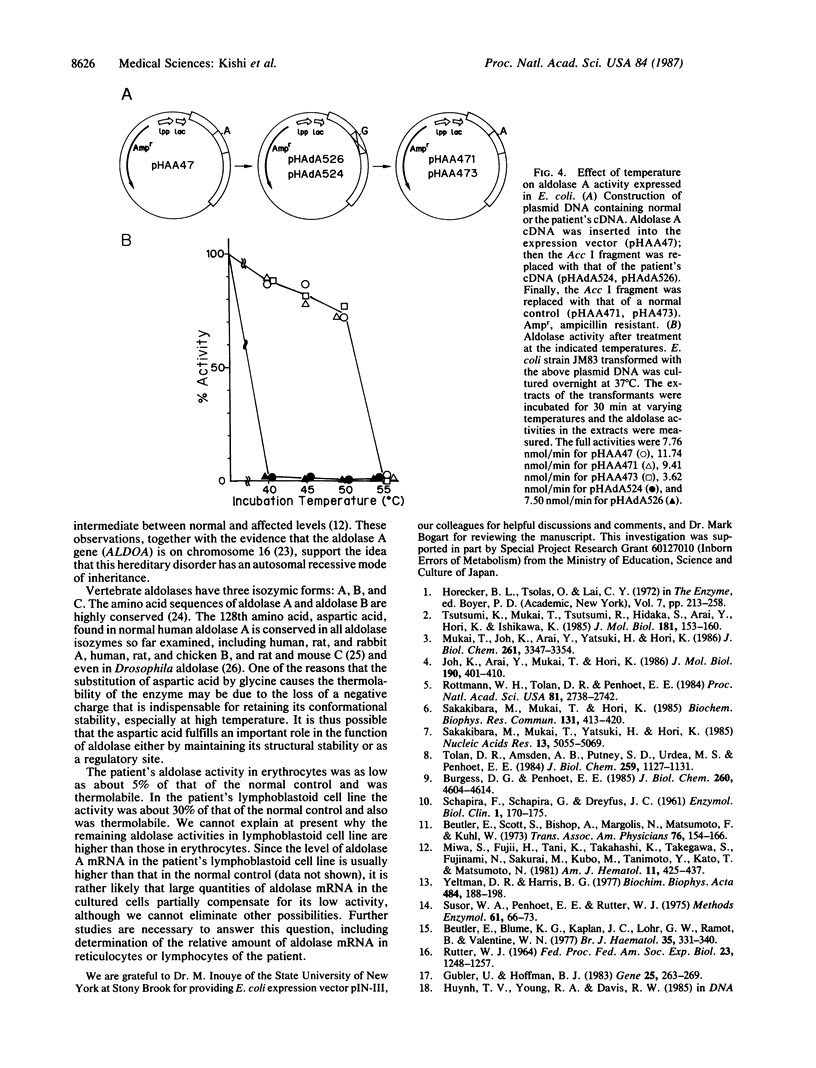
Abstract
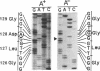
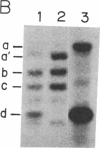
Fructose-1,6-bisphosphate aldolase A (fructose-bisphosphate aldolase; EC 4.1.2.13) deficiency is an autosomal recessive disorder associated with hereditary hemolytic anemia. To clarify the molecular mechanism of the deficiency at the nucleotide level, we have cloned aldolase A cDNA from a patient's poly(A)+ RNA that was expressed in cultured lymphoblastoid cells. Nucleotide analysis of the patient's aldolase A cDNA showed a substitution of a single nucleotide (adenine to guanine) at position 386 in a coding region. As a result, the 128th amino acid, aspartic acid, was replaced with glycine (GAT to GGT). Furthermore, change of the second letter of the aspartic acid codon extinguished a F ok I restriction site (GGATG to GGGTG). Southern blot analysis of the genomic DNA showed the patient carried a homozygous mutation inherited from his parents. When compared with normal human aldolase A, the patient's enzyme from erythrocytes and from cultured lymphoblastoid cells was found to be highly thermolabile, suggesting that this mutation causes a functional defect of the enzyme. To further examine this possibility, the thermal stability of aldolase A of the patient and of a normal control, expressed in Escherichia coli using expression plasmids, was determined. The results of E. coli expression of the mutated aldolase A enzyme confirmed the thermolabile nature of the abnormal enzyme. The Asp-128 is conserved in aldolase A, B, and C of eukaryotes, including an insect, Drosophila, suggesting that the Asp-128 of the aldolase A protein is likely to be an amino acid residue with a crucial role in maintaining the correct spatial structure or in performing the catalytic function of the enzyme.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler E., Blume K. G., Kaplan J. C., Löhr G. W., Ramot B., Valentine W. N. International Committee for Standardization in Haematology: recommended methods for red-cell enzyme analysis. Br J Haematol. 1977 Feb;35(2):331–340. doi: 10.1111/j.1365-2141.1977.tb00589.x. [DOI] [PubMed] [Google Scholar]
- Beutler E., Scott S., Bishop A., Margolis N., Matsumoto F., Kuhl W. Red cell aldolase deficiency and hemolytic anemia: a new syndrome. Trans Assoc Am Physicians. 1973;86:154–166. [PubMed] [Google Scholar]
- Burgess D. G., Penhoet E. E. Characterization of the chicken aldolase B gene. J Biol Chem. 1985 Apr 25;260(8):4604–4614. [PubMed] [Google Scholar]
- Cooper D. N., Schmidtke J. Diagnosis of genetic disease using recombinant DNA. Hum Genet. 1986 May;73(1):1–11. doi: 10.1007/BF00292654. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Hori K., Mukai T., Joh K., Arai Y., Sakakibara M., Yatsuki H. Structure and expression of human and rat aldolase isozyme genes: multiple mRNA species of aldolase A produced from a single gene. Isozymes Curr Top Biol Med Res. 1987;14:153–175. [PubMed] [Google Scholar]
- Joh K., Arai Y., Mukai T., Hori K. Expression of three mRNA species from a single rat aldolase A gene, differing in their 5' non-coding regions. J Mol Biol. 1986 Aug 5;190(3):401–410. doi: 10.1016/0022-2836(86)90011-2. [DOI] [PubMed] [Google Scholar]
- Joh K., Mukai T., Yatsuki H., Hori K. Rat aldolase A messenger RNA: the nucleotide sequence and multiple mRNA species with different 5'-terminal regions. Gene. 1985;39(1):17–24. doi: 10.1016/0378-1119(85)90102-7. [DOI] [PubMed] [Google Scholar]
