatory mediator expression, and myocardial MPO activity. The protective effects of EP may be attributable, in part, to suppression of the inflammatory response via down-regulation of NF-κB-induced inflammation by I/R injury after reperfusion. Other studies have reported that inflammatory cytokines and MPO activity are elevated between 30 and 60 min after reperfusion.15-17 NF-κB levels increased biphasically in post-ischemic tissue with peak levels at 15 min and again at 3 hours after reperfusion18; thus, we checked NF-κB translocation and MPO activity in myocardial tissue and the serum inflammatory cytokine levels at 2 hours after reperfusion.
NF-κB increases the expression of the genes for many cytokines, enzymes, and adhesion molecules in an inflammatory situation. The activation of NF-κB therefore leads to a coordinated increase in the expression of many genes whose products mediate inflammatory responses.19 NF-κB has been identified as a transcription factor and plays an important role in the onset of inflammatory responses stimulated by pro-inflammatory cytokines such as TNF-α, IL-1, IL-6, and IL-8.10-12 Because EP inhibits NF-κB activation in a variety of in vivo and in vitro systems, the myocardial protective effect of EP may be related to NF-κB inactivation by anti-inflammatory agents.11,12,20 EP pre-treatment resulted in the attenuation of NF-κB translocation and the levels of TNF-α and IL-1β. The protective effects of EP may be partially attributable to suppression of the inflammatory response via down-regulation of NF-κB.
EP reduced myocardial I/R injury by inactivating NF-κB. Our results suggest that EP exerts myocardial protection by inhibiting the inflammatory process and that it may be related to down-regulation of NF-κB translocation. A previous study showed that I/R injury is associated with activation of NF-κB in various organs such as the liver,21 kidney,22 and brain.11 The present results show that myocardial I/R injury-induced NF-κB activation are consistent with these observations.
Myocardial I/R injury initiates an acute inflammatory response.23 Accumulation of polymorphonuclear leukocytes (PMNs) is major evidence of acute inflammation, and the infiltration of PMNs initiates myocardial damage by releasing oxygen free radicals, proteases, and leukotrienes.23,24 The MPO activity assay for PMN activation should be checked because MPO is mainly released from PMNs.24,25 In the present study, MPO activity was inhibited by EP treatment; thus, EP may exert a protective effect by inhibiting PMN activity.
We also showed that EP improved cardiac function and reduced myocardial infarct size after regional I/R injury in an in vivo rat heart model after a 24 hours reperfusion. Myocardial infarction reaches a peak at 24 hours, whereas myocardial ischemia progressively develops during the late reperfusion period.26,27 Myocardial infarction was progressively extended between 6 and 24 hours, so we obtained the hemodynamic parameters and checked the infarction size 24 hours after reperfusion to identify the myocardial protective effect of EP. Other studies have reported reperfusion injury and infarction less than 60 min after ischemia, but our results showed a delayed phase in the myocardial reperfusion injury 24 hours after ischemia. So, EP can be used to protect myocardium in a clinical situation such as coronary artery bypass graft operation, heart transplantation, and many cardiac reperfusion procedures. EP dissolved in Ringer's solution may be helpful fluid therapy for these situations.
Our study has some limitations. First, a time gap existed between the anti-inflammatory markers and hemodynamic function/infarct size, so we did not clearly correlate the relationship and assumed that the myocardial protection was caused by the anti-inflammatory effect of EP. Second, because of technical difficulty, we could not check the hemodynamic parameters during the baseline or the ischemic period, so we only checked the hemodynamic parameters 24 hours after reperfusion. Third, we used EP at 25 and 50 mg/kg. In a future study, increased EP concentrations should be evaluated for further myocardial protective effects.
In conclusion, EP inhibited MPO activity, inflammatory cytokine release, and NF-κB translocation during the anti-inflammatory response. EP was associated with a delayed myocardial protective effect after regional I/R injury in an in vivo rat heart model. This protective effect may have been attributable at least in part to suppression of the inflammatory response.
ACKNOWLEDGEMENTS
This study was supported by grants from Gyeongsang National University Hospital (2008). This study was presented at the Annual Meeting of the European Society of Anaesthesiologists in Milano, Italy, June 2009.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Mallet RT, Sun J, Knott EM, Sharma AB, Olivencia-Yurvati AH. Metabolic cardioprotection by pyruvate: recent progress. Exp Biol Med (Maywood) 2005;230:435–443. doi: 10.1177/153537020523000701. [DOI] [PubMed] [Google Scholar]
- 2.Brand KA, Hermfisse U. Aerobic glycolysis by proliferating cells: a protective strategy against reactive oxygen species. FASEB J. 1997;11:388–395. doi: 10.1096/fasebj.11.5.9141507. [DOI] [PubMed] [Google Scholar]
- 3.Dobsak P, Courderot-Masuyer C, Zeller M, Vergely C, Laubriet A, Assem M, et al. Antioxidative properties of pyruvate and protection of the ischemic rat heart during cardioplegia. J Cardiovasc Pharmacol. 1999;34:651–659. doi: 10.1097/00005344-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Yu YM, Kim JB, Lee KW, Kim SY, Han PL, Lee JK. Inhibition of the cerebral inchemic injury by ethyl pyruvate with a wide therapeutic window. Stroke. 2005;36:2238–2243. doi: 10.1161/01.STR.0000181779.83472.35. [DOI] [PubMed] [Google Scholar]
- 5.Cicalese L, Lee K, Schraut W, Watkins S, Borle A, Stanko R. Pyruvate prevents ischemia-reperfusion mucosal injury of rat small intestine. Am J Surg. 1996;171:97–100. doi: 10.1016/S0002-9610(99)80081-6. [DOI] [PubMed] [Google Scholar]
- 6.Sileri P, Schena S, Morini S, Rastellini C, Pham S, Benedetti E, et al. Pyruvate inhibits hepatic ischemia-reperfusion injury in rats. Transplantation. 2001;72:27–30. doi: 10.1097/00007890-200107150-00008. [DOI] [PubMed] [Google Scholar]
- 7.Fink MP. Ethyl pyruvate. Curr Opin Anaesthesiol. 2008;21:160–167. doi: 10.1097/ACO.0b013e3282f63c2e. [DOI] [PubMed] [Google Scholar]
- 8.Taylor MD, Grand TJ, Cohen JE, Hsu V, Liao GP, Zentko S, et al. Ethyl pyruvate enhances ATP levels, reduces oxidative stress and preserves cardiac function in a rat model of off-pump coronary bypass. Heart Lung Circ. 2005;14:25–31. doi: 10.1016/j.hlc.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Woo YJ, Taylor MD, Cohen JE, Jayasankar V, Bish LT, Burdick J, et al. Ethyl pyruvate preserves cardiac function and attenuates oxidative injury after prolonged myocardial ischemia. J Thorac Cardiovasc Surg. 2004;127:1262–1269. doi: 10.1016/j.jtcvs.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 10.Kim HS, Cho IH, Kim JE, Shin YJ, Jeon JH, Kim Y, et al. Ethyl pyruvate has an anti-inflammatory effect by inhibiting ROS-dependent STAT signaling in activated microglia. Free Radic Biol Med. 2008;45:950–963. doi: 10.1016/j.freeradbiomed.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Kim JB, Yu YM, Kim SW, Lee JK. Anti-inflammatory mechanism is involved in ethyl pyruvate-mediated efficacious neuroprotection in the postischemic brain. Brain Res. 2005;1060:188–192. doi: 10.1016/j.brainres.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 12.Yang R, Han X, Delude RL, Fink MP. Ethyl pyruvate ameliorates acute alcohol-induced liver injury and inflammation in mice. J Lab Clin Med. 2003;142:322–331. doi: 10.1016/S0022-2143(03)00138-0. [DOI] [PubMed] [Google Scholar]
- 13.Shin IW, Jang IS, Lee SH, Baik JS, Park KE, Sohn JT, et al. Propofol has delayed myocardial protective effects after a regional ischemia/reperfusion injury in an in vivo rat heart model. Korean J Anesthesiol. 2010;58:378–382. doi: 10.4097/kjae.2010.58.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin YC, Kim CW, Kim YM, Nizamutdinova IT, Ha YM, Kim HJ, et al. Cryptotanshinone, a lipophilic compound of Salvia miltiorrhiza root, inhibits TNF-alpha-induced expression of adhesion molecules in HUVEC and attenuates rat myocardial ischemia/ reperfusion injury in vivo. Eur J Pharmacol. 2009;614:91–97. doi: 10.1016/j.ejphar.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 15.Gwechenberger M, Mendoza LH, Youker KA, Frangogiannis NG, Smith CW, Michael LH, et al. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation. 1999;99:546–551. doi: 10.1161/01.cir.99.4.546. [DOI] [PubMed] [Google Scholar]
- 16.Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, et al. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci U S A. 2002;99:12351–12356. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zingarelli B, Hake PW, Yang Z, O'Connor M, Denenberg A, Wong HR. Absence of inducible nitric oxide synthase modulates early reperfusion-induced NF-kappaB and AP-1 activation and enhances myocardial damage. FASEB J. 2002;16:327–342. doi: 10.1096/fj.01-0533com. [DOI] [PubMed] [Google Scholar]
- 18.Chandrasekar B, Freeman GL. Induction of nuclear factor kappaB and activation protein 1 in postischemic myocardium. FEBS Lett. 1997;401:30–34. doi: 10.1016/s0014-5793(96)01426-3. [DOI] [PubMed] [Google Scholar]
- 19.Barnes PJ, Karin M. Nuclear factor-kappaB; a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 20.Han Y, Englert JA, Yang R, Delude RL, Fink MP. Ethyl pyruvate inhibits nuclear factor-kappaB-dependent signaling by directly targeting p65. J Pharmacol Exp Ther. 2005;312:1097–1105. doi: 10.1124/jpet.104.079707. [DOI] [PubMed] [Google Scholar]
- 21.Tsung A, Kaizu T, Nakao A, Shao L, Bucher B, Fink MP, et al. Ethyl pyruvate ameliorates liver ischemia-reperfusion injury by decreasing hepatic necrosis and apoptosis. Transplantation. 2005;79:196–204. doi: 10.1097/01.tp.0000151681.07474.2e. [DOI] [PubMed] [Google Scholar]
- 22.Reade MC, Fink MP. Bench-to-bedside review: Amelioration of acute renal impairment using ethyl pyruvate. Crit Care. 2005;9:556–560. doi: 10.1186/cc3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen PR. Role of neutrophils in myocardial ischemia and reperfusion. Circulation. 1995;91:1872–1885. doi: 10.1161/01.cir.91.6.1872. [DOI] [PubMed] [Google Scholar]
- 24.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 25.Goldmann BU, Rudolph V, Rudolph TK, Holle AK, Hillebrandt M, Meinertz T, et al. Neutrophil activation precedes myocardial injury in patients with acute myocardial infarction. Free Radic Biol Med. 2009;47:79–83. doi: 10.1016/j.freeradbiomed.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Zhao ZQ, Nakamura M, Wang NP, Velez DA, Hewan-Lowe KO, Guyton RA, et al. Dynamic progression of contractile and endothelial dysfunction and infarct extension in the late phase of reperfusion. J Surg Res. 2000;94:133–144. doi: 10.1006/jsre.2000.6029. [DOI] [PubMed] [Google Scholar]
- 27.Zhao ZQ, Velez DA, Wang NP, Hewan-Lowe KO, Nakamura M, Guyton RA, et al. Progressively developed myocardial apoptotic cell death during late phase of reperfusion. Apoptosis. 2001;6:279–290. doi: 10.1023/a:1011335525219. [DOI] [PubMed] [Google Scholar]