Abstract
Heartburn is the most common and characteristic symptom of gastroesophageal reflux disease. It ultimately results from contact of refluxed gastric acid with nociceptors within the esophageal mucosa and transmission of this peripheral signal to the central nervous system for cognition. Healthy esophageal epithelium provides an effective barrier between refluxed gastric acid and esophageal nociceptors; but this barrier is vulnerable to attack and damage, particularly by acidic gastric contents. How gastric acid is countered by defensive elements within the esophageal mucosa is a major focus of this discussion. When the defense is successful, the subject is asymptomatic and when unsuccessful, the subject experiences heartburn. Those with heartburn commonly fall into one of three endoscopic types: nonerosive reflux disease, erosive esophagitis and Barrett's esophagus. Although what determines endoscopic type remains unknown; it is proposed herein that inflammation plays a key, modulating role.
Keywords: tissue resistance, barrier function, pH regulation, ion transport, dilated intercellular spaces, nonerosive reflux disease, gastroesophageal reflux disease, erosive esophagitis, Barrett's esophagus, inflammation
Introduction
Gastroesophageal reflux is a common daily occurrence in healthy subjects – and these by definition experience no symptoms. The reasons for this are mulitfactorial. For instance, the number and volume of episodes of reflux are limited by the antireflux barriers, principally the lower esophageal sphincter and the diaphragm, and, after an episode of reflux, the time of contact between the noxious elements in the refluxate, principally hydrochloric acid and pepsin, and the esophageal epithelium limited to a few minutes by luminal clearance mechanisms. Contributing to luminal clearance are gravity and peristalsis, for volume clearance, and secretions from salivary and esophageal submucosal glands, for acid clearance. Since reflux frequency and acid clearance are far from zero, particularly at night when both gravity, peristalsis and glandular secretions ebb during sleep, the esophageal epithelium routinely comes into contact with acidic refluxates. The mechanisms available to the epithelium for defense against acid injury during this time have come to known collectively as ‘tissue resistance’. In a sense, tissue resistance, as defense against the acidic refluxate, is the final arbiter of health and (reflux) disease because when ineffective, it leads to the most commonly recognized symptoms and signs of gastroesophageal reflux disease (GERD), that is heartburn and esophageal erosions(1, 2).
Offensive Factors in the Refluxate
The refluxate contains numerous products with noxious potential, including: hydrochloric acid (HCl), pepsin, bile salts, and pancreatic enzymes(3). However, it is clear that the most noxious of these is HCl whose gastric secretion can produce values as low as pH 1.0. Support for this is provided by the general effectiveness of anti-acid medications in controlling the symptoms and signs of GERD while absence of acid is associated with general lack of injury in the upper gastrointestinal tract, including the esophagus. Additionally, support for the noxious acidity within the esophagus being derived from the stomach through reflux rather than through ingestion of acidic products with meals is provided by the effectiveness of both medications that reduce gastric acid secretion, e.g. histamine-2 receptor antagonists and proton pump inhibitors (PPIs), and surgical fundoplication. Other factors such as pepsin and conjugated bile salts may, in selected individuals, contribute to the noxious quality of acidic refluxates. This is based on studies showing that pepsin and conjugated bile salts increase esophageal damage at acidic pH (4-6).
Defensive Factors in Esophageal Mucosa
Since the major injurious agent in the refluxate is gastric acid, this section will focus on those factors within the esophageal mucosa that provide defense against acidity. That this tissue defense can be formidable is best illustrated by revisiting the Bernstein Test. During the Bernstein test, the esophagus of a healthy subject can be continuously perfused with HCl, pH 1.0, for up to 30 min and this without producing heartburn(7). Since this technique bypasses both antireflux and luminal clearance mechanisms for protection against acid injury, it speaks directly to the presence and effectiveness of mucosal factors for protection when in contact with luminal acid. And among these factors, the most important are those contained within the esophageal epithelium proper, a non-keratinized stratified squamous epithelium (see Table 1). This is because, unlike stomach and duodenum, the esophagus has no viscoelastic surface mucous layer and its epithelial cells secrete no bicarbonate(2). Consequently, the esophagus has no effective means of trapping luminal bicarbonate that can serve as a means for buffering gastric acid as it back diffuses from lumen toward epithelium.
TABLE 1. Properties within the esophageal epithelium for protection against luminal acidity.
| Apical Cell Membrane |
| Hydophobic lipid bilayer |
| Nonselective sodium channel |
| Apical Junctional Complex |
| Tight junction |
| Adherens junction |
| Desmosome |
| Ion Transport |
| Na+/H+ exchanger |
| Na+-dependent Cl-/HCO3- exchanger |
| Buffers |
| Bicarbonate ions |
| Phosphate ions |
| Basic proteins |
| Reparative Processes |
| Cell replication |
| Cell regeneration |
| Inflammation |
Esophageal Epithelium
The multilayered, non-keratinized stratified squamous epithelium, has several surface layers of flat cells constituting the stratum corneum, several more layers of actively transporting cells constituting the stratum spinosum, and a single or double cell layer of mitotically-active cells constituting the stratum (basalis) germinativum. The cells of the most luminal layers, the stratum corneum, initially provide defense in the form of a permeability barrier(8, 9). This permeability barrier is comprised of apical cell membranes and apical junctional complexes (AJCs) that prevent the diffusion of luminal acid directly into the cells or intercellular spaces, respectively. The apical cell membrane does this because of its hydrophobicity and because its cation (sodium) channels, though non-selective, are inhibited at acidic luminal pH; and the apical junctional complex does this by creating an aqueous permselective pathway formed by tight junctions, adherens junctions and desmosomes that greatly limits the rate of paracellular ion diffusion(8-11). In particular the tight junction forms the narrowest component of the pathway, a function supported by the underlying adherens junction, while structural integrity with close apposition of adjacent cell membranes is provided by the desmosomes. Within the intercellular space of esophageal epithelium is also a matrix of glycoproteins that was once considered an aide to the apical junctional complex in limiting paracellular ion diffusion(8). Recent studies, however, have shown that their removal by enzymatic digestion had no effect on the esophageal electrical resistance, a sensitive marker of paracellular permeability to ions. In contrast disruption of desmosomal binding to intermediate filaments was shown to markedly increase paracellular permeability(12). Taken together, the combination of apical junctional complexes and apical cell membranes in the stratum corneum are largely what account for the esophageal epithelium being ‘electrically tight’ with resistances in human and animal esophageal epithelium of ≥1000 ohms.cm2 (13, 14). [Note: the electrical resistance of healthy human esophageal epithelium is much lower when measured in esophageal biopsies mounted in mini-Ussing chambers, i.e. ∼345 ohms.cm2(15). This difference is largely technical and due to the greater ratio of circumferential edge damage to epithelial surface area in mini-chambered specimens compared to standard-chambered specimens(14). The greater the ratio the lower is the recorded electrical resistance.]
Apical Junctional Complex
The trio of tight junctions, adherens junctions and desmosomes, comprising the apical junctional complex, are individually highly complex organelles. While detailed descriptions of each are beyond the scope of this discussion (and can be found elsewhere – (16, 17)), each has an extracellular (equivalent of intercellular), transmembrane and intracellular domain useful for cell signaling as well as barrier function. Noteworthy is that the barrier function for these junctions ultimately falls to those proteins that bridge the intercellular space. For the tight junctions these proteins are occludin, and members of a family of claudins(18). In esophageal epithelium, claudin 1 and claudin 4 are the most prominent(19). For the adherens junction, the major protein is e-cadherin(20, 21); and for the desmosome, the major proteins are desmogleins and desmocollin(22). Importantly, the proteins of the tight junction and adherens junction encircle the entire perimeter of the cell creating a seal that separates the lumen from intercellular space of the epithelium. The desmosome, however, produces only a ‘spot weld’ that does not encircle the cell. Nonetheless, the desmosome contributes to barrier function indirectly by maintaining close apposition of adjacent cell membranes throughout the multilayered epithelium. In effect, the desmosomes enable formation of a long serpiginous intercellular pathway that contributes to barrier function by its narrow width, long length and electrical charges present within the membranous proteins of adjacent cells(12).
Acid Buffering and Neutralization
Although the apical cell membrane and apical junctional complex provide the structural barriers that prevent penetration of luminal ions, particular hydrogen ions (H+) derived from refluxed gastric acid, into the cell or intercellular space, this defensive alignment remains imperfect. Therefore, H+ can diffuse slowly into the cell cytosol or into the intercellular space requiring an alternative defense to prevent acidification of these two compartments. This is accomplished by the presence within these compartments of substances that buffer and/or neutralize H+. Such substances include proteins, phosphates and bicarbonate, with bicarbonate being the most versatile and replenishable(2). This is because bicarbonate can readily diffuse from blood into the extracellular space and so intercellular spaces within the epithelium and then be transported by proteins located in the basolateral cell membranes from extracellular space to cell cytosol. In addition to diffusion from blood to extracellular space and transport from extracellular space to cell cytosol, bicarbonate can also be generated de nova within the cell cytosol and intercellular compartments by an enzymatic reaction. This reaction is mediated by carbonic anhydrases and converts water and carbon dioxide to carbonic acid and carbonic acid ionizes to H+ and bicarbonate(23). In effect, slow rates of H+ back diffusing from the lumen to the cell cytosol and intercellular spaces are buffered or neutralized and so in a healthy epithelium have little to no impact on cytosolic or intercellular pH.
Acid Transport
When H+ generated metabolically and/or diffusing into the cell cytosol by back diffusion from refluxed gastric acid overwhelm cell buffer capacity, cytosolic pH declines to acidic levels. To keep such acidity from injuring the cell, cell membranes come equipped with ion transporters capable of removing excess H+ from the cell and restoring pH to neutrality. In esophageal epithelial cells, these transporters are localized to the basolateral membrane and include a sodium-dependent, chloride-bicarbonate, exchanger and a sodium-hydrogen ion exchanger of isotype-1(24-27). As noted by the nomenclature, these transporters utilize the transmembrane gradient for sodium ions to move sodium into the cell either to promote the exchange of intracellular chloride for extracellular bicarbonate ions to neutralize cytosolic H+, or to promote the exchange of extracellular sodium directly for removal of intracellular H+. In both instances, the end result of the action of these transporters is to increase cytosolic pH toward neutrality. Moreover, if these actions overshoot their mark, i.e. cytosolic pH exceeds its set point of ∼ 7.4, the basolateral membrane of esophageal cells possess a third acid transporter known as the Na-independent, chloride-bicarbonate exchanger(25). However, and in contrast to the other two exchangers, this transporter operates to bring H+ into the cell by promoting the exchange of extracellular chloride for intracellular bicarbonate – and removal of intracellular bicarbonate is effectively the same as the addition of H+ to the cell cytosol. Thus, these three transporters cooperate in esophageal cells to tightly regulate the cytosolic pH within the neutral range.
Blood Flow
The barrier and transport functions of the esophageal epithelium are integral processes for protection of the tissue against injury upon exposure to gastric acid. These processes, however, would not be durable were it not for an adequate blood supply. For it is the blood supply that provides the oxygen and nutrients for growth and repair and for acid-base balance the delivery of electrolytes and removal of carbon dioxide. Moreover, the blood supply can adapt to such threatening environments as acid exposure and acid injury to esophageal epithelium with an increase in flow rate. This process is mediated both neutrally and non-neural chemical signaling and serves an important protective function(28, 29).
Pathophysiology of Reflux Esophagitis
The ability of luminal acid to injure the esophageal epithelium is well established. In humans, this is based on the positive clinical response to acid suppressing agents such as proton pump inhibitors; and in animals this is based on morphologic and functional changes to the esophageal epithelium induced by acid perfusion. In the rabbit model, it has been shown that acid (HCl) initially injures the esophageal epithelium by altering the AJC; and this in turn causes an early increase in paracellular permeability and a morphologic lesion known as dilated intercellular spaces (DIS)(2). DIS is a sensitive marker of GERD(30-34); but it is not specific, having been produced by acidified bile salts, hypertonic media, cold water-restraint stress and exposure to the zwitterionic detergent, empigen. It is also observed in patients with eosinophilic esophagitis, candida esophagitis, and esophageal cancer(6, 12, 33, 35-39). To date the alterations in the AJC of acid-damaged esophagi include changes in the tight junction proteins, occludin and claudins 1,3, and 4, changes in the number of desmosomes and changes to the adherens junction protein, e-cadherin(40-43). As a consequence, paracellular permeability increases and this enables greater concentrations of luminal H+ to diffuse into and acidify the intercellular space. In addition, there is an increase in paracellular diffusion of chloride ions that results in an osmotic gradient for water movement into the intercellular spaces(44). It is the hydrostatic force created by this osmotic action that is responsible for generation of DIS. [Note: When carefully sought in subjects with GERD or in animal models of acid damage to esophageal epithelium, DIS are evident in all layers. However, they are particularly prominent and so more readily apparent in the basal layers. While not formally studied, this is likely a reflection of greater compliance and so separation of basal cell membranes versus luminal cell membranes in response to the increase in intercellular hydrostatic force. Unlike basal cells, differentiation produces luminal cells that are flat, rigid and tightly compacted, thus yielding little room for membrane separation.] Further, acidification of the intercellular space by paracellular diffusion of H+ triggers the firing of pH-sensitive nociceptors within the esophageal mucosa, e.g. the transient receptor potential vanilloid channel, type 1 and acid sensing ion channel, type 3(45-48). Where in the mucosa these nerve endings reside has not been resolved; but evidence exists in both primates and humans that sensory fibers can extend into the intercellular spaces of the epithelium(45, 49). Also unresolved is how luminal acidity activates these sensory nerves; that is, do back diffusing H+ acidify the intercellular space and so directly activate the nociceptors as proposed above or are the nociceptors activated by carbon dioxide formed after the reaction of luminal or back diffusing H+ with bicarbonate ions(50)? In either case, transmission of this peripheral signal to the brain then results in the symptom of ‘heartburn’(51). Acidification of the intercellular space not only triggers heartburn but can cause cell acidification, the latter the proximate cause of cell injury and necrosis. The reason this occurs is because, unlike apical cell membranes, the basolateral cell membranes contain an acid-absorbing transporter, that is, the Na-independent, chloride/bicarbonate exchanger(2). Because this transporter is driven by the transmembrane concentration gradients for chloride and bicarbonate, a low intercellular pH is readily translated into low intracellular pH(52, 53). It is persistence of a low intracellular pH that sets in motion a series of events leading to cell injury and necrosis.
Tissue Repair
Although prolonged acidification results in cell injury and necrosis, this process can be contained by two epithelial reparative mechanisms. One is epithelial restitution and the other is epithelial replication. Restitution is a rapid form of repair, 30 – 60 minutes, because it doesn't require deoxyribonucleic acid (DNA) and protein synthesis; instead it relies on the migration of viable cells adjacent to those that are necrotic to maintain the barrier function of the epithelium(54, 55). In contrast, regeneration is a slower form of repair, days to weeks depending upon the size of the necrotic area, because it depends on mitosis and so synthesis of DNA and proteins(56). Notably, during repair, injury may still be ongoing if acid exposure is repeated and prolonged. Consequently, the balance between rate of injury and rate of repair determine whether the epithelial changes remain microscopic or are magnified into the macroscopic changes of erosive esophagitis.
Inflammation
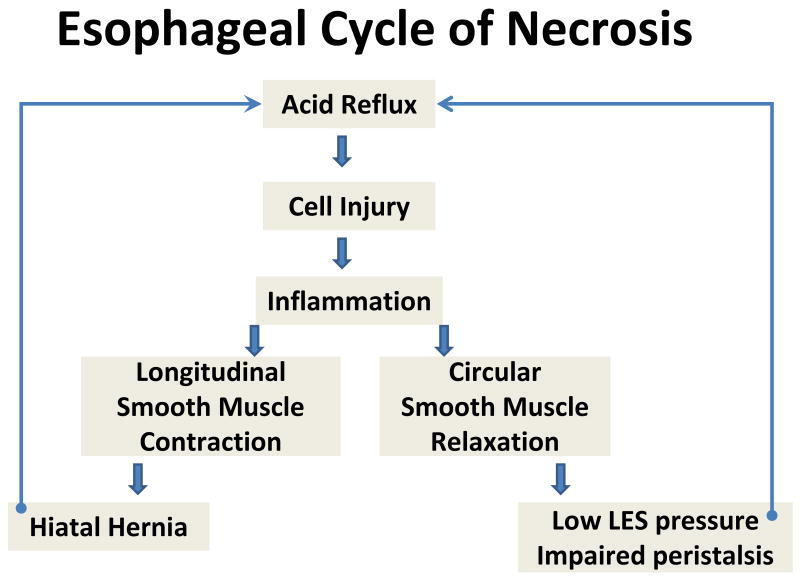
Following acid-induced cell injury, there is an important modulator of the rate of cell injury and repair – and this is inflammation. Inflammation is a well-established tissue response whose pattern varies with the degree, extent, type and duration of injury. It is triggered by the release of chemokines and cytokines from injured cells, and these chemicals through diffusion to adjacent areas and to the systemic circulation attract immune cells to the area. Within the area of injury, immune cells are capable of digestion and removal of necrotic debris, and by so doing help ultimately in repair of the area. Inflammation, however, is not a highly tuned process, such that the influx of immune cells may result in additional damage even while acting as the means of repair. This phenomena was first shown in the acid-perfused rabbit esophagus and more recently in a rat model where damage was induced by creation of a esophagoduodenostomy(57-59). In the rabbit model, esophageal injury was reduced by blocking white blood cell migration to the area with ketotifen and, by blocking their production of oxygen-derived free radicles with either superoxide dismutase or catalase. In the rat model, release of interleukin-8 was shown to be one of the signaling molecules released from esophageal squamous cells for recruitment of inflammatory cells to the area of injury. These data indicate that inflammation, per se, is a double-edged sword with the capacity to directly injure as well as to repair squamous epithelium. Moreover, and perhaps more importantly, inflammation promotes and perpetuates injury indirectly; this is done by altering neuromuscular transmission of esophageal smooth muscle(60-67). Consequently, inflammation can reduce lower esophageal sphincter pressure, impair peristaltic contractility, and contract longitudinal muscle, the latter a means of promoting creation of a hiatal hernia (see Figure 1). In effect, inflammation can both augment acid reflux and delay acid clearance, producing a vicious cycle in which ‘reflux-induced cell damage begets inflammation which begets more reflux-induced cell damage which begets more inflammation’. As cornerstone of this cycle, inflammation is an attractive candidate to serve as modulator of endoscopic type of GERD (see below).
Figure 1.
The diagram depicts a proposed means by which acid injury to esophageal epithelium is perpetuated by inflammation. Inflammation is shown to alter both longitudinal and circular smooth muscle function in distal esophagus, resulting in impairment in both antireflux mechanisms [reduction in lower esophageal sphincter (LES) pressure and creation of a hiatal hernia] and luminal clearance (impaired peristalsis). These defects in turn are shown to create more damage and inflammation by cycling back to induce more acid reflux.
Endoscopic Types in GERD
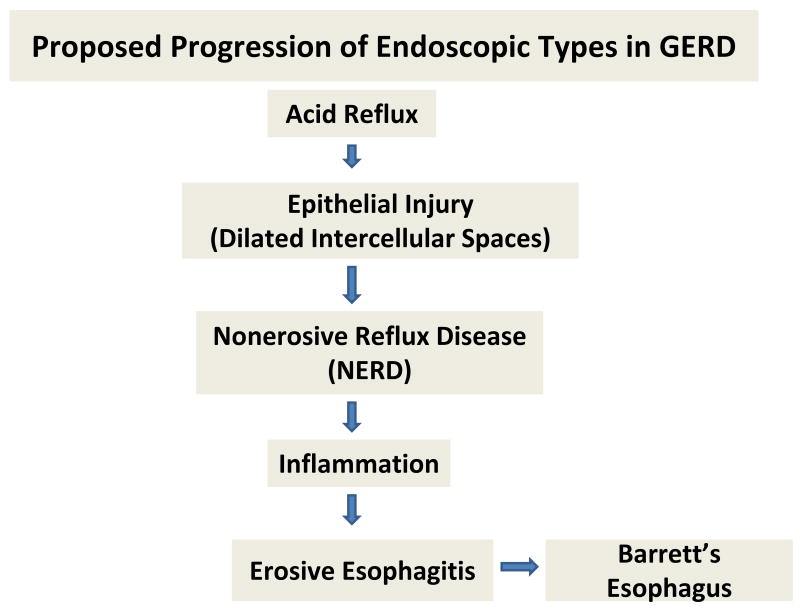
The fact that there is such seeming stability among the three common endoscopic types of GERD – NERD, erosive esophagitis, and Barrett's epithelium – has lead to the concept that these are different disorders rather than a continuum that progresses from NERD to erosive esophagitis to Barrett's epithelium(68, 69). Yet, there is clear evidence in support of the continuum among types. Certainly NERD progresses to erosive esophagitis and erosive esophagitis can revert to NERD(70, 71). Barrett's esophagus is more of an enigma since it is typically present fully-formed on initial endoscopy rather than appearing to progress gradually in those with erosive disease(72). Nonetheless, Barrett's has been produced in a rat model of reflux, is more commonly found in GERD than in healthy subjects and is reported to appear in those with erosive esophagitis that did not initially have Barrett's esophagus(73-75). Moreover, there is evidence that NERD, erosive esophagitis and Barrett's esophagus share a common histopathologic feature within esophageal (squamous) epithelium, that is DIS(34, 76). Since DIS is a reflection of a break in barrier function, it supports the concept that these three forms of GERD share a common pathogenesis (see Figure 2). As proposed above, the type and extent of inflammation induced by acid injury to esophageal epithelium that may be the modulating key as to who develops NERD, erosive esophagitis and Barrett's esophagus. For instance, subjects in which esophageal acid exposure, either by quantity of acid reflux, environmental factors that can amplify mucosal injury such as alcohol, stress, smoking, or by a programmed host (genetic) response, produces little or no inflammation presents as NERD while those with significant inflammation present with erosive esophagitis. Barrett's esophagus, an aberrant form of repair, emerges in those with erosive esophagitis as a means of defense against acid injury(74, 77). Based on differences in frequency between Caucasian and African American populations in the United States, the emergence of Barrett's esophagus likely involves a distinct set of host genetics(78, 79).
Figure 2.
The diagram depicts a proposed means by which acid injury to esophageal epithelium leads to the three common endoscopic types of gastroesophageal reflux disease (GERD): nonerosive reflux disease (NERD), erosive esophagitis, and Barrett's esophagus. Notably, all three have in common the histopathologic lesion of dilated intercellular spaces within esophageal ‘squamous’ epithelium; and inflammation is shown to be the promoter of progression from NERD to erosive esophagitis. Barrett's esophagus is an aberrant form of repair that occurs in some subjects and this is shown to extend from erosive esophagitis.
Summary
Heartburn is the most common and characteristic symptom of GERD. It results from sufficient contact of refluxed gastric acid with the esophageal epithelium that it ‘breaks the barrier’. The break in the barrier is manifest functionally by an increase in paracellular permeability and structurally by the appearance of DIS. The increase in paracellular permeability provides a pathway for luminal acid to diffuse into the esophageal mucosa where it can acidify the intercellular space. Acidification of this space is hypothesized to trigger the sensory nociceptors responsible for heartburn and to enable acid to penetrate the cell across the basolateral membrane on a sodium-independent, chloride/bicarbonate exchanger. Cell acidification leads to cell injury and necrosis. Cell injury and necrosis lead to activation of cell reparative phenomena, a component of which is tissue inflammation. Inflammation, however, is a double-edged sword, producing additional injury even as it aids repair. Moreover, inflammation alters esophageal motor function, promoting greater reflux and delaying acid clearance. This creates a vicious cycle that may serve to modulate the outcome as to endoscopic type of GERD.
Research Agenda.
Research is needed to define the mechanisms that cause alteration in the apical junctional complex in those with GERD and strategies for their restoration beyond acid suppression.
Research is needed to better define the nature of the esophageal nocieptors responsible for the symptom of heartburn.
Research is needed to better define the mechanisms that determine whether subjects with GERD have NERD or erosive esophagitis and whether subjects with GERD progress to Barrett's esophagus.
Footnotes
Conflict of interest: The author has recently been recipient of research grants from Proctor and Gamble; Astra Zeneca and Takeda Pharmaceuticals. He has also served as consultant for Astra Zeneca and Takeda Pharmaceuticals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Orlando RC. Reflux esophagitis. In: Yamada T, Alpers D, Owyang C, Powell D, Laine L, editors. Textbook of Gastroenterology. 3rd. Philadelphia: JB Lippincott Williams & Wilkins; 1999. pp. 1235–63. [Google Scholar]
- 2.Orlando RC. Pathophysiology of gastroesophageal reflux disease: Esophageal epithelial resistance. In: Castell DO, Richter JE, editors. The Esophagus. 4th. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 421–33. [Google Scholar]
- 3.Gotley DC, Morgan AP, Ball D, Owen RW, Cooper MJ. Composition of gastrooesophageal refluxate. Gut. 1991 Oct;32(10):1093–9. doi: 10.1136/gut.32.10.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tobey NA, Hosseini SS, Caymaz-Bor C, Wyatt HR, Orlando GS, Orlando RC. The role of pepsin in acid injury to esophageal epithelium. Am J Gastroenterol. 2001 Nov;96(11):3062–70. doi: 10.1111/j.1572-0241.2001.05260.x. [DOI] [PubMed] [Google Scholar]
- 5.Harmon JW, Johnson LF, Maydonovitch CL. Effects of acid and bile salts on the rabbit esophageal mucosa. Dig Dis Sci. 1981 Jan;26(1):65–72. doi: 10.1007/BF01307977. [DOI] [PubMed] [Google Scholar]
- 6.Farre R, van Malenstein H, De Vos R, Geboes K, Depoortere I, Vanden Berghe P, et al. Short exposure of oesophageal mucosa to bile acids, both in acidic and weakly acidic conditions, can impair mucosal integrity and provoke dilated intercellular spaces. Gut. 2008 Oct;57(10):1366–74. doi: 10.1136/gut.2007.141804. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein LM, Baker LA. A clinical test for esophagitis. Gastroenterology. 1958 May;34(5):760–81. [PubMed] [Google Scholar]
- 8.Orlando RC, Lacy ER, Tobey NA, Cowart K. Barriers to paracellular permeability in rabbit esophageal epithelium. Gastroenterology. 1992 Mar;102(3):910–23. doi: 10.1016/0016-5085(92)90177-z. [DOI] [PubMed] [Google Scholar]
- 9.Elias PM, McNutt NS, Friend DS. Membrane alterations during cornification of mammalian squamous epithelia: a freeze-fracture, tracer, and thin-section study. Anat Rec. 1977 Dec;189(4):577–94. doi: 10.1002/ar.1091890404. [DOI] [PubMed] [Google Scholar]
- 10.Tobey NA, Argote CM, Awayda MS, Vanegas XC, Orlando RC. Effect of luminal acidity on the apical cation channel in rabbit esophageal epithelium. Am J Physiol Gastrointest Liver Physiol. 2007 Mar;292(3):G796–805. doi: 10.1152/ajpgi.00385.2005. [DOI] [PubMed] [Google Scholar]
- 11.Awayda MS, Bengrine A, Tobey NA, Stockand JD, Orlando RC. Nonselective cation transport in native esophageal epithelia. Am J Physiol Cell Physiol. 2004 Aug;287(2):C395–402. doi: 10.1152/ajpcell.00412.2003. [DOI] [PubMed] [Google Scholar]
- 12.Tobey NA, Djukic Z, Brighton LE, Gambling TM, Carson JL, Orlando RC. Lateral cell membranes and shunt resistance in rabbit esophageal epithelium. Dig Dis Sci Jul. 55(7):1856–65. doi: 10.1007/s10620-010-1215-4. [DOI] [PubMed] [Google Scholar]
- 13.Tobey NA, Argote CM, Awayda MS, Vanegas XC, Orlando RC. Effect of Luminal Acidity on the Apical Cation Channel in Rabbit Esophageal Epithelium. Am J Physiol Gastrointest Liver Physiol. 2007 Apr 13;292:G796–805. doi: 10.1152/ajpgi.00385.2005. [DOI] [PubMed] [Google Scholar]
- 14.Orlando RC, Powell D. Studies of esophageal epithelial electrolyte transport and potential difference in man. In: Allen A, Flemstrom G, Garner A, Silen W, editors. Mechanisms of Mucosal Protection in the Upper Gastrointestinal Tract. New York: Raven Press; 1984. pp. 75–9. [Google Scholar]
- 15.Tobey NA, Argote CM, Vanegas XC, Barlow W, Orlando RC. Electrical parameters and ion species for active transport in human esophageal stratified squamous epithelium and Barrett's specialized columnar epithelium. Am J Physiol Gastrointest Liver Physiol. 2007 Jul;293(1):G264–70. doi: 10.1152/ajpgi.00047.2007. [DOI] [PubMed] [Google Scholar]
- 16.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008 Mar;1778(3):660–9. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrod D, Chidgey M. Desmosome structure, composition and function. Biochim Biophys Acta. 2008 Mar;1778(3):572–87. doi: 10.1016/j.bbamem.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Angelow S, Yu AS. Claudins and paracellular transport: an update. Curr Opin Nephrol Hypertens. 2007 Sep;16(5):459–64. doi: 10.1097/MNH.0b013e32820ac97d. [DOI] [PubMed] [Google Scholar]
- 19.Jovov B, Van Itallie CM, Shaheen NJ, Carson JL, Gambling TM, Anderson JM, et al. Claudin-18: a dominant tight junction protein in Barrett's esophagus and likely contributor to its acid resistance. Am J Physiol Gastrointest Liver Physiol. 2007 Dec;293(6):G1106–13. doi: 10.1152/ajpgi.00158.2007. [DOI] [PubMed] [Google Scholar]
- 20.Tobey NA, Argote CM, Hosseini SS, Orlando RC. Calcium-switch technique and junctional permeability in native rabbit esophageal epithelium. Am J Physiol Gastrointest Liver Physiol. 2004 Jun;286(6):G1042–9. doi: 10.1152/ajpgi.00387.2003. [DOI] [PubMed] [Google Scholar]
- 21.Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988 Oct;107(4):1575–87. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garrod DR, Merritt AJ, Nie Z. Desmosomal cadherins. Curr Opin Cell Biol. 2002 Oct;14(5):537–45. doi: 10.1016/s0955-0674(02)00366-6. [DOI] [PubMed] [Google Scholar]
- 23.Christie KN, Thomson C, Xue L, Lucocq JM, Hopwood D. Carbonic anhydrase isoenzymes I, II, III, and IV are present in human esophageal epithelium. J Histochem Cytochem. 1997 Jan;45(1):35–40. doi: 10.1177/002215549704500105. [DOI] [PubMed] [Google Scholar]
- 24.Tobey NA, Koves G, Orlando RC. Human esophageal epithelial cells possess an Na+/H+ exchanger for H+ extrusion. Am J Gastroenterol. 1998 Nov;93(11):2075–81. doi: 10.1111/j.1572-0241.1998.00596.x. [DOI] [PubMed] [Google Scholar]
- 25.Tobey NA, Reddy SP, Khalbuss WE, Silvers SM, Cragoe EJ, Jr, Orlando RC. Na(+)-dependent and -independent Cl-/HCO3- exchangers in cultured rabbit esophageal epithelial cells. Gastroenterology. 1993 Jan;104(1):185–95. doi: 10.1016/0016-5085(93)90851-3. [DOI] [PubMed] [Google Scholar]
- 26.Shallat S, Schmidt L, Reaka A, Rao D, Chang EB, Rao MC, et al. NHE-1 isoform of the Na+/H+ antiport is expressed in the rat and rabbit esophagus. Gastroenterology. 1995 Nov;109(5):1421–8. doi: 10.1016/0016-5085(95)90626-6. [DOI] [PubMed] [Google Scholar]
- 27.Layden TJ, Schmidt L, Agnone L, Lisitza P, Brewer J, Goldstein JL. Rabbit esophageal cell cytoplasmic pH regulation: role of Na(+)-H+ antiport and Na(+)-dependent HCO3- transport systems. Am J Physiol. 1992 Sep;263(3 Pt 1):G407–13. doi: 10.1152/ajpgi.1992.263.3.G407. [DOI] [PubMed] [Google Scholar]
- 28.Feldman MJ, Morris GP, Dinda PK, Paterson WG. Mast cells mediate acid-induced augmentation of opossum esophageal blood flow via histamine and nitric oxide. Gastroenterology. 1996 Jan;110(1):121–8. doi: 10.1053/gast.1996.v110.pm8536848. [DOI] [PubMed] [Google Scholar]
- 29.Feldman MJ, Morris GP, Paterson WG. Role of substance P and calcitonin gene-related peptide in acid-induced augmentation of opossum esophageal blood flow. Dig Dis Sci. 2001 Jun;46(6):1194–9. doi: 10.1023/a:1010646809166. [DOI] [PubMed] [Google Scholar]
- 30.Solcia E, Villani L, Luinetti O, Trespi E, Strada E, Tinelli C, et al. Altered intercellular glycoconjugates and dilated intercellular spaces of esophageal epithelium in reflux disease. Virchows Arch. 2000 Mar;436(3):207–16. doi: 10.1007/s004280050032. [DOI] [PubMed] [Google Scholar]
- 31.Villanacci V, Grigolato PG, Cestari R, Missale G, Cengia G, Klersy C, et al. Dilated intercellular spaces as markers of reflux disease: histology, semiquantitative score and morphometry upon light microscopy. Digestion. 2001;64(1):1–8. doi: 10.1159/000048833. [DOI] [PubMed] [Google Scholar]
- 32.Caviglia R, Ribolsi M, Maggiano N, Gabbrielli AM, Emerenziani S, Guarino MP, et al. Dilated intercellular spaces of esophageal epithelium in nonerosive reflux disease patients with physiological esophageal acid exposure. Am J Gastroenterol. 2005 Mar;100(3):543–8. doi: 10.1111/j.1572-0241.2005.40978.x. [DOI] [PubMed] [Google Scholar]
- 33.Ravelli AM, Villanacci V, Ruzzenenti N, Grigolato P, Tobanelli P, Klersy C, et al. Dilated Intercellular Spaces: A Major Morphological Feature of Esophagitis. J Pediatr Gastroenterol Nutr. 2006 May;42(5):510–5. doi: 10.1097/01.mpg.0000215312.78664.b9. [DOI] [PubMed] [Google Scholar]
- 34.Tobey NA, Carson JL, Alkiek RA, Orlando RC. Dilated intercellular spaces: a morphological feature of acid reflux--damaged human esophageal epithelium. Gastroenterology. 1996 Nov;111(5):1200–5. doi: 10.1053/gast.1996.v111.pm8898633. [DOI] [PubMed] [Google Scholar]
- 35.Bove M, Vieth M, Dombrowski F, Ny L, Ruth M, Lundell L. Acid challenge to the human esophageal mucosa: effects on epithelial architecture in health and disease. Dig Dis Sci. 2005 Aug;50(8):1488–96. doi: 10.1007/s10620-005-2867-3. [DOI] [PubMed] [Google Scholar]
- 36.Farre R, De Vos R, Geboes K, Verbecke K, Vanden Berghe P, Depoortere I, et al. Critical role of stress in increased oesophageal mucosa permeability and dilated intercellular spaces. Gut. 2007 Sep;56(9):1191–7. doi: 10.1136/gut.2006.113688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long JD, Marten E, Tobey NA, Orlando RC. Effects of luminal hypertonicity on rabbit esophageal epithelium. Am J Physiol. 1997 Sep;273(3 Pt 1):G647–54. doi: 10.1152/ajpgi.1997.273.3.G647. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigo S, Abboud G, Oh D, DeMeester SR, Hagen J, Lipham J, et al. High intraepithelial eosinophil counts in esophageal squamous epithelium are not specific for eosinophilic esophagitis in adults. Am J Gastroenterol. 2008 Feb;103(2):435–42. doi: 10.1111/j.1572-0241.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- 39.Takubo K, Honma N, Aryal G, Sawabe M, Arai T, Tanaka Y, et al. Is there a set of histologic changes that are invariably reflux associated? Arch Pathol Lab Med. 2005 Feb;129(2):159–63. doi: 10.5858/2005-129-159-ITASOH. [DOI] [PubMed] [Google Scholar]
- 40.Jovov BJ, Djukic Z, Shaheen NJ, Orlando RC. E-cadherin cleavage in GERD. Gastroenterology. 2009;136(May):M1834. [Google Scholar]
- 41.Asaoka D, Miwa H, Hirai S, Ohkawa A, Kurosawa A, Kawabe M, et al. Altered localization and expression of tight-junction proteins in a rat model with chronic acid reflux esophagitis. J Gastroenterol. 2005 Aug;40(8):781–90. doi: 10.1007/s00535-005-1628-6. [DOI] [PubMed] [Google Scholar]
- 42.Miwa H, Oshima T, Sakurai J, Tomita T, Matsumoto T, Iizuka S, et al. Experimental oesophagitis in the rat is associated with decreased voluntary movement. Neurogastroenterol Motil. 2009 Mar;21(3):296–303. doi: 10.1111/j.1365-2982.2008.01221.x. [DOI] [PubMed] [Google Scholar]
- 43.Li FY, Li Y. Interleukin-6, desmosome and tight junction protein expression levels in reflux esophagitis-affected mucosa. World J Gastroenterol. 2009 Aug 7;15(29):3621–30. doi: 10.3748/wjg.15.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tobey NA, Gambling TM, Vanegas XC, Carson JL, Orlando RC. Physicochemical basis for dilated intercellular spaces in non-erosive acid-damaged rabbit esophageal epithelium. Dis Esophagus. 2008;21(8):757–64. doi: 10.1111/j.1442-2050.2008.00841.x. [DOI] [PubMed] [Google Scholar]
- 45.Bhat YM, Bielefeldt K. Capsaicin receptor (TRPV1) and non-erosive reflux disease. Eur J Gastroenterol Hepatol. 2006 Mar;18(3):263–70. doi: 10.1097/00042737-200603000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Bielefeldt K, Davis BM. Differential effects of ASIC3 and TRPV1 deletion on gastroesophageal sensation in mice. Am J Physiol Gastrointest Liver Physiol. 2008 Jan;294(1):G130–8. doi: 10.1152/ajpgi.00388.2007. [DOI] [PubMed] [Google Scholar]
- 47.Boudaka A, Worl J, Shiina T, Neuhuber WL, Kobayashi H, Shimizu Y, et al. Involvement of TRPV1-dependent and -independent components in the regulation of vagally induced contractions in the mouse esophagus. Eur J Pharmacol. 2007 Feb 5;556(1-3):157–65. doi: 10.1016/j.ejphar.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Yu S, Undem BJ, Kollarik M. Vagal afferent nerves with nociceptive properties in guinea-pig oesophagus. J Physiol. 2005 Mar 15;563(Pt 3):831–42. doi: 10.1113/jphysiol.2004.079574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodrigo J, Hernandez CJ, Vidal MA, Pedrosa JA. Vegetative innervation of the esophagus. III. Intraepithelial endings. Acta Anat (Basel) 1975;92(2):242–58. doi: 10.1159/000144444. [DOI] [PubMed] [Google Scholar]
- 50.Akiba Y, Mizumori M, Kuo M, Ham M, Guth PH, Engel E, et al. CO2 chemosensing in rat oesophagus. Gut. 2008 Dec;57(12):1654–64. doi: 10.1136/gut.2007.144378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kern M, Hofmann C, Hyde J, Shaker R. Characterization of the cerebral cortical representation of heartburn in GERD patients. Am J Physiol Gastrointest Liver Physiol. 2004 Jan;286(1):G174–81. doi: 10.1152/ajpgi.00184.2003. [DOI] [PubMed] [Google Scholar]
- 52.Tobey NA, Orlando RC. Mechanisms of acid injury to rabbit esophageal epithelium. Role of basolateral cell membrane acidification. Gastroenterology. 1991 Nov;101(5):1220–8. doi: 10.1016/0016-5085(91)90070-2. [DOI] [PubMed] [Google Scholar]
- 53.Tobey NA, Reddy SP, Keku TO, Cragoe EJ, Jr, Orlando RC. Mechanisms of HCl-induced lowering of intracellular pH in rabbit esophageal epithelial cells. Gastroenterology. 1993 Oct;105(4):1035–44. doi: 10.1016/0016-5085(93)90946-a. [DOI] [PubMed] [Google Scholar]
- 54.Jimenez P, Lanas A, Piazuelo E, Esteva F. Effect of growth factors and prostaglandin E2 on restitution and proliferation of rabbit esophageal epithelial cells. Dig Dis Sci. 1998 Oct;43(10):2309–16. doi: 10.1023/a:1026687126937. [DOI] [PubMed] [Google Scholar]
- 55.Jimenez P, Lanas A, Piazuelo E, Esteva F. Effects of extracellular pH on restitution and proliferation of rabbit oesophageal epithelial cells. Aliment Pharmacol Ther. 1999 Apr;13(4):545–52. doi: 10.1046/j.1365-2036.1999.00491.x. [DOI] [PubMed] [Google Scholar]
- 56.Tsuji H, Fuse Y, Kawamoto K, Fujino H, Kodama T. Healing process of experimental esophageal ulcers induced by acetic acid in rats. Scand J Gastroenterol Suppl. 1989;162:6–10. doi: 10.3109/00365528909091112. [DOI] [PubMed] [Google Scholar]
- 57.Lanas A, Soteras F, Jimenez P, Fiteni I, Piazuelo E, Royo Y, et al. Superoxide anion and nitric oxide in high-grade esophagitis induced by acid and pepsin in rabbits. Dig Dis Sci. 2001 Dec;46(12):2733–43. doi: 10.1023/a:1012735714983. [DOI] [PubMed] [Google Scholar]
- 58.Souza RF, Huo X, Mittal V, Schuler CM, Carmack SW, Zhang HY, et al. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology. 2009 Nov;137(5):1776–84. doi: 10.1053/j.gastro.2009.07.055. [DOI] [PubMed] [Google Scholar]
- 59.Naya MJ, Pereboom D, Ortego J, Alda JO, Lanas A. Superoxide anions produced by inflammatory cells play an important part in the pathogenesis of acid and pepsin induced oesophagitis in rabbits. Gut. 1997 Feb;40(2):175–81. doi: 10.1136/gut.40.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biancani P, Barwick K, Selling J, McCallum R. Effects of acute experimental esophagitis on mechanical properties of the lower esophageal sphincter. Gastroenterology. 1984 Jul;87(1):8–16. [PubMed] [Google Scholar]
- 61.Rieder F, Cheng L, Harnett KM, Chak A, Cooper GS, Isenberg G, et al. Gastroesophageal reflux disease-associated esophagitis induces endogenous cytokine production leading to motor abnormalities. Gastroenterology. 2007 Jan;132(1):154–65. doi: 10.1053/j.gastro.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 62.Cheng L, Harnett KM, Cao W, Liu F, Behar J, Fiocchi C, et al. Hydrogen peroxide reduces lower esophageal sphincter tone in human esophagitis. Gastroenterology. 2005 Nov;129(5):1675–85. doi: 10.1053/j.gastro.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 63.Cheng L, Cao W, Fiocchi C, Behar J, Biancani P, Harnett KM. Platelet-activating factor and prostaglandin E2 impair esophageal ACh release in experimental esophagitis. Am J Physiol Gastrointest Liver Physiol. 2005 Sep;289(3):G418–28. doi: 10.1152/ajpgi.00024.2005. [DOI] [PubMed] [Google Scholar]
- 64.Cao W, Cheng L, Behar J, Fiocchi C, Biancani P, Harnett KM. Proinflammatory cytokines alter/reduce esophageal circular muscle contraction in experimental cat esophagitis. Am J Physiol Gastrointest Liver Physiol. 2004 Dec;287(6):G1131–9. doi: 10.1152/ajpgi.00216.2004. [DOI] [PubMed] [Google Scholar]
- 65.White RJ, Zhang Y, Morris GP, Paterson WG. Esophagitis-related esophageal shortening in opossum is associated with longitudinal muscle hyperresponsiveness. Am J Physiol Gastrointest Liver Physiol. 2001 Mar;280(3):G463–9. doi: 10.1152/ajpgi.2001.280.3.G463. [DOI] [PubMed] [Google Scholar]
- 66.Paterson WG. Role of mast cell-derived mediators in acid-induced shortening of the esophagus. Am J Physiol. 1998 Feb;274(2 Pt 1):G385–8. doi: 10.1152/ajpgi.1998.274.2.G385. [DOI] [PubMed] [Google Scholar]
- 67.Paterson WG, Kolyn DM. Esophageal shortening induced by short-term intraluminal acid perfusion in opossum: a cause for hiatus hernia? Gastroenterology. 1994 Dec;107(6):1736–40. doi: 10.1016/0016-5085(94)90814-1. [DOI] [PubMed] [Google Scholar]
- 68.Fass R. Gastroesophageal reflux disease revisited. Gastroenterol Clin North Am. 2002 Dec;31(4 Suppl):S1–10. doi: 10.1016/s0889-8553(02)00046-8. [DOI] [PubMed] [Google Scholar]
- 69.Fass R, Ofman JJ. Gastroesophageal reflux disease--should we adopt a new conceptual framework? Am J Gastroenterol. 2002 Aug;97(8):1901–9. doi: 10.1111/j.1572-0241.2002.05912.x. [DOI] [PubMed] [Google Scholar]
- 70.Labenz J, Nocon M, Lind T, Leodolter A, Jaspersen D, Meyer-Sabellek W, et al. Prospective follow-up data from the ProGERD study suggest that GERD is not a categorial disease. Am J Gastroenterol. 2006 Nov;101(11):2457–62. doi: 10.1111/j.1572-0241.2006.00829.x. [DOI] [PubMed] [Google Scholar]
- 71.Pace F, Pallotta S, Vakil N. Gastroesophageal reflux disease is a progressive disease. Dig Liver Dis. 2007 May;39(5):409–14. doi: 10.1016/j.dld.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 72.Cameron AJ, Lomboy CT. Barrett's esophagus: age, prevalence, and extent of columnar epithelium. Gastroenterology. 1992 Oct;103(4):1241–5. doi: 10.1016/0016-5085(92)91510-b. [DOI] [PubMed] [Google Scholar]
- 73.Hanna S, Rastogi A, Weston AP, Totta F, Schmitz R, Mathur S, et al. Detection of Barrett's esophagus after endoscopic healing of erosive esophagitis. Am J Gastroenterol. 2006 Jul;101(7):1416–20. doi: 10.1111/j.1572-0241.2006.00631.x. [DOI] [PubMed] [Google Scholar]
- 74.Orlando RC. Pathogenesis of reflux esophagitis and Barrett's esophagus. In: Cappell MS, editor. Medical Clinics of North America. Philadelphia: WB Saunders Company; 2005. pp. 219–42. [DOI] [PubMed] [Google Scholar]
- 75.Su Y, Chen X, Klein M, Fang M, Wang S, Yang CS, et al. Phenotype of columnar-lined esophagus in rats with esophagogastroduodenal anastomosis: similarity to human Barrett's esophagus. Lab Invest. 2004 Jun;84(6):753–65. doi: 10.1038/labinvest.3700079. [DOI] [PubMed] [Google Scholar]
- 76.Alvaro-Villegas JC, Sobrino-Cossio S, Hernandez-Guerrero A, Alonso-Larraga JO, de-la-Mora-Levy JG, Molina-Cruz A, et al. Dilated intercellular spaces in subtypes of gastroesophagic reflux disease. Rev Esp Enferm Dig May. 102(5):302–7. doi: 10.4321/s1130-01082010000500003. [DOI] [PubMed] [Google Scholar]
- 77.Orlando RC. Mucosal defense in Barrett's esophagus. In: Sharma P, Sampliner R, editors. Barrett's esophagus and esophageal adenocarcinoma. 2nd. Malden: Blackwell Publishing; 2006. [Google Scholar]
- 78.El-Serag HB, Petersen NJ, Carter J, Graham DY, Richardson P, Genta RM, et al. Gastroesophageal reflux among different racial groups in the United States. Gastroenterology. 2004 Jun;126(7):1692–9. doi: 10.1053/j.gastro.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 79.Wang A, Mattek NC, Holub JL, Lieberman DA, Eisen GM. Prevalence of complicated gastroesophageal reflux disease and Barrett's esophagus among racial groups in a multi-center consortium. Dig Dis Sci. 2009 May;54(5):964–71. doi: 10.1007/s10620-009-0742-3. [DOI] [PMC free article] [PubMed] [Google Scholar]