- Kukita A., Yoshida M. C., Fukushige S., Sakakibara M., Joh K., Mukai T., Hori K. Molecular gene mapping of human aldolase A (ALDOA) gene to chromosome 16. Hum Genet. 1987 May;76(1):20–26. doi: 10.1007/BF00283044. [DOI] [PubMed] [Google Scholar]
- Malek A. A., Suter F. X., Frank G., Brenner-Holzach O. Amino acid sequence of an invertebrate FBP aldolase (from Drosophila melanogaster). Biochem Biophys Res Commun. 1985 Jan 16;126(1):199–205. doi: 10.1016/0006-291x(85)90591-1. [DOI] [PubMed] [Google Scholar]
- Miwa S., Fujii H., Tani K., Takahashi K., Takegawa S., Fujinami N., Sakurai M., Kubo M., Tanimoto Y., Kato T. Two cases of red cell aldolase deficiency associated with hereditary hemolytic anemia in a Japanese family. Am J Hematol. 1981 Dec;11(4):425–437. doi: 10.1002/ajh.2830110412. [DOI] [PubMed] [Google Scholar]
- Mukai T., Joh K., Arai Y., Yatsuki H., Hori K. Tissue-specific expression of rat aldolase A mRNAs. Three molecular species differing only in the 5'-terminal sequences. J Biol Chem. 1986 Mar 5;261(7):3347–3354. [PubMed] [Google Scholar]
- Paolella G., Buono P., Mancini F. P., Izzo P., Salvatore F. Structure and expression of mouse aldolase genes. Brain-specific aldolase C amino acid sequence is closely related to aldolase A. Eur J Biochem. 1986 Apr 15;156(2):229–235. doi: 10.1111/j.1432-1033.1986.tb09572.x. [DOI] [PubMed] [Google Scholar]
- RUTTER W. J. EVOLUTION OF ALDOLASE. Fed Proc. 1964 Nov-Dec;23:1248–1257. [PubMed] [Google Scholar]
- Rottmann W. H., Tolan D. R., Penhoet E. E. Complete amino acid sequence for human aldolase B derived from cDNA and genomic clones. Proc Natl Acad Sci U S A. 1984 May;81(9):2738–2742. doi: 10.1073/pnas.81.9.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAPIRA F., SCHAPIRA G., DREYFUS J. C. [The enzymatic defect of benign fructosuria]. Enzymol Biol Clin (Basel) 1961;1:170–175. [PubMed] [Google Scholar]
- Sakakibara M., Mukai T., Hori K. Nucleotide sequence of a cDNA clone for human aldolase: a messenger RNA in the liver. Biochem Biophys Res Commun. 1985 Aug 30;131(1):413–420. doi: 10.1016/0006-291x(85)91818-2. [DOI] [PubMed] [Google Scholar]
- Sakakibara M., Mukai T., Yatsuki H., Hori K. Human aldolase isozyme gene: the structure of multispecies aldolase B mRNAs. Nucleic Acids Res. 1985 Jul 25;13(14):5055–5069. doi: 10.1093/nar/13.14.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susor W. A., Penhoet E., Rutter W. J. Fructose-diphosphate aldolase, pyruvate kinase, and pyridine nucleotide-linked activities after electrophoresis. Methods Enzymol. 1975;41:66–73. doi: 10.1016/s0076-6879(75)41017-5. [DOI] [PubMed] [Google Scholar]
- Tolan D. R., Amsden A. B., Putney S. D., Urdea M. S., Penhoet E. E. The complete nucleotide sequence for rabbit muscle aldolase A messenger RNA. J Biol Chem. 1984 Jan 25;259(2):1127–1131. [PubMed] [Google Scholar]
- Tsutsumi K., Mukai T., Tsutsumi R., Hidaka S., Arai Y., Hori K., Ishikawa K. Structure and genomic organization of the rat aldolase B gene. J Mol Biol. 1985 Jan 20;181(2):153–160. doi: 10.1016/0022-2836(85)90081-6. [DOI] [PubMed] [Google Scholar]
- Yeltman D. R., Harris B. G. Purification and characterization of aldolase from human erythrocytes. Biochim Biophys Acta. 1977 Sep 15;484(1):188–198. doi: 10.1016/0005-2744(77)90124-3. [DOI] [PubMed] [Google Scholar